Abstract
Pomegranate arils were dehydrated at 55, 65, 75 °C using hot-air technique, and the impacts of drying on color, texture, rehydration ratio, shrinkage of arils were evaluated as well as bioactive compounds like total phenolics, total monomeric anthocyanins, total tannins and radical scavenging activity. Sigmoid model gave the best results at all studied temperatures for drying kinetics. Effective moisture diffusivities of arils were 3.5689 × 10−11 m2 s−1 (at 55 °C), 9.3950 × 10−11 m2 s−1 (at 65 °C), 1.9330 × 10−10 m2 s−1 (at 75 °C), and activation energy was 80.33 kJ mol−1. Averaged convective mass transfer coefficients and moisture extraction rates were also calculated, and their highest values were observed at 75 °C. Rehydration was only conducted at 25 °C, and two-term exponential decay model was the most suitable equation for describing rehydration phenomenon. Thermal operation caused important changes in L*, b*, hardness and shrinkage (p < 0.05). Total phenolics, total monomeric anthocyanins and total tannins of dried arils were changed between 5512.37–6895.80 mg gallic acid equivalent, 163.87–324.58 mg cyanidin-3-glucoside equivalent and 1024.99–2467.77 mg per kg dry matter, respectively. The radical scavenging activity was decreased from the initial value of 77.42% to circa 24.79% by drying. Because high temperature had harmful effect on fruit, temperature of 65 °C may be advisable for dehydration of pomegranate arils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pomegranate (Punica granatum L.) belongs to Punicaceae family, and Iran, India and Mediterranean countries are the major producers of this ancient and nutritive fruit [1]. Edible seeds (about 50% of total fruit mass) substantially include various bioactive compounds such as polysaccharides (pectin, sugars), various vitamins (ascorbic acid, vitamin E and K) and minerals (potassium, calcium, magnesium, sodium) [2]. The bioactive compounds like phenolic acids, anthocyanins and tannins have the ability of reducing cancer, obesity, diabetes risks [3], threatening headache, dysentery and leucorrhea [4]. Due to these potential therapeutic benefits, pomegranate could be accepted as a functional food [1]. However, these bioactive compounds are greatly sensitive to thermal degradation [5], and they make pomegranate “antioxidant”.
Drying is a common process in food industry, and the main objective of this operation is the removal of moisture from food in order to prevent microbial spoilage and extend the shelf life. Hot-air drying is still a widely used dehydration technique [6] because of low energy requirement, simple and cheap equipment and less drying time in comparison with sun drying which is so popular in fruit dehydration [7]. Besides convectional drying, microwave and infrared drying has been used in recent years [8].
When water contacts with dried material, rehydration process starts. Rehydration is a complicated procedure consisting of returning into raw material properties of dehydrated substance. Fruit tissue absorbs moisture; however, valuable compounds transfer into tissue at the same time [9]. Hence, some physical and chemical alterations would occur in the product depending on its porosity [10].
Process controls and operation of instruments are able to be monitored by mathematical models easily. The semi-theoretical models have been mostly used for describing drying and rehydration phenomena, and these equations are derived from either Fick’s second law of diffusion or Newton’s law of cooling. Temperature (of water/air) and velocity of drying air, moisture content of material at the beginning and sample thickness/porosity are the significant factors which determine the application of these type of models [11]. In the literature, semi-theoretical equations were applied into the drying data of apricot pomace [12], red pepper [13], paddy [14], turmeric [15], and so on.
The color of a dried fruit is an important quality criterion for the consumers’ first choices. Any deteriorations or abnormalities in color properties will affect the demands of the dried products. During a drying operation, vivid color of fruit converts into darker by reason of reducing sugars or oxidation of polyphenols based on time and method [16]. Textural features of foods are specified either holding by hand or chewing. When defects are available in the fruit tissue, costumers may probably reject the product. Insoluble solids are responsible for creating structure in food [17]; while drying, increased ratio of dry matter induces hardening. Changes in width, length and/or height of fruit are not at the same level and depend on density, porosity, etc. [18]. If there is a proportional relation between mass loss and shrinkage, the volume reduction could be accepted as ideal [19].
In this framework, the purposes of this research were (1) to reveal drying and rehydration kinetics, (2) to compute effective diffusivities, mass transfer coefficients and activation energy of conventionally dried pomegranate arils (cv. Hicaznar), (3) to determine the change in both the physical (color, texture, rehydration ratio, shrinkage) and chemical (total phenolics, anthocyanins, tannins, antioxidant activity) properties in the arils after hot-air drying. It is believed that, this study focuses on both drying and rehydration kinetics with the aid of 22 and 5 semi-empirical models available in the literature, respectively, and it will be unique in processing of pomegranate (without any pretreatment or preservative) which regards also some quality changes, especially significant for consumers’ diet. Furthermore, this comprehensive research will provide benefits for design engineers, fruit producers and nutritionists.
