Abstract
The effect of different air-drying temperatures on drying kinetics of apricot pomace was investigated. The apricot pomaces were dried at 40, 50, 60 and 70 °C in the cabinet dryer. It is observed that drying characteristics of apricot pomace were greatly influenced by air temperature. Color values were examined for samples with dried apricot pomace at different temperatures. Ten well-known thin-layer models were used to predict drying kinetics by nonlinear analysis of regression. The Aghbashlo et al. and Midilli et al. models best fitted the experimental data for the whole range of temperatures. Effective moisture diffusivity ranged from 1.014×10−9 to 1.859×10−9 m2 s−1 and calculated using the Fick’s second law. Activation energy was estimated by a modified Arrhenius-type equation and found to be 19.41 kJ mol−1. Color analysis showed that the values of the parameters L, a, b, C and H decreased with drying temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Food and Agriculture Organization of the United Nations (FAO) estimates that roughly one-third of all edible food produced for human consumption is wasted or otherwise lost from the food supply per year, or about 1.3 billion metric tonnes (M t) [1]. The most economical and environmentally friendly production processes with increasing population consist of production waste, so it is important to develop and evaluate the most efficient way of preventing waste of resources [2]. Wine, juice and soft beverage industry has a large quantity of wastes, such as peel, seed, pomace, rags, kernels. Pomace is the pulp residue remaining after fruit has been crushed in order to extract its juice [3]. Food industry waste is divided into solid and liquid waste. Different methods for evaluating the food industry waste are available in the literature: Anaerobic digestion, fermentation, obtained of compost, pyrolysis, burn to obtain fuel, production animal feed or silage and feed supplements extraction of valuable components such as pectin and dietary fiber [4,5,6,7].
Fruit pomace can be used in food as flour substitutes in bread, cookies, macaroni muffin, they have also been added in soups, sauces, mayonnaise, jams, fruit bars, yogurt obtained from waste fruit, and vegetable pomace compounds such as dietary fiber, phenolic, carotenoid, tocopherol and ascorbic acid provide cheap and natural raw material for functional food production [8, 9].
Apricot (Prunus armeniaca L.) is included in the genus prunus of the subfamily Prunoideae in the family Rosaceae. It is mainly distributed throughout Central Asia, West Asia, the Mediterranean region and Western China. According to Food and Agricultural Organization (FAO), the total global production of apricots is about 4.1 million tons. Turkey, Iran, Uzbekistan, Algeria and Italy are the major producers of apricot [10]. Apricot is a climacteric and seasonal fruit with a very short storage life due, in part, to a high respiration rate and a rapid ripening process. To extend the shelf life of this fruit, several preservation methods have been improved, such as drying, canning, packing in a controlled/modified atmosphere, and processing to produce fruit juice, fruit puree, jam, marmalade or pestil [11, 12].
Apricot pomace is a by-product of apricot juice processing industry. The processed apricot pomace has generally high moisture contents, and the removal of moisture before the production of high value-added products such as carbohydrates, acids, vitamins, minerals and dietary fiber [13] is needed. Drying has always been of great importance to the preservation of agricultural products and their by-products.
Drying is the most widely used commercial process to preserve foods because, compared to other long-term preservation methods, it is less costly and easier to operate. The main purpose of drying products is to allow longer periods of storage, minimize packaging requirements and reduce shipping weights [14]. There are numerous studies and data available in the literature regarding the thin-layer drying of various agricultural products [15,16,17]. However, there is no information in the literature about hot-air drying of apricot pomace and effect of drying on quality criteria and color of final product. The main objectives of this study were to investigate the effect of hot air on the drying rate and time, fit the experimental data to ten thin-layer drying models, compute effective moisture diffusivity and determine chemical composition of apricot pomace.
Experimental
Materials
The apricot pomace, which is a waste material of apricot juice processing, was obtained from “Döhler Natural Food and Beverage Ingredients Factory, Karaman-Turkey” in ice bags and stored in a refrigerator (Arcelik 1050T, Eskisehir, Turkey) until usage at −18 °C.
