Abstract
The aim of the present work is to bring out an exergy analysis of the hydrocarbon refrigerant mixture of R290/R600a as an alternative to R134a on the performance of a domestic refrigerator which is originally designed to work with R134a. The performance of both refrigerants was evaluated using an exergy analysis. The effects of evaporator temperature on the coefficient of performance (COP), exergy loss, exergic efficiency, and efficiency defect in the four major components of the system for R134a and R290/R600a mixture were experimentally investigated. The results obtained showed that the COP of R290/R600a mixture was improved up to 28.5 % than that of R134a. The highest average exergic efficiency of the system (42.1 %) was obtained using R290/R600a mixture at an evaporator temperature of 263 K (−10 °C). The overall efficiency defect in the refrigeration cycle working with R290/R600a mixture was consistently better than R134a.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Domestic refrigerators are major energy consuming appliance in household environment [1]. R134a is the most widely used refrigerant in domestic refrigerators, due to its excellent thermodynamics and thermophysical properties. But, it must be phased out soon according to Kyoto protocol due to its high global warming potential (GWP) of 1,300 times more than CO2. Many researchers [2–4] have reported that GWP of hydrofluorocarbon (HFC) refrigerants is more significant even though it has less than that of chlorofluorocarbons (CFC) refrigerants. Regarding the issue of high GWP of R134a, thermal storage using secondary refrigerants or phase change materials can relieve this issue by reduction of R134a charge for domestic refrigerators [5–7]. Another option for this issue is to find promising environmentally-friendly R134a alternatives. Some R134a refrigerant substitutes that meet these requirements are a key process in this investigation. Many researchers have reported that hydrocarbon-mixed refrigerants are found to be an energy efficient and environmentally-friendly alternative option in household refrigerators.
Pure hydrocarbon refrigerants are not suitable as drop-in substitutes for R134a due to its mismatch in volumetric cooling capacity and operating pressure [8]. Jung et al. [9] experimented with R290/R600a (in the ratio of 60:40, by mass fraction) as an alternative to R12 in 299 and 465 l domestic refrigerators and reported that coefficient of performance (COP) and energy efficiency were improved by 2.3 and 4 %, respectively. Akash and Said [10] experimented with LPG (composed of R290, R600, and R600a, in the ratio of 30:55:15, by mass) as an alternative to R12 in domestic refrigerators at various mass charges 50, 80, and 100 g. The results reported that 80 g of LPG showed the best performance compared to that of R12. Lee and Su [11] carried out an experimental study on the performance of a domestic vapor compression refrigeration system with isobutane (R600a) as the refrigerant. The COP of the system was improved better than R12 and R134a. Wongwises and Chimres [12] investigated with hydrocarbon mixtures and HC/HFC mixtures at different mass ratios in a 239 l domestic refrigerator at an ambient temperature of 298 K to replace R134a. It has been reported that R290/R600 mixture (in the ratio of 60:40, by mass fraction) is the most appropriate alternative. Fatouh and Kafafy [13] experimented with LPG (composed of 60 % of R290 and 40 % of commercial butane) as an alternative to R134a in a 280 l domestic refrigerator at 316 K ambient temperature. The results reported that COP of the refrigerator using LPG was higher than that of R134a by about 7.6 % with lower values of energy consumption by about 10.8 %. Based on the theoretical investigation, it has been reported that capillary tube length using ternary hydrocarbon refrigerant mixture (composed of R290, R600, and R600a) with 60 % R290 mass fraction requires 30 % increase in capillary tube length compared to R134a.
Considering the performance study of commercial refrigeration equipments and comparative studies of the behavior of R134a have shown that R134a exhibits the worst performance when comparing with R290 at the same original working conditions in a system designed for R12. Mani and Selladurai [14] carried out a performance study of vapor compression refrigeration system with the new R290/R600a refrigerant mixture as drop-in substitute compared with R12 and R134a. The experimental results showed that the R290/R600a refrigerant mixture had 28.6–87.2 % higher refrigerating capacity than R134a. Mohanraj et al. [15] conducted an experimental investigation with R290/R600a mixture as an alternative to R134a in a domestic refrigerator system and reported that the COP of the system was improved with R290/R600a mixture than R134a. The results also revealed that the life of the compressor is improved due to lesser discharge temperature of R290/R600a mixture.