Materials and methods
Materials
Pomegranate fruits (Punica granatum L.) cultivar “Hicaznar” were harvested from the fields of Alata Horticulture Research Institute near Erdemli in Mersin, Turkey, in November 2015 and stored at + 7 °C until drying experiments, and trials were completed in a few days to eliminate moisture and quality losses. Arils were separated from their bodies manually, and the average moisture contents of fresh arils were determined as 78.10 ± 0.81% according to the method of AOAC, 1990 [20].
Hot-air drying
Just before drying, nearly 200 grams of arils was taken apart from their husks. Experiments were conducted at 55, 65 and 75 °C (± 1 °C) in a laboratory tray dryer (Eksis, Isparta, Turkey) having dimensions of 50 cm × 110 cm × 50 cm with 3 kW nominal power. Air velocity was 1.5 m s−1, and relative humidity was 5% in all trials. Dryer was run as idle for 15 min until the desired temperature was accessed. After that, arils were put onto the perforated aluminum trays (30 × 30 cm) and drying began. The trays were placed in the fourth and sixth shelves of dryer, and sampling was done at certain time intervals (durations of dehydration processes were different at all temperatures and sampling times were also varied) in order to determine the moisture losses. Approximately 1 g of arils from each tray was taken at specific times during drying so that homogenous sampling could be provided. In order to prevent longer drying times, keep the degradation of bioactives at a reasonable level and decrease microbial risks; final moisture contents of the dried arils were reduced to 10.20 ± 1.42%. Dehydrated samples were stored in glass jars with locked plastic bags at 4 °C till physical and chemical analysis. All drying experiments were performed in triplicate.
The moisture ratio (MR) values were determined by using the following formula [21];
In the above equation, M and M0 denote moisture content at any time and initial moisture content, respectively. Equilibrium moisture content was considerably small, and because of this, it was neglected. Experimental MRs were fitted into 22 different thin-layer mathematical models (listed in supplementary file, Table 1) to choose the best equation which describes the drying phenomena. The highest determination coefficient (R2) and the lowest root mean square error (RMSE) and Chi-square (χ2) values were the indicators of a good model.
Effective moisture diffusivity, convective mass transfer coefficient, activation energy, moisture extraction rate
Fick’s second law is a useful tool for calculating effective moisture diffusivities of foods. It was assumed that arils had spherical geometry and uniform initial moisture distribution, symmetric mass transfer happened in accordance with the center of aril, there was negligible surface resistant to mass transfer, no chemical reactions occurred, and diffusion coefficient was constant throughout the process. Equation 2 [22] expresses the correlation between MR and diffusion coefficient.
The slope of linearized plot of Eq. 2 gives effective moisture diffusivity (Deff) (m2 s−1). In Eq. 2, t and r represent time (s) and radius of aril (m), respectively.
Kaya et al. [23] reported a formula about convective mass transfer coefficient, and it is given in Eq. 3;
where V is the sample volume (m3), A is surface area of sample (m2) and α is surface (convective) mass transfer coefficient (m s−1).
Arrhenius-type equation is beneficial for temperature-dependent activation energy. Activation energy can be confirmed from the slope of the linearized plot of Eq. 4 [12].
where Ea is activation energy (kJ mol−1), R is universal gas constant (8.3143 J mol−1 K−1), T is medium temperature (K) and D0 is the predominant exponential factor (m2 s−1).
The moisture extraction rate (MER) was calculated by Eq. 5 [24];
where Awrd was amount of water removed during drying (kg) and DT was total drying time (h).
Rehydration
Two grams of dried arils was immersed into a water bath at room temperature (25 ± 1 °C). The water was drained and the mass of arils recorded at every 45 min until no changes were observed. The rehydration ratio (RR) value was calculated from Eq. 6 [24];
where Wr is the mass of rehydrated arils at any time and Wd is the mass of dried arils. Experimental RRs were fitted into five different mathematical equations available in the literature (listed in supplementary file, Table 2) for selecting the most appropriate one which described the rehydration behavior. R2, RMSE and χ2 were used for model adequacy as well as in drying kinetics. Triplicate measurements were done.
Color
Arils were put into a weighing dish as a single layer without leaving any space between them, and measurements were taken from three different regions of that layer. The colorimetric values (L*, a* and b*) of fresh and dried arils were measured by a digital color meter (Minolta Chroma Meter CR 400, Japan), and total color change (∆E) (Eq. 7) and chroma or intensity (C*) (Eq. 8) were calculated using CIELAB coordinates [2].
In those equations, L*, a* and b* indicate lightness, redness-greenness and yellowness-blueness, respectively. “Ref” values belong to fresh arils.