Experimental setup
Drying experiments were performed in a cabinet type dryer (API & PASILAC Limited of Carlisle, Cumbria, UK). The cabinet dryer was described previously by Doymaz [18]. It was constructed from stainless steel sheets formed as a rectangular tunnel of dimensions 0.54 m × 1.4 m × 1.02 m. The drying tray had an area of size 0.3 m × 0.3 m. In this dryer, air is flowing horizontally through the samples. The dryer was adjusted to the selected temperature for about half an hour before the start of the experiments in order to achieve the steady-state conditions. Then, the samples were spread thickness of 1±0.1 cm in a single layer on a tray in the dryer and dried at 40, 50, 60 and 70 °C, a constant air velocity of 2±0.1 m s−1. The relative humidity of air was determined by using wet and dry bulb temperatures from the psychrometric chart. The velocity of air passing through the system was measured by a 0.4–30-m s−1-range anemometer (model AM-4201, Lutron Electronic, Taipei, Taiwan). The moisture losses of apricot pomace were recorded at 30 min intervals during the drying process by a digital balance (Mettler, model BB3000, Mettler-Toledo AG, Grefensee, Switzerland) and an accuracy of ±0.1 g. Drying was stopped when the moisture contents of the samples were about 0.001 kg water kg−1 dry matter. The product was cooled and packed in low-density polyethylene (LDPE). The experiments were repeated twice, and the average of the moisture ratio at each value was used for the drawing of the drying curves.
Chemical compositional analysis of apricot pomaces
The initial moisture content of the fresh apricot pomace was determined according to AOAC 934.06 [19]. The pH was determined by using pH meter (Inolab/Level), and acidity (% malic acid) was determined according to Demir and Özcan [20]. Crude oil was determined by extraction Soxhlet apparatus with petroleum ether by using AOAC 983.23 [19]. AOAC 990.03 [21] was used to determine crude protein as total nitrogen in Leco FP-528 apparatus and converted into protein using conversion factor (N × 6.25). Crude cellulose, ash and ash insoluble in hydrochloric acid were analyzed according to AOAC 920.86 [19] and AOAC 940.26 [22], respectively.
Color
The color attributes of fresh and dried apricot pomace samples were analyzed with a chroma meter (CR-400, Konica Minolta Co, Osaka, Japan), which was calibrated with a white standard board (L = 93.49, a = 0.25, b = 0.09). Before and after the drying process, four different points were measured from the surface of each sample. The color values of the samples were described as L (brightness/blackness), a (redness/greenness) and b (yellowness/blueness). In this study, the total color change (ΔE), chrome (C) and hue angle (H) values were calculated by using Eqs. 1, 2 and 3, respectively.
Mathematical modeling
The data derived from drying of apricot pomace were fitted with ten thin-layer drying models (Table 1) typically used for the modeling of drying curves. The moisture ratio (MR) of the apricot pomace during drying experiments was calculated by using the following equation:
where M t, M 0 and M e are moisture content at any time, initial moisture content, equilibrium moisture content (kg water kg−1 dry matter), respectively, and t is drying time (min). The equilibrium moisture content (M e) is relatively small compared with M 0. Therefore, M e was numerically set to zero in this study. So MR can be simplified to MR = M t/M 0 [26, 33].
The drying rate (DR) was calculated using Eq. (5)
where M t+Δt is moisture content at t + Δt (kg water kg−1 dry matter), and t is time (min).
Data analysis
Data were analyzed using Statistica 8.0.550 (StatSoft Inc., Tulsa, OK, USA) software package. The parameters of models were estimated using a nonlinear regression procedure based on the Levenberg–Marquardt algorithm. The fitting quality of the experimental data to all models was evaluated using the coefficient of determination (R 2), mean relative percent error (P), reduced Chi-square (χ 2), and root mean square error (RMSE). The R 2, P, χ 2 and RMSE were calculated from the following formulas:
where MR exp,i and MR pre,i are experimental and predicted dimensionless moisture ratios, respectively; N is number of observations; z is number of constants. The best model was chosen based on the following four criteria: the highest value of R 2 and the least values of P, χ 2 and RMSE [34, 35].
Determination of effective moisture diffusivity
Fick’s second law of diffusion equation, symbolized as a mass-diffusion equation for drying of agricultural products drying in a falling-rate period, is shown in the following equation:
Fick’s second law of unsteady-state diffusion given in Eq. (10) can be used to determine the moisture ratio in Eq. (11). The solution of diffusion equation for infinite slab given by Crank [36], and supposed uniform initial moisture distribution, negligible external resistance, constant diffusivity and negligible shrinkage, is:
where D eff is the effective moisture diffusivity (m2 s−1), t is the time (s), L is the half-thickness of samples (m), and n is a positive integer. For longer drying periods, the above equation can be simplified to only first term of series, without much affecting the accuracy of the prediction.