Exergy analysis of a thermal system can be performed by analyzing the components of the system separately. Identifying the major components of exergy loss gives the solution for the improvement of a system efficiency. The main objective of the exergy analysis is to find the minimum power required for a desired output and to minimize the energy loss in the system components. There are few articles in the literature related to thermodynamic analysis which deals with exergy analysis of the refrigerating systems [16–18]. Said and Ismail [19] assessed the theoretical performances of R123, R134a, R11, and R12 as refrigerants. It was reported that for a specific amount of desired exergy, more compression work is required for R123 and R134a than R11 and R12. The differences are not very significant at high evaporation temperatures and hence R123 and R134a should not be excluded as alternative refrigerants. Also, in the study they obtained an optimum evaporation temperature for each condensation temperature, which yields the highest exergic efficiency.
Aprea and Greco [20] compared the performance between R22 and R407C and suggested that R407C is a promising drop-in substitute for R22. Experimental tests were performed in a vapor compression plant with a reciprocating compressor to evaluate the compressor performance using R407C in comparison to R22. The plant overall exergic performance was also evaluated and revealed that R22 performance is consistently better than that of R407C.
Aprea and Renno [21] studied experimentally the performance of a commercial vapor compression refrigeration plant, generally adopted for preservation of foodstuff, using R22 and its candidate substitute R417A as working fluids. They reported that the substitute refrigerant (R417A), which is a non-azeotropic mixture and non-ozone depleting, can serve as a long term replacement for R22; it can be used in new and existing direct expansion R22 systems using traditional R22 lubricants. Also in their analysis, the best exergic performances of R22 in comparison with those of R417A were determined in terms of the coefficient of performance, exergic efficiency, and exergy destroyed in the plant components.
Padilla et al. [22] performed an experimental and exergy analysis in a domestic refrigerator with R413A as a replacement of R12. They used fans in the condenser and evaporator to vary the air flows and reported that the overall exergy efficiency of the system working with R413A is consistently better than that of R12. Kumar et al. [23] derived a method to perform a exergy analysis of a vapor compression system using R11 and R12. Arora and Kaushik [24] did a detailed exergy analysis in the vapor compression cycle and developed a computational model to calculate COP, exergy loss, exergy efficiency, and efficiency defects for R502, R404, and R507A. They reported that R507a is a better substitute to R502a than R404a.
Based on the above results, the exergy analysis of domestic refrigeration system using hydrocarbon mixture (HCM) composed of 45 % of R290 and 55 % of R600a as an alternative to R134a is carried out. During the test the evaporator temperature (\( T_{\text{eva}} \)) was maintained at 263 K and the ambient temperature (\( T_{0} \)) was measured as 305 K. Usually, the factors like COP, volumetric efficiency, compressor power consumption, and the condenser and the evaporator temperatures are considered for the analysis. In this study, a different approach has been adopted for comparing the performance of new refrigerant mixture with R134a at the same environment conditions in the system. A comparative exergy analysis based on experimental tests was carried out to evaluate the thermodynamic performance of R290/R600a refrigerant mixture as an alternative to R134a in an unmodified R134a domestic refrigerator. These results cannot be obtained in a traditional energy analysis which leads to an incomplete thermodynamic analysis. Hence, this study includes an exergy-based analysis which suits well between the classical thermodynamic approach based on the first law and the exergy approach based on the second law for the evaluation of a refrigeration system.
Experimental setup and procedure
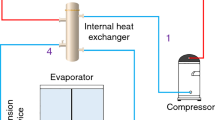
The photographic view and the line diagram of a domestic refrigerator test rig unit is shown in Fig. 1a, b. The experimental setup has a 200 l, top-freezer mounted single-door domestic refrigerator with single evaporator. It consists of a hermetically sealed reciprocating compressor with 280 W nominal input power at 220 V in 50 HZ, a wire mesh air-cooled condenser, a capillary strainer (filter), and five capillary tubes of different lengths with ball valves and an evaporator. In order to optimize the capillary tube length for hydrocarbon refrigerant mixture, five capillary tubes of 0.78 mm diameter with different lengths (3, 3.5, 4, 4.5, and 5 m) were provided. To estimate the actual COP and refrigeration capacity of the domestic refrigerator, the evaporator similar to one used in refrigerator was kept in a calorimeter filled with brine solution as a secondary refrigerant. The calorimeter was insulated to reduce the ambient heat infiltration. Four ball valves were fixed in the circuit between the capillary tube outlet and compressor suction to divert the refrigerant flow to either one of the evaporator (in the refrigerator cabin or calorimeter). Another five ball valves were fixed at the capillary tube inlet for choosing different capillary tube lengths. The refrigerator was instrumented with four compound pressure gauges with an accuracy of ±0.25 % at the inlet and outlet of the compressor for measuring the suction and discharge pressures and at the inlet and outlet of the capillary tubes. Four calibrated RTD (Pt100) temperature sensors with an accuracy of ±0.25 K were provided in the compressor outlet, condenser outlet, evaporator inlet, and inlet of the compressor. The power consumption of the compressor was measured by using an energy meter with 0.01 kWh of accuracy.