Texture (hardness)
Hardness of fresh and dried arils was recorded by puncture test [25] with a texture analyzer (Brookfield, CT3) using Texturepro CT V1.4 Build 17 software. A 2-mm cylindrical probe (TA 39, 20 mm length) with a speed of 1 mm s−1 penetrated into 3 mm of aril. Trigger load was 0.20 N, and load cell of device was 4500 g. The data were presented by the way of 40 different measurements for each sample type.
Shrinkage
Moisture removal in dehydration process causes volume change in pomegranate arils. Shrinkage (S) is calculated as follows (Eq. 9):
where V0 and Vf are the initial and final volume of sample (m3), respectively. In order to calculate the exact volume of samples, geometric mean diameter was used because of spheroid shape of aril [26]. The samples were accepted as homogeneous, although some heterogeneities were in fact present. The data represented the mean of 40 different measurements.
Extraction of bioactive compounds
Extraction procedure was modified from Bennett et al. [27]. One gram of grinded fruit sample was analyzed with 10 mL of 80% methanol. The mixture was vortexed for 15 s, sonicated for 20 min at 25 °C (J.P. Selecta Ultrasons HD, Barcelona) and centrifuged at 3500 g for 15 min (Hettich Zentrifugen, Tuttlingen). The supernatants were filtered and stored at 4 °C until spectrophotometric analysis. 80% methanol was accepted as blank solution in all chemical analysis, and each test was conducted in triplicate.
Total phenolics (TP), radical scavenging activity (RSA), total monomeric anthocyanin (TMA) and total tannins (TT) content
Folin–Ciocalteu method mentioned by Li et al. (with some modifications) [28] gave good results about total phenolic content determinations of pomegranate arils. 0.5 mL of extract was mixed with 0.5 mL of Folin–Ciocalteu reagent; then 3 mL of 10% Na2CO3 was added. After 30-min waiting in dark place, absorbances were read at 760 nm using a spectrophotometer (Shimadzu, UV 1800, Japan). Results were expressed as mg GAE (gallic acid equivalent)/kg dry matter (DM). R2 of gallic acid standard curve was 0.9952.
The method of radical scavenging activity (DPPH assay) described by Aghraz et al. [29] was used for determination of anti-oxidant activity. Briefly, 2 mL of DPPH (2,2-diphenyl-1-picrylhydrazyl) solution (0.025 g L−1, prepared in 100% methanol) was added to 0.1 mL of extract. After incubating for 30 min in dark place, absorbances were measured at 517 nm using a spectrophotometer (Shimadzu, UV 1800, Japan). Results were given as DPPH inhibition (%) (Eq. 10).
where ADPPH and Asample were the absorbances of DPPH (initial) and extract, respectively. The analysis was run in triplicate.
pH differential method was partially modified from Li et al. [28] and conducted to determine total anthocyanin concentration. Two samples of 0.5 mL of extract were added into 4.5 mL of KCl–HCl solution (0.025 mol L−1, pH = 1) and 4.5 mL of NaAc–HAc solution (0.4 mol L−1, pH = 4.5) separately. Absorbances of mixture solutions (after keeping 10 min in dark place) were measured at 510 nm and at 700 nm, respectively, using a spectrophotometer (Shimadzu, UV 1800, Japan). Results were stated as mg cy-3-GE (cyanidin-3-glucoside equivalent)/kg DM.
Total tannins (TT) were determined according to Hosu et al. [30]. Two sample sets including 0.2 ml of each extract, 3 mL of concentrated HCl and 1 mL of distilled water were prepared. One set was boiled at 100 °C in a water bath (Wisd WiseBath WB-11, Germany), and 0.5 mL methanol was added into another one. Absorbances of all sample sets were read at 470, 520 and 570 nm using a spectrophotometer (Shimadzu, UV 1800, Japan), and total tannins content was calculated from the following formulas (Eqs 11, 12 and 13);
where ΔA470, ΔA520 and ΔA570 were the differences in absorbances of samples at 470, 520 and 570 nm, respectively.
Statistical analysis
The nonlinear regression analysis was performed by OriginPro 2016 (Origin Lab, USA) software for investigating both drying and rehydration kinetics of pomegranate arils. An analysis of variance (ANOVA) (at 95% confidence interval) was applied to the results of physical and chemical analysis by using SPSS (version 18). In order to determine the homogenous groups, Duncan test was used.
Results and discussion
Hot-air drying
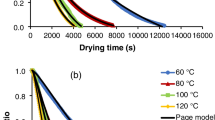
The alterations in moisture levels of pomegranate arils with time are shown in Fig. 1a. It was obvious that the moisture ratios of arils decreased with dehydration time, but they did not reach an equilibrium because of not being carried out the process until no mass changes were observed. The drying times were 660, 240 and 150 min for 55, 65 and 75 °C, respectively. An increase in temperature shortened drying time nearly 64% from 55 °C to 65 °C and 37% from 65 °C to 75 °C. Several authors proved this situation previously [2, 31]. Among 22 thin-layer drying models available in the literature, sigmoid model [32] (Eq. 14) was the best to describe drying phenomena of arils.