The effective moisture diffusivity (D eff ) was also typically calculated by using the slope of Eq. (12). A straight line with a slope of K was obtained when ln (MR) was plotted versus time:
Using the slope value (Eq. 13), the effective moisture diffusivity could be determined.
Computation of activation energy
The relationship between the effective moisture diffusivity and drying temperature was described using the Arrhenius-type equation:
Here D 0 is the pre-exponential factor (m2 s−1), E a is the activation energy (kJ mol−1), R is the universal gas constant (kJ mol−1 K−1), and T is drying air temperature (°C).
Results and discussion
Physicochemical characterization
The initial moisture of fresh pomace was determined as 4.6179 kg water kg−1 dry matter (d.b.). The values of pH and titratable acidity were found to be 3.66 and 2.56, respectively. Table 2 shows the chemical composition of fresh and dried apricot pomace at 50 °C. Eksi and Artik [37] reported that the crude protein, crude fat, crude fiber, the total ash and HCl ash of apricot pulp were 3.02, 0.2, 2.81, 0.50 and 0.17%, respectively. In another study, Ucuncu et al. [38] found protein content 1.29% and ash content 0.6% in fresh apricot pomace from Turkey. The acidity, protein, fat, ash and mineral composition of the fruits of cultivated conditions change to grow in the region and the product year. However, it is generally reported that the proportion of protein in wild fruits is less than 5% and the fat content is less than 2%. Haciseferogullari et al. [39] reported that similar chemical compositions such as crude protein 2.84 and 4.29%, crude oil 0.55–3.12%, ash 2.72–5.34%, pH and acidity 0.17–0.79% (malic acid) for various apricot varieties in the Malatya region. Ash contents of the Malatya apricot varieties varied from 0.5 to 0.89% and titratable acidity (% citric acid) 0.1–1.0, and the values of pH from 2.83 to 5.63 were reported by Akin et al. [40].
Color evaluation
The CIE Lab color coordinates and the chrome and hue angle color attributes have been widely used to describe the optical properties of fruits and vegetables [11]. The color parameters for apple pomace samples as a function of drying temperature are reported in Table 3. The color values, namely L, a and b, of the pomace samples decreased after drying process, and they were decreased with increasing drying temperature. Dried samples at 40 °C exhibited the brightest product color (highest L value), most red color (highest a value) and most yellow color (highest b value). Karabulut et al. [41] studied the effect of hot-air drying and sun drying on color values of apricot. All color values decreased with high temperature. This is the reason for the occurrence of browning reactions during long-term drying at high temperature. Similar results were also reported by some authors [42, 43].
The color change (ΔE) in the apricot pomace at different temperatures is observed to increase with the increase in drying temperature, but lower color change was favorable. Alam et al. [44] reported color change (ΔE) in carrot pomace increased with the increase in drying temperature from 55 to 65 °C. In other some studies, L, a and b values decreased and color change (ΔE) increased with the increase in temperature [44,45,46].
Analysis of drying curves
The effect of temperature on drying curves of the apricot pomace during drying is shown in Fig. 1. The drying curves are typical to ones for similar pomaces of fruit and vegetable. All drying curves showed that the moisture content of apricot pomace decreased continuously with the increase in drying time. Furthermore, it was found that the drying time decreased with the increase in drying temperature. The drying times required to reach the final moisture content of samples were 660, 570, 450 and 360 min at air temperature of 40, 50, 60 and 70 °C, respectively. The average drying rate of samples increased 1.83 times as air-drying temperature increased from 40 to 70 °C. This is due to the increasing energy of the water molecules at increased temperature, which can escape easier and faster [47]. The effect of temperature on drying behavior of various agricultural by-product, pomace and waste has been investigated by some researches [17, 26, 47, 48].
Drying rate
The drying rate curves of apricot pomaces under different drying temperatures are illustrated in Fig. 2. The drying rates were higher in the beginning of the process and then decreased with the increase in drying time. The reduction in the drying is mainly due to the reduction in moisture content as drying advances. The rate of migration of moisture from inner surface to outer surface decreases at the final stage of drying and hence lower drying rates [17]. The apricot pomace did not exhibit a constant drying rate period, and all the drying operations are seen to occur in the falling drying rate period except a very short accelerating period at the beginning. This observation is in agreement with previous results on thin-layer drying of biological products [17, 18, 26, 48].