Initially, the test rig was thoroughly checked by flushing with nitrogen gas to eliminate impurities, moisture, and other foreign materials inside the system, which may affect the accuracy of the experimental results. The evacuation was carried out with the help of a vacuum pump and refrigerant was charged into the system with the help of charging unit. The refrigerator was first charged with R134a of 115 g and tested at the intended various conditions. During experimentation with R134a, 3.5 m capillary tube length which is normally used in the domestic refrigerator was used. The tests were carried out by connecting the evaporator inside the calorimeter with the system. During the test the ambient temperature and condenser temperature are measured as 305 and 313 K, respectively, and the evaporator temperature is maintained at 263 K. The actual refrigeration capacity and COP of the refrigerator were calculated as per the procedure followed by Sekhar and Lal [25]. The energy consumption of the compressor was measured using energy meter. All the experimental observations were made after attaining the steady-state conditions. After completing the baseline reference test with R134a, the refrigerant was recovered from the system. Before experimenting with R290/R600a mixture, the length of the capillary tube was optimized for maximum COP. During capillary tube optimization, the refrigerator was charged with various masses like 40, 50, 60, 70, and 80 g of R290/R600a mixture and the maximum COP was observed with 4.5 m capillary tube length and 60 g of R290/R600a mixture. Hence, 4.5 m capillary tube was taken for the experimentation with R290/R600a mixture. Then, the refrigerator was charged with 60 g of R290/R600a mixture and these tests were repeated. Since the mixture is zoetrope, the refrigerant is charged in liquid state and the charge quantity was ensured with the help of electronic balance having an accuracy of ±0.01 g. In order to reduce the experimental uncertainties, experiments were repeated for three times and average values were considered. The variation in experimental values from the average value is within ±5 %. Temperatures and pressures are recorded at frequent intervals. The power consumption of the refrigerator during tests was measured after attaining the steady-state condition. The measured values were used to study the performance characteristics of the refrigerator.
Exergy analysis
Exergy (or availability) is a thermodynamic property that represents the maximum work that can be obtained from a fluid stream in a reversible process until it reaches the thermodynamic equilibrium with the surroundings. Exergy analysis can be used to evaluate the performance of thermodynamic system. Unlike energy, exergy is not conserved, it can be destroyed. With the experimental data obtained from the tests using both R134a and R290/R600a mixture, the exergy analysis is carried out to find exergy loss of the domestic refrigerator in order to obtain a quantitative measurement of the system inefficiency. Under the assumption that the change of kinetic and potential energy is negligible and the ambient temperature is \( T_{0} \), the exergy is given by the equation:
For the system shown in Fig. 1b, component wise the exergy balance equation can be written as follows:
-
(a)
For compressor:
$$ {\text{Compressor work}},\quad W_{\text{c}} = \dot{m}(h_{2} - h_{1} ) $$(3)$$ {\text{The exergy at inlet}}\quad \psi_{1} = \dot{m}(h_{1} - T_{0} s_{1} ) +W_{\text{c}} $$(4)$$ {\text{The exergy at outlet}}\quad \psi_{2} = \dot{m}(h_{2} - T_{0} s_{2} ) $$(5)The exergy loss (due to irreversibility) in the compressor
$$ I_{\text{comp}} = \dot{m}(h_{1} - T_{0} s_{1} ) + W_{\text{c}} - \dot{m}(h_{2} - T_{0} s_{2} ) $$(6) -
(b)
For condenser:
$$ {\text{Heat removed at condenser,}}\quad Q_{\text{cond}} = \dot{m}(h_{2} - h_{3} ) $$(7)$$ {\text{The exergy at inlet}}\quad \psi_{2} = \dot{m}(h_{2} - T_{0} s_{2} ) $$(8)$$ {\text{The exergy at outlet}}\quad \psi_{3} = \dot{m}(h_{3} - T_{0} s_{3} ) $$(9)The exergy loss in the condenser
$$ I_{\text{cond}} = \dot{m}(h_{2} - T_{0} s_{2} ) - \dot{m}(h_{3} - T_{0} s_{3} ) - Q_{\text{cond}} \left( {1 - \frac{{T_{0} }}{{T_{\text{cond}} }}} \right) $$(10) -
(c)
For capillary tube (expansion device):
$$ {\text{The exergy at inlet}}\quad \psi_{3} = \dot{m}(h_{3} - T_{0} s_{3} ) $$(11)$$ {\text{The exergy at outlet}}\quad \psi_{4} = \dot{m}(h_{4} - T_{0} s_{4} ) $$(12)The exergy loss in the capillary tube
$$ I_{\exp } = \dot{m}(h_{3} - T_{0} s_{3} ) - \dot{m}(h_{4} - T_{0} s_{4} ) $$(13)Since the enthalpy is constant during the expansion process, \( h_{3} = h_{4} \) the above equation can be written as
$$ I_{\exp } = \dot{m}T_{0} (s_{4} - s_{3} ) $$(14) -
(d)
For evaporator:
$$ {\text{Heat addition in evaporator}},\quad Q_{\text{eva}} = \dot{m}(h_{1} - h_{4} ) $$(15)$$ {\text{The exergy at inlet}}\quad \psi_{4} = \dot{m}(h_{4} - T_{0} s_{4} ) + Q_{\text{eva}} \left( {1 - \frac{{T_{0} }}{{T_{\text{eva}} }}} \right) $$(16)$$ {\text{The exergy at outlet}}\quad \psi_{1} = \dot{m}(h_{1} - T_{0} s_{1} ) $$(17)The exergy loss in the evaporator
$$ I_{\text{eva}} = \dot{m}(h_{4} - T_{0} s_{4} ) + Q_{\text{eva}} \left( {1 - \frac{{T_{0} }}{{T_{\text{eva}} }}} \right) - \dot{m}(h_{1} - T_{0} s_{1} ) $$(18)The total exergy loss of the system
$$ I_{\text{Total}} = I_{\text{comp}} + I_{\text{cond}} + I_{ \exp } + I_{\text{eva}} $$(19)
Also, the exergy efficiency is given by
The efficiency defect (\( \delta_{\text{i}} \)) is evaluated for each device of the system, considering the ratio of exergy used in each component (\( \psi_{\text{i}} \)) to the exergy required to sustain the process (exergy input through the compressor,\( W_{\text{c}} \)).
The overall performance of a refrigeration system is determined by evaluating its COP and is calculated as the ratio between the refrigeration capacity (\( Q_{\text{eva}} \)) and the electrical power supplied to the compressor (\( W_{\text{c}} \))
Results and discussion
Experimental values of temperature, pressure, and power consumption obtained during the test were used to calculate various parameters like COP, exergy efficiency, and efficiency defects of various components and the results are discussed in this section. Figure 2 shows the variation of COP with varying evaporator temperature of R134a and R290/R600a mixture. It can be observed that the COP increases with increase in evaporator temperature for both the refrigerants. This is due to decrease in work of compression when the temperature difference between the evaporator and the ambient is reduced. The reduced temperature difference results in reduced load on the compressor. The results obtained showed that the COP of R290/R600a mixture is higher than that of R134a. Refrigerator working with R134a exhibited significantly lower COP which requires higher electric power consumption in order to provide the same refrigerating effect. Apart from direct costs, this would create environmental pollution, since more fuel must be burned and higher amount of carbon dioxide would be released into the atmosphere.
Figure 3 shows the variation of exergy efficiency with evaporator temperature for R134a and R290/R600a mixture. Exergy efficiency decreases with increase in evaporator temperature. This is due to the increase of irreversibility of the components when the evaporator temperature increases. The exergy efficiency of R290/R600a mixture is higher than that of R134a from 7.1 to 28 % which depends on the evaporator temperature. Exergy efficiency of 38.6 and 42.1 % were obtained at evaporator temperature of 263 K for R134a and R290/R600a mixture, respectively. R134a refrigerant exhibits very less exergy efficiency for higher evaporator temperature than R290/R600a mixture.