In the above model, k is drying rate constant (min−1), a, b and c are constants, and t is time (second). Results of sigmoid model are given in Table 1. R2, χ2 and RMSE values ranged between 0.9974–0.9995, 0.0001–0.0004 and 0.0068–0.0154, respectively. There is a good agreement between the experimental drying data and sigmoid model equation. When drying temperature increased, k also increased; however, c decreased. “a” constant had a negative value. The meaning of this asymptotic parameter was that the theoretical MR achieved in infinite time, not in real drying [33]. Furthermore, a and b did not have a distinct trend. Midilli et al. model [34, 35], Page model [21] and sigmoid model [33] were satisfactorily fitted to conventional drying data of pomegranate arils in previous researches.
Effective moisture diffusivity, convective mass transfer coefficient, activation energy, moisture extraction rate
Effective moisture diffusivities were 3.5689 × 10−11 m2 s−1 for 55 °C, 9.3950 × 10−11 m2 s−1 for 65 °C, and 1.9330 × 10−10 m2 s−1 for 75 °C. As such in drying rate constant, temperature caused an augmentative effect in Deff because of growing vapor pressure inside arils [34]. The values of Deff determined in this research are in line with 10−12–10−8 m2 s−1 which have been reported as a general range for dehydration of foods [36].
Table 2 depicts average mass transfer coefficients and related equations. Mathematical equations were fourth-order polynomial models, and R2 values were ranged between 0.9914 and 0.9989. From Table 2, αav was 6.1217 × 10−9, 1.2288 × 10−8 and 2.8665 × 10−8 m s−1 for 55, 65 and 75 °C, respectively. In the current literature, reported mass transfer coefficients of food materials are very limited. Because of different dehydration/calculation techniques, process conditions and product geometry, comparison has become difficult [37]. For lemon slices, αav was stated in the range of 3.6770 × 10−8–7.727 × 10−8 m s−1 (at 50, 55 and 60 °C) [38] and for celeriac slices in the range of 6.3127 × 10−7–1.1236 × 10−6 m s−1 (at 55, 65 and 75 °C, 17 kPa vacuum pressure) [39].
Activation energy of pomegranate arils was determined as 80.33 kJ mol−1 (R2 = 0.9955). 66.12 kJ mol−1 was reported by Mundada et al. [34], 49.7 kJ mol−1 was stated by Doymaz [32], and 60.34 kJ mol−1 was determined by Doymaz [35] for conventionally dried aril samples without any pretreatment. These variations in activation energy of pomegranate arils could be resulted from differences in cultivars and initial moisture content. Samples with high initial moisture content require more energy in order to evaporate water when compared to materials with low moisture level [34].
MER is the first step for evaluating the energy efficiency of a dryer, and it could change depending on sample mass. In this study, the total mass of arils in every trial was approximately 200 g. Calculated MER values were 0.0099 kg h−1 for 55 °C, 0.0276 kg h−1 for 65 °C and 0.0392 kg h−1 for 75 °C. Caliskan and Dirim [24] dried pumpkin slices (the total amount of sample was 38 g) in both convective and freeze dryer and stated the MER values as 0.0900 and 0.0150 kg h−1, respectively. Prasertsan and Saen-saby [40] dehydrated banana by using a heat pump dryer in open (MERs were between 1.8700 and 2.7070 kg h−1) and partially closed systems (MERs were in the range of 1.9050 and 2.011 kg h−1) with different fruit loadings (sample amounts were between 87.3 kg and 210.9 kg) and maximum MER level (2.710 kg h−1) obtained when the drying load was highest. Certainly, type of drying technique and food material are able to affect MER values, but unfortunately there are no enough data for making a good comparison.
Rehydration
The rehydration curves of conventionally dried pomegranate arils are shown in Fig. 1b. The moisture level progressively increased, while rehydration was proceeding. The impact of drying temperature was important, and arils dehydrated at 75 °C absorbed more water than other samples. Cellular and structural deterioration designates rehydration degree, and mostly irreversible retrogressions can occur [41]. More porous structure probably arose in samples dried at 75 °C in contrast with their high shrinkage. Similarly, Bilbao-Sainz et al. [42] stated that apple samples dehydrated at high microwave power level absorbed more moisture than samples dried at lower powers.
Stabilization time of rehydration was nearly 680 min at room temperature, and the RRs were 2.5199, 2.6194 and 2.8777 for 55, 65 and 75 °C, respectively. Nevertheless, the final moisture contents of dried arils did not reach their initial values before drying. Observations in this study are in agreement with the results reported by Mulik and Bhosale [43] who determined RRs of osmo-convective dried (30, 40 and 50˚ brix and temperature of 50, 60 and 70 °C) pomegranate arils. They stated RRs between the range of 2.5500–3.3800.