Evaluation of the models
The moisture content data obtained at different infrared powers were converted into the MR and fitted to ten thin-layer drying models listed in Table 1. The best model was selected based on the highest R 2 and the lowest P, χ 2 and RMSE values. Results of the statistical computing are shown in Table 4. The R 2 values for all models were above 0.96. Among the thin-layer drying models, the Aghbashlo et al. and Midilli et al. models were found to represent the drying kinetics of apricot pomace with high R 2 values and low P, χ 2 and RMSE values for all air temperatures (Table 4). The P, χ 2 and RMSE also showed best results with the smaller values. It is clear that the R 2, P, χ 2 and RMSE values of this model were changed between 0.9993 and 0.9999, 1.7117–17.2464, 0.000011 and 0.000094, 0.007448 and 0.029740, respectively. To validate the selected models, plots of experimental MR and predicted MR by Aghbashlo et al. and Midilli et al. models are shown in Figs. 3 and 4. Obviously, a good agreement was observed between experimental and predicted MR values. That is, the data points generally banded around a 45° straight line on the plots. This trend provides extra evidence for the suitability of the models to forecast the drying characteristics of apricot pomaces.
Effective moisture diffusivity
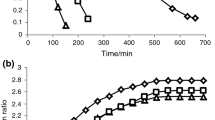
The effective moisture diffusivity (D eff) values for different temperatures, calculated from Eq. (13), are given in Fig. 5 and ranged from 1.014×10−9 to 1.859×10−9 m2 s−1. It was observed that D eff values increased with the increase in air temperature. It may be due to the increase in vapor pressure inside the pomace at higher air temperature. The highest value of effective moisture diffusivity was found to be 1.859×10−9 m2 s−1 at 70 °C. The D eff values of the apricot pomace were within the general ranges of 10−12 to 10−8 m2 s−1 for biological materials [49]. These values are comparable to 0.347–0.868 × 10−8 m2 s−1 for watermelon pomace [18], 3.47–6.47 × 10−9 m2 s−1 for apple pomace [13], 2.74–4.64 × 10−9 m2 s−1 for carrot pomace [17] and 3.24 × 10−9–1.24 × 10−8 m2 s−1 for tomato by-products [48].
Activation energy
The activation energy was calculated by plotting ln (D eff) versus the reciprocal of the temperature (1/(T + 273.15)) and is presented in Fig. 6. The effect of temperature on the D eff of apricot pomace is shown in Eq. (15):
The value of activation energy was found to be 19.41 kJ mol−1. The value of activation energy lies within the general range of 12.7–110 kJ mol−1 for food materials [49]. Similar value to those proposed in the literature for the drying of different agricultural by-products, pomaces and wastes is as follows: 16.5 kJ mol−1 for apricot kernels [50], 24.51 kJ mol−1 for apple pomace [15], 23.05 kJ mol−1 for carrot pomace [17] and 19.27 kJ mol−1 for industrial grape by-products [51].
Conclusions
In this study, the drying characteristics of apricot pomace were investigated in a cabinet dryer at different air-drying temperatures. The drying of apricot pomaces at each air-drying temperature occurred in falling-rate period. The drying time decreased with the increase in air-drying temperature. To explain the drying behavior of apricot pomace, ten thin-layer models were applied. Among these models, the Aghbashlo et al. and Midilli et al. models gave the best results and showed good agreement with experimental data obtained from the experiments. The effective moisture diffusivity was computed from Fick’s second law, the values of which varied between 1.014×10−9 and 1.859×10−9 m2 s−1, over the air-drying temperature range. The values of D eff increased with the increase in drying temperature. Activation energy was estimated by a modified Arrhenius-type equation and found to be 19.41 kJ mol−1. Color analysis showed that the values of the parameters L, a, b, C and H decreased with drying temperature except ΔE.
References
Buzby JC, Hyman J. Total and per capita value of food loss in the United States. Food Policy. 2012;37:561–70.
Borges KC, Azevedo JC, Medeiros MF, Correia RTP. Physicochemical characterization and bioactive value of tropical berry pomaces after spouted bed drying. J Food Qual. 2016;39:192–200.
Nawirska A, Kwasniewska M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005;91:221–5.
Follonier S, Goyder MS, Silvestri AC, Crelier S, Kalman F, Riesen R, Zinn M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int J Biol Macromol. 2014;71:42–52.