Figure 4 gives the comparison of efficiency defect in compressor for R134a and R290/R600a refrigerant mixture with varying evaporator temperature. As shown in the Fig. 4, efficiency defect in compressor decreases with decrease in evaporator temperature. This is because the exergy used by the compressor is more when the evaporator temperature is reduced because of higher temperature difference. The result obtained showed that efficiency defect in compressor with R290/R600a refrigerant mixture varies from 0.96 to 4.04 % lower than R134a and depends on the evaporator temperature. For lower temperature R290/R600a refrigerant mixture gives considerable amount of less efficiency defect in compressor. Figure 4 shows that for higher evaporator temperature between 273 and 298 K, the difference in efficiency defect of both the refrigerants are very less which means that the exergy loss in the compressor is more irrespective of refrigerant used.
Figure 5 shows the variation of efficiency defect in condenser with evaporator temperature for R134a and R290/R600a mixtures. As shown in the figure, efficiency defect in condenser decreases with decrease in evaporator temperature. This is because the exergy used by the condenser is more when the evaporator temperature is reduced due to higher temperature difference. The result obtained showed that efficiency defect in condenser is 31.03 % lower for R290/R600a mixture than R134a.
Figure 6 shows the variation of efficiency defect in capillary tube with evaporator temperature for R134a and R290/R600a mixtures. As shown in Fig. 6, efficiency defect in capillary tube decreases with increase in evaporator temperature, because the exergy used by the capillary tube is more when the evaporator temperature is reduced due to higher temperature difference. The result obtained showed that efficiency defect in capillary tube is 30.4 % lower for R290/R600a mixture than R134a.
Figure 7 shows the variation of efficiency defect in evaporator with evaporator temperature for R134a and R290/R600a mixtures. The figure showed that the efficiency defect in evaporator decreases with decrease in evaporator temperature, because of more compressor work input due to less cooling coil temperature. The efficiency defects in evaporator were 33.3 % lower for R290/R600a mixture than R134a. As shown in Fig. 7, the overall efficiency defect in evaporator is marginal in comparison with those of other components in the system (Figs. 5, 6, 7). This refers that transferring heat at lower temperature difference can further reduce the efficiency defect in the evaporator.
Conclusions
An exergy analysis was conducted in a single-evaporator domestic refrigerator between the evaporation and condensation temperatures ranging from −10 to 40 °C by using R134a and R290/R600a mixture refrigerants. Based on both experimental study and exergy analysis on the performance of R134a retrofit with R290/R600a mixture in domestic refrigerator, the following conclusions were drawn:
-
The COP of the domestic refrigerator using R290/R600a mixture as a refrigerant has higher value than R134a. It is obvious that the refrigerant with lower COP will consume more energy, which will have great adverse effect on the environment.
-
The highest exergy efficiency was obtained using R290/R600a refrigerant mixture in the system. The average exergy efficiencies of the system using R290/R600a refrigerant mixture was higher than that of the R134a.
-
The overall efficiency defect in the system working with R290/R600a refrigerant mixture is consistently better (lower) than R134a.
-
Among the four major components the highest efficiency defects were obtained in the compressor, condenser, and capillary tube.
-
Generally, the experimental domestic refrigeration system performed well using R290/R600a refrigerant mixture than using R134a as working fluid.
Thus, it can be concluded that R290/R600a mixture could be an ozone-friendly, exergy efficient, and safe viable alternative to R134a for domestic refrigeration systems with the main advantage that it can be replaced directly without the need to replace or modify any system component.
Abbreviations
- COP:
-
Coefficient of performance
- GWP:
-
Global warming potential
- h :
-
Specific enthalpy (J kg−1)
- T 0 :
-
Ambient temperature (K)
- s :
-
Specific entropy (J kg−1 K−1)
- \( \dot{m} \) :
-
Mass (refrigerant) flow rate (kg s−1)
- \( W_{\text{c}} \) :
-
Compressor work input (W)
- \( Q_{\text{cond}} \) :
-
Heat removed at condenser (W)
- \( Q_{\text{eva}} \) :
-
Refrigerating effect (W)
- \( T_{\text{cond}} \) :
-
Condenser temperature (K)
- \( T_{\text{eva}} \) :
-
Evaporator temperature (K)
- ψ:
-
Exergy flow (J kg−1)
- η x :
-
Exergy efficiency (%)
- δ:
-
Efficiency defect
- comp:
-
Compressor
- cond:
-
Condenser
- exp:
-
Expansion device
- eva:
-
Evaporator
References
Radermacher R, Kim K. Domestic refrigerators: recent developments. Int J Refrig. 1996;19:61–9.