Among five rehydration models available in the current literature, two-term exponential decay model (Eq. 15) [44] was sufficient to define rehydration behavior of pomegranate arils at 25 °C.
where d, e, f, g and y0 are the constants and tt is time (second). Results of two-term exponential decay model are given in Table 3. R2, χ2 and RMSE values ranged between 0.9976–0.9995, 0.0002–0.0009 and 0.0122–0.0231, respectively. The relatively high coefficient of determination suggests that the constants fit adequately to two-term exponential decay equation. When drying temperature decreased, e, f, g also decreased. There were no specific trends observed for y0 and d.
Color, texture (hardness) and shrinkage
Maskan et al. [45] claimed that the dried arils available in the current markets had generally brownish color and this might not be charming for consumers, because anthocyanins degraded due to drying process. Hence, color attributes play an important role in customers’ purchasing choices. Table 4 demonstrates the values of L*, a*, b*, ΔE and C* after drying in comparison with the fresh fruit. The values of L* ranged between 23.99 and 26.96, a* varied in the range of 20.55–23.67, and b* values were between 8.02 and 10.89 (except controls). Firstly, it could be said that, after drying, the luminosities of arils decreased; however, redness increased with increasing temperature. It may probably be arisen from the degradation of red pigments and/or their conversion into dark ones because of Maillard reaction [46]. Also, there was no specific relation between yellowness-blueness and dehydration temperature. Drying conditions had significant effects on the color of the dried product (p < 0.05).
The values of ΔE can be interpreted as “not noticeable” (0–0.5), “slightly noticeable” (0.5–1.5), “noticeable” (1.5–3.0), “well visible” (3.0–6.0) and “great” (6.0–12.0) [47]. Total color changes of dried arils were in the fifth category which was mentioned above. The C* value provides the saturation and strength level of color [48]. The untreated arils had the lowest C* compared to hot-air dried samples at specific temperatures. Similar results were reported by Mphahlele et al. [5] for pomegranate peel.
One of the important textural characteristics of fruits is hardness. Because the storability, product losses during processing and transport are related to hardness (N) and firmness (N mm−1) of food material. On the other hand, hardness helps to define the mechanical properties of fruit tissues [49]. The hardness values of arils are shown in Table 4. The fresh samples had a hardness of 9.12 N, and drying process enhanced the level of stiffness nearly four times because losses of both cell adhesion and turgor pressure of parenchyma cells occurred due to dehydration [50]. There were no significant differences between dried samples (p > 0.05) and arils dehydrated at 75 °C were the hardest because of fast moisture diffusion in a shorter drying time [51].
The changes in the volume and surface area could be revealed by the reason of moisture loss. Moreover, diffusion coefficient, accordingly the drying rate of the product, is pertained to shrinkage phenomenon [52]. Shrinkage percentages of the dried arils were in the range of 72.88–79.74 (Table 4) and level of contraction increased with increasing drying temperature. There have been two contrast explanations about shrinkage of fruits and vegetables while drying. Abbasi [53] claimed the same approach as for this study that higher dehydration temperature could cause more violent evaporation of moisture, and thus, high vapor pressure could lead to more destruction. Higher pore formation was observed in onion slices dried at 90 °C rather than 60, 70 and 80 °C. As opposed to this, Horuz and Maskan [54] stated the shrinkage values of the pomegranate arils as 78.57%, 77.06% and 75.62% at 50, 60 and 70 °C, respectively. Shrinkage levels are nearly similar to the results of this research, but they declared that longer drying time (at 50 °C) allowed more shrinkage. In addition, drying conditions had significant effects on the shrinkage of the dried arils in this study (p < 0.05).
Total phenolics (TP), radical scavenging activity (RSA), total monomeric anthocyanin (TMA) and total tannins (TT) content
The TP contents of fresh and dried arils were 12,061.24 mg GAE/kg DM and 5512.37, 5783.23, 6895.80 mg GAE/kg DM at 55, 65 and 75 °C, respectively (Fig. 2a). There was no clear distinction in the TP levels of dried arils statistically (p > 0.05). The initial TP amount of pomegranate varies based on cultivar, soil properties and storage time after harvest [55]. During dehydration, the reduction in TP levels ranged nearly between 42 and 52% which meant that thermal operation destroyed phenolic compounds in pomegranate depending on both drying air temperature and time. Also, polyphenol molecules may be bounded to proteins or they could change their chemical structure by force of drying [55]. Loss of phenolics in pomegranate at higher temperature was lower because of non-enzymatic conversion of some bioactive compounds into other phenolics. The availability of precursor phenolics were able to increase; hence, new phenolic substances could occur at high temperatures (i.e., 75 °C). In an earlier attempt to determine the TP content of vacuum-dried pomegranates, TP stages of the pretreated arils dropped to 64, 67 and 73% at 75, 65 and 55 °C, respectively, and TP of the untreated samples declined to 51, 56 and 58% in the same conditions [56].
DPPH method is very effective and common in order to determine RSA (%) of various fruits and vegetables. Figure 2b gives an opinion about the antioxidant activities of arils. Significant differences were observed the levels of RSA among samples (p < 0.05). The antioxidant activity of fresh sample extract was identified as 77.42%, and in extracts of dried arils, this decreased to 23.96% at 55 °C, 24.55% at 65 °C and 25.87% at 75 °C. As such in TP, dehydration operation caused a reducing effect on RSA and the lowest antioxidant activity was defined in extracts obtained from arils dried at 55 °C. Karaaslan et al. [56] determined the antioxidant activity of vacuum-dried pomegranate arils beside TP contents and stated the scavenging activity as 37, 42 and 58% for treated and 29, 34 and 47% for untreated samples at 75, 65 and 55 °C, respectively.
Figure 2c depicts the TMA amounts and 1100.92, 324.58, 282.74 and 163.87 ppm cy-3-GE (in DM) were recorded for fresh and dried arils at 55, 65 and 75 °C, respectively. The drying temperature was a statistically important parameter on TMA (p < 0.05). Anthocyanins are responsible for creating reddish colors in fruits and applications in high temperature which trigger the degradation of anthocyanins. Thus, aglycone, anthocyanidins and/or sugar molecules can be damaged in food systems [57]. Moreover, polyphenol oxidase (PPO) enzyme causes enzymatic degradations of anthocyanin pigments and inactivation of PPO is intensely related to temperature [58] and duration of dehydration. Temperature × time effect on PPO might probably be more dominant in samples dried 55 °C than 75 °C, because anthocyanin molecules were preserved in higher amount at 55 °C. Tontul and Topuz [51] studied the TMA contents of pomegranate leathers (pestil) which were dried at 50, 60 and 70 °C and expressed that the maximum TMA was observed in pestil dehydrated at 50 °C.
Hydrolysable and condensed tannins are two of the major groups of tannin molecules, and pomegranate contains a different sequence of hydrolysable tannins. Punicalagins are the most found tannins in this fruit whose molecular weights are 1038 Dalton [59]. The TT levels are illustrated in Fig. 2d, and only arils dried at 55 °C were significantly different from the others (p < 0.05). Fresh samples had 2857.11 ppm (in DM) of tannins, and this value reduced nearly in the rates of 64% at 55 °C, 24% at 65 °C and 14% at 75 °C by drying. Because of containing phenolic groups, tannins are also heat-sensitive substances. Hence, temperature has a negative effect on TT. Nevertheless, no any studies focused on tannins of dried pomegranate arils and/or seed. Also, the diversity of testing methods makes difficult the comparisons. Mphahlele et al. [5] dried pomegranate peels in oven at 40, 50, 60 °C and determined the TT levels by using Folin–C method. They concluded that heating had a harmful effect on tannins, the lowest content of TT was designated in peels dried at 50 °C, and there were no significant differences between dried samples (p > 0.05). Also, Larrauri et al. [60] dried red grape pomace peels at 60, 100 and 140 °C and found the condensed tannins as 26.2%, 24.0% and 22.5%, respectively.
Conclusions
This research revealed the hot-air drying kinetics, mass transfer characteristics, rehydration and the changes in the bioactivity of pomegranate arils which included no preservatives at different temperatures. For the first time, 22 thin-layer drying equations and 5 rehydration models were analyzed together in a study and it was inferred that sigmoid model for drying and two-term exponential decay equation for rehydration were the suitable mathematical expressions. Also effective moisture diffusivity, averaged convective mass transfer coefficient and moisture extraction rate showed their maximum levels at 75 °C. Except rehydration ratio, all physical analysis gave their best results at 55 °C. However, the contents of bioactive substances (apart from total anthocyanins) enhanced at 75 °C when compared to other processing temperatures. On the other hand, anthocyanins were observed as the most heat-sensitive substances by thermal operation. As a result, the dehydration temperature of 65 °C may be advised as the most appropriate condition in order for drying of pomegranate arils (cv. Hicaznar) among tested aspects.
References
Viuda-Martos M, Fernández-Lóaez J, Pérez-álvarez JA. Pomegranate and its many functional components as related to human health: a review. Compr Rev Food Sci Food Saf. 2010;9(6):635–54.
Bchir B, Besbes S, Karoui R, Attia H, Paquot M, Blecker C. Effect of air-drying conditions on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food Bioprocess Technol. 2012;5(5):1840–52.
Alexandre EMC, Araújo P, Duarte MF, Freitas V, Pintado M, Saraiva JA. Experimental design, modeling and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food Bioprocess Technol. 2017;10(5):886–900.
Dhinesh KV, Ramasamy D. Pomegranate processing and value addition: review. J Food Process Technol. 2016;7(3):565.
Mphahlele RR, Fawole OA, Makunga NP, Opara UL. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement Altern Med. 2016;16(1):143.
Ju HY, Zhao SH, Mujumdar AS, Fang XM, Gao ZJ, Zheng ZA, Xiao HW. Energy efficient improvements in hot air drying by controlling relative humidity based on Weibull and Bi-Di models. Food Bioprod Process. 2018;111:20–9.
Igual M, García-Martínez E, Martín-Esparza ME, Martínez-Navarrete N. Effect of processing on the drying kinetics and functional value of dried apricot. Food Res Int. 2012;47(2):284–90.
Movagharnejad K, Vahdatkhoram F, Nanvakenari S. Optimization of microwave and infrared drying process of nettle leaves using design of experiments. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7511-5.
Rastogi NK, Angersbach A, Niranjan K, Knorr D. Rehydration kinetics of high pressure pretreated and osmotically dehydrated pineapple. J Food Sci. 2000;65(5):838–41.
Fasogbon BM, Gbadamosi SO, Talwo KA. Studies on the osmotic dehydration and rehydration characteristics of pineapple slices. J Food Process Technol. 2013;4(4):1–8.
Onwude DI, Hashim N, Janius RB, Nawi NM, Abdan K. Modeling the thin-layer drying of fruits and vegetables: a review. Compr Rev Food Sci Food Saf. 2016;15(3):599–618.
Kayran S, Doymaz İ. Determination of drying kinetics and physicochemical characterization of apricot pomace in hot-air dryer. J Therm Anal Calorim. 2017;130(2):1163–70.
Tekin ZH, Baslar M. The effect of ultrasound-assisted vacuum drying on the drying rate and quality of red peppers. J Therm Anal Calorim. 2018;132(2):1131–43.
Zhang J, Ma P, Zhang X, Wang B, Wu J, Xing X. Isothermal drying kinetics of paddy using thermogravimetric analysis. J Therm Anal Calorim. 2018;134(3):2359–65.
Surendhar A, Sivasubramanian V, Vidhyeswari D, Deepanraj B. Energy and exergy analysis, drying kinetics, modeling and quality parameters of microwave-dried turmeric slices. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7791-9.
Chong CH, Law CL, Figiel A, Wojdylo A, Oziemblowski M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013;141(4):3889–96.
Barrett DM, Beaulieu JC, Shewfelt R. Color, flavor, texture and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement and the effects of processing. Crit Rev Food Sci Nutr. 2010;50(5):369–89.
Porciuncula BDA, Segura LA, Laurindo JB. Processes for controlling the structure and texture of dehydrated banana. Dry Technol. 2016;34(2):167–76.
Madiouli J, Lecomte D, Nganya T, Chavez S, Sghaier J, Sammouda H. A method for determination of porosity change from shrinkage curves of deformable materials. Dry Technol. 2007;25:621–8.
AOAC. AOAC method no 985.29. Official methods of analysis. 15th ed. Arlington: Association of Official Analytical Chemists; 1990.
Kingsly RP, Singh DB. Drying kinetics of pomegranate arils. J Food Eng. 2007;79:741–4.
Malekjani N, Emam-Djomeh Z, Hashemabadi SH, Askari, GR. Modeling thin layer drying kinetics, moisture diffusivity and activation energy of hazelnuts during microwave-convective drying. Int J Food Eng. 2017. https://doi.org/10.1515/ijfe-2017-0100.
Kaya A, Aydin O, Demirtas C. Concentration boundary conditions in the theoretical analysis of convective drying process. J Food Process Eng. 2007;30(5):546–77.
Caliskan G, Dirim SN. Drying characteristics of pumpkin (Cucurbita moschata) slices in convective and freeze dryer. Heat Mass Transf. 2017;53(6):2129–41.
Said LBH, Bellagha S, Allaf K. Measurements of texture, sorption isotherms and drying/rehydration kinetics of dehydrofrozen-textured apple. J Food Eng. 2015;165:22–33.
Aral S, Beşe AV. Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage and rehydration capacity. Food Chem. 2016;210:577–84.
Bennett LE, Jegasothy H, Konczak I, Frank D, Sudharmarajan S, Clingeleffer PR. Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions. J Funct Foods. 2011;3(2):115–24.
Li X, Wasila H, Liu L, Yuan T, Gao Z, Zhao B, Ahmad I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 chinese cultivars and the environmental factors analysis. Food Chem. 2015;175:575–84.
Aghraz A, Gonçalves S, Rodríguez-Solana R, Dra LA, Di Stefano V, Dugo G, Cicero N, Larhsini M, Markouk M, Romano A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S Afr J Bot. 2018;118:58–64.
Hosu A, Cristea VM, Cimpoiu C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in romanian red wines: prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014;150:113–8.
Doymaz I. Prediction of drying characteristics of pomegranate arils. Food Anal Methods. 2012;5(4):841–8.
Figiel A. Drying kinetics and quality of vacuum-microwave dehydrated garlic cloves and slices. J Food Eng. 2009;94:98–104.
Calín-Sanchez A, Figiel A, Szarycz M, Lech M, Nuncio-Jáuregui N, Carbonell-Barrachina AA. Drying kinetics and energy consumption in the dehydration of pomegranate (Punica granatum L.) arils and rind. Food Bioprocess Technol. 2014;7(7):2071–83.
Mundada M, Hathan BS, Maske S. Convective dehydration kinetics of osmotically pretreated pomegranate arils. Biosyst Eng. 2010;107(4):307–16.
Doymaz I. Drying of pomegranate arils and selection of a suitable drying model. Food Biophys. 2011;6(4):461–7.
Zogzas NP, Maroulis ZB, Marinos-Kouris D. Moisture diffusivity data compilation in foodstuffs. Dry Technol. 1996;14:2225–53.
Białobrzewski I. Determination of the mass transfer coefficient during hot-air-drying of celery root. J Food Eng. 2007;78(4):1388–96.
Sadeghi M, Kesbi OM, Mireei SA. Mass transfer characteristics during convective, microwave and combined microwave-convective drying of lemon slices. J Sci Food Agric. 2013;93(3):471–8.
Beigi M. Mathematical modelling and determination of mass transfer characteristics of celeriac slices under vacuum drying. Period Polytech Chem Eng. 2017;61(2):109–16.
Prasertsan S, Saen-saby P. Heat pump drying of agricultural materials. Dry Technol. 1998;16(1–2):235–50.
Cox S, Gupta S, Abu-Ghannam N. Effect of different rehydration temperatures on the moisture content of phenolic compounds, antioxidant capacity and textural properties of edible Irish brown seaweed. LWT Food Sci Technol. 2012;47(2):300–7.
Bilbao-Sainz C, Andres A, Fito P. Hydration kinetics of dried apple as affected by drying conditions. J Food Eng. 2005;68:369–76.
Mulik SV, Bhosale MG. Effect of process variable in osmo-convective dehydration of pomegranate arils. Int J Innov Eng Technol. 2015;5(4):285–93.
Lee JH, Rhim JW. Rehydration kinetics of vacuum-dried Salicornia herbacea. Food Sci Biotechnol. 2010;19(4):1083–7.
Maskan A, Kaya S, Maskan M. Hot air and sun drying of grape leather (pestil). J Food Eng. 2002;54:81–8.
Deng L, Yang X, Mujumdar AS, Zhao J, Wang D. Red pepper (Capsicum annuum L.) drying: effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity and microstructure. Dry Technol. 2018;36(8):893–907.
Cserhalmi Z, Sass-Kiss À, Tόth-Markus M, Lechner N. Study of pulsed electric field treated citrus juices. Innov Food Sci Emerg Technol. 2006;7(1–2):49–54.
Maskan M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J Food Eng. 2001;48:169–75.
Vega-Gàlvez A, Ah-Hen K, Chacana M, Vergara J, Martínez-Monzό J, Garcìa-Segovia P, Lemus-Mondaca R, Di Scala K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny smith) slices. Food Chem. 2012;132(1):51–9.
Ferreira D, Silva JALD, Pinto G, Santos C, Delgadillo I, Coimbra MA. Effect of sun-drying on microstructure and texture of S. Bartolomeu pears (Pyrus communis L.). Eur Food Res Technol. 2008;226:1545–52.
Tontul I, Topuz A. Effects of different drying methods on the physicochemical properties of pomegranate leather (pestil). LWT Food Sci Technol. 2017;80:294–303.
Koua BK, Koffi PME, Gbaha P. Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans. J Saudi Soc Agric Sci. 2019;18(1):72–82.
Abbasi S. Investigation of changes in physical properties and microstructure and mathematical modeling of shrinkage of onion during hot air drying. Iran Food Sci Technol. 2011;7(1):92–8.
Horuz E, Maskan M. Hot air and microwave drying of pomegranate (Punica granatum L.) arils. J Food Sci Technol. 2015;52(1):285–93.
Lopez J, Vega-Gálvez A, Torres MJ, Lemus-Mondaca R, Quispe-Fuentes I, Di Scala K. Effect of dehydration temperature on physico-chemical properties and antioxidant capacity of goldenberry (Physalis peruviana L.). Chil J Agric Res. 2013;73(3):293–300.
Karaaslan M, Yilmaz FM, Cesur Ö, Vardin H, Ikinci A, Dalgic AC. Drying kinetics and thermal degradation of phenolic compounds and anthocyanins in