Paraman I, Sharif MK, Supriyadi S, Rizvi SSH. Agro-food industry byproducts into value-added extruded foods. Food Bioprod Proces. 2015;96:78–85.
Dhillon GS, Kaur S, Brar SK. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: a review. Renew Sustain Energy Rev. 2013;27:789–805.
Serrano A, Siles JA, Chica AF, Martín MÁ. Agri-food waste valorization through anaerobic co-digestion: fish and strawberry residues. J Clean Prod. 2013;54:125–32.
Aguedo M, Kohnen S, Rabetafika N, Bossche SV, Sterckx J, Blecker C, Beauve C, Paquota M. Composition of by-products from cooked fruit processing and potential use in food products. J. Food Comp Anal. 2012;27:61–9.
Tseng A, Zhao Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013;138:356–65.
FAO, Food and Agriculture Organization. FAO statistical yearbook for world food and agriculture. Rome: FAO; 2015.
Garcia-Martinez E, Igual M, Martin-Esparza ME, Martinez-Navarrete N. Assessment of the bioactive compounds, color, and mechanical properties of apricots as affected by drying treatment. Food Bioprocess Technol. 2013;6:3247–55.
Incedayi B, Tamer CE, Sinir GO, Suna S, Copur OU. Impact of different drying parameters on color, β-carotene, antioxidant activity and minerals of apricot (Prunus armeniaca L.). Food. Sci Technol. 2016;36:171–8.
Sun J, Hu X, Zhao G, Wu J, Wang Z, Chen F, Liao X. Characteristics of thin-layer infrared drying of apple pomace with and without hot air-drying. Food Sci Technol Int. 2007;13:91–7.
Zielinska M, Markowski M. Air drying characteristics and moisture diffusivity of carrots. Chem Eng Process. 2010;49:212–8.
Wang Z, Sun J, Liao X, Chen F, Zhao G, Wu J, Hu X. Mathematical modeling on hot air drying of thin layer apple pomace. Food Res Int. 2007;40:39–46.
Wang Z, Sun J, Liao X, Chen F, Zhao G, Wu J, Hu X. Mathematical modelling on thin layer microwave drying of apple pomace with and without hot air pre-drying. J Food Eng. 2007;80:536–44.
Kumar N, Sarkar BC, Sharma HK. Mathematical modelling of thin layer hot air drying of carrot pomace. J Food Sci Technol. 2012;49:33–41.
Doymaz I. Effect of pre-treatments using potassium metabisulphide and alkaline ethyl oleate on the drying kinetics of apricots. Biosyst Eng. 2004;89:281–7.
AOAC, Official Methods of Analysis. Association of official analytical chemists. Arlington: AOAC; 1990.
Demir F, Ozcan M. Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J Food Eng. 2001;47:333–6.
AOAC, Official Methods of Analysis. Association of official analytical chemists. Washington DC: AOAC; 2002.
AOAC, Official Methods of Analysis. Association of official analytical chemists. Washington DC: AOAC; 1984.
Roberts JS, Kidd DR, Padilla-Zakour O. Drying kinetics of grape seeds. J Food Eng. 2008;89:460–5.
Ghirişan A, Drãgan S. Kinetic study of carrots drying. Studia Ubb Chemia. 2013;58(2):35–42.
Faal S, Tavakoli T, Ghobadian B. Mathematical modelling of thin layer hot air drying of apricot with combined heat and power dryer. J Food Sci Technol. 2015;52:2950–7.
Togrul IT, Pehlivan D. Modelling of thin layer drying kinetics of some fruits under open-air sun drying process. J Food Eng. 2004;65:413–25.
Sharma GP, Prasad S. Effective moisture diffusivity of garlic cloves undergoing microwave-convective drying. J Food Eng. 2004;65:609–17.
Baloda S, Goel V, Sharma N. Experimental investigation of the performance of an indirect mode natural convection solar dryer for drying fenugreek leaves. J Therm Anal Calorim. 2014;118:523–31.
Chen DY, Zhang D, Zhu XF. Heat/mass transfer characteristics and nonisothermal drying kinetics at the first stage of biomass pyrolysis. J Therm Anal Calorim. 2012;109:847–54.
Shringi V, Surendra Kothari S, Panwar NL. Experimental investigation of drying of garlic clove in solar dryer using phase change material as energy storage. J Therm Anal Calorim. 2014;118:533–9.