Ashford P, Clodic D, McCulloch A, Kuijpers L. Emission from the foam and refrigeration sectors comparison with atmospheric concentrations, Part 2: results and discussion. Int J Refrig. 2004;27:701–16.
McCulloch A, Lindley AA. From mine to refrigeration: a life cycle inventory analysis of the production of HFC134a. Int J Refrig. 2003;26:865–72.
McCulloch A, Midgley PM, Ashford P. Release of refrigerant gases (CFC12, HCFC22, HFC 134a) to the atmosphere. Atmospheric Environ. 2003;37:889–902.
Li G, Hwang Y, Radermacher R. Review of cold storage materials for air conditioning application. Int J Refrig. 2012;35:2053–77.
Li G, Hwang Y, Radermacher R, Chun H-H. Review of cold storage materials for subzero applications. Energy. 2012. doi:10.1016/j.energy.2012.12.0025 Available online 8 Jan 2013.
Li G, Liu D, Xie Y. Study on thermal properties of TBAB-THF hydrate mixture for cold storage by DSC. J Therm Anal Calorim. 2010;102(2):819–26.
Fatouh M, ElKafafy M. Assessment of propane/commercial butane mixtures as possible alternatives to R134a in domestic refrigerators. Energy Convers Manag. 2006;47:2644–58.
Jung DS, Kim C-B, Song K, Park BJ. Testing of propane/isobutene mixture in domestic refrigerators. Int J Refrig. 2000;23:517–27.
Akash BA, Said SA. Assessment of LPG as a possible alternative toR-12 in domestic refrigerator. Energy Convers Manag. 2003;44:381–8.
Lee YS, Su CC. Experimental studies of isobutane (R600a) as the refrigerant indomestic refrigeration system. Appl Therm Eng. 2002;22:507–19.
Wongwises S, Chimres N. Experimental study of hydrocarbon mixtures to replace HFC134a in domestic refrigerators. Energy Convers Manag. 2005;46:85–100.
Fatouh M, ElKafafy M. Experimental evaluation of a domestic refrigerator working with LPG. Appl Therm Eng. 2006;26:1593–603.
Mani K, Selladurai V. Experimental analysis of new refrigerant mixture asdrop-in replacement for CFC12 and HFC134a. Int J Therm Sci. 2008;47:1490–5.
Mohanraj M, Jeyaraj S, Muraleedaran C. Experimental investigation of R290/R600a as an alternative to R134a in a domestic refrigerator. Int J Therm Sci. 2009;48:1036–42.
Anand S, Tyagi SK. Exergy analysis and experimental study of a vapor compression refrigeration cycle: a technical note. J Therm Anal Calorim. 2012;110:961–71.
Anand S, Gupta A, Tyagi SK. Simulation studies of refrigeration cycles: a review. Renew Sustain Energy Rev. 2013;17:260–77.
Tyagi SK, Park SR, Tyagi VV, Anand S. Second law based performance evaluation and parametric study of a sea water source cascade heat pump. Int J Exergy. 2010;7(3):369–86.
Said SAM, Ismail B. Exergetic assessment of the coolants HCFC 123, HFC 134a, CFC 11and CFC 12. Energy. 1994;2011(19):1181–6.
Aprea C, Greco A. An exergetic analysis of R22 substitution. Appl Therm Eng. 2002;22:1455–69.
Aprea C, Renno C. Experimental comparison of R22 with R417A performance in a vapor compression refrigeration plant subjected to a cold store. Energy Convers Manag. 2004;45:1807–19.
Padilla M, Remi R, Jocelyn B. Exergy analysis of R413A as replacement of R12 in a domestic refrigeration system. Energy Convers Manag. 2010;51:2195–201.
Kumar S, Prevost M, Bugarel R. Exergy analysis of a compression refrigeration system. Heat Recovery Syst CHP. 1989;9:151–7.
Arora A, Kaushik SC. Theoretical analysis of vapour compression system with R502, R404A and R507A. Int J Refrig. 2008;31:998–1005.
Sekhar SJ, Lal DM. HFC134a/HC600a/HC290 mixture a retrofit for CFC12systems. Int J Refrig. 2005;28:735–43.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saravanakumar, R., Selladurai, V. Exergy analysis of a domestic refrigerator using eco-friendly R290/R600a refrigerant mixture as an alternative to R134a. J Therm Anal Calorim 115, 933–940 (2014). https://doi.org/10.1007/s10973-013-3264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3264-3