Corzo O, Bracho N, Pereira A, Vásquez A. Weibull distribution for modelling air drying of coroba slices. LWT Food Sci Technol. 2008;41:2023–8.
Aghbashlo M, Kianmehr MH, Khani S, Ghasemi M. Mathematical modeling of carrot thin-layer drying using new model. Int Agrophys. 2009;23:313–7.
Calín-Sánchez A, Figiel A, Wojdylo A, Szaryez M, Carbonell-Barrachina AA. Drying of garlic slices using convective pre-drying and vacuum-microwave finishing drying: kinetics, energy consumption, and quality studies. Food Bioprocess Technol. 2014;7:398–408.
Fahloul D, Lahbari M, Benmoussa H, Mezdour S. Effect of osmotic dehydration on the freeze dryig kinetics of apricots. J Food Agric Environ. 2009;7:117–21.
Falade KO, Ogunwolu OS. Modeling of drying patterns of fresh and osmotically pretreated cooking banana and plantain slices. J Food Process Preserv. 2014;38:373–88.
Crank J. The mathematics of diffusion. London: Oxford University Press; 1975.
Eksi A, Artik N. Kayısı ve şeftali palper posası ile atılan besin öğesi miktarı. Gıda. 1982;7:99–102.
Ucuncu C, Tari C, Demir H, Buyukkileci AO, Oguz B. Dilute-acid hydrolysis of apple, orange, apricot and peach pomaces as potential candidates for bioethanol production. J Biobased Mater Bio. 2013;7:376–89.
Haciseferogullari H, Gezer I, Ozcan MM, Asma BM. Post-harvest chemical and physical–mechanical properties of some apricot varieties cultivated in Turkey. J Food Eng. 2007;79:364–73.
Akin EB, Karabulut I, Topcu A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008;107:939–48.
Karabulut I, Topcu A, Duran A, Turan S, Ozturk B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT Food Sci Technol. 2007;40:753–8.
Ihns R, Diamante LM, Savage PG, Vanhanen L. Effect of temperature on the drying characteristics, colour, antioxidant and beta-carotene contents of two apricot varieties. Int J Food Sci Technol. 2011;46:275–83.
Albanese D, Cinquanta L, Cuccurullo G, Matteo MD. Effects of microwave and hot-air drying methods on colour, β-carotene and radical scavenging activity of apricots. Int J Food Sci Technol. 2013;48:1327–33.
Alam S, Singh B, Khaira H, Kaur J, Singh SK. Optimization of extrusion process variables for development of pulse-carrot pomace incorporated rice based snacks. J Food Legumes. 2013;25:1–18.
Yan W, Zhang M, Huang L, Tang J, Mujumdar AS, Sun J. Studies on different combined microwave drying of carrot pieces. Int J Food Sci Technol. 2010;45:2141–8.
Nóbrega M, Oliveira EL, Genovese MI, Correia RTP. The impact of hot air drying on the physical-chemical characteristics, bioactive compounds and antioxidant activity of acerola (Malphigia emarginata) residue. J Food Proces Preserva. 2015;39:131–41.
Al-Muhtaseb AH, Al-Harahsheh M, Hararah M, Magee TRA. Drying characteristics and quality change of unutilized-protein rich-tomato pomace with and without osmotic pre-treatment. Ind Crop Prod. 2010;31:171–7.
Celma AR, Francisco C, Fernando LR, Aida R. Thin layer drying behaviour of industrial tomato by-products in a convective dryer at low temperatures. Res J Biotechnol. 2013;8(2):50–60.
Zogzas NP, Maroulis ZB, Marinos-Kouris D. Moisture diffusivity data compilation in foodstuffs. Dry Technol. 1996;14:2225–53.
Zhang QA, Song Y, Wang X, Zhao WQ, Fan XH. Mathematical modeling of debittered apricot (Prunus armeniaca L) kernels during thin-layer drying. CyTA J Food. 2016;14:509–17.
Celma AR, López-Rodríguez F, Blázquez FC. Experimental modelling of infrared drying of industrial grape by-products. Food Bioprod Process. 2009;87:247–53.
Acknowledgements
Support from Döhler Natural Food and Beverage Ingredients Factory in providing the apricot pomace is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kayran, S., Doymaz, İ. Determination of drying kinetics and physicochemical characterization of apricot pomace in hot-air dryer. J Therm Anal Calorim 130, 1163–1170 (2017). https://doi.org/10.1007/s10973-017-6504-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6504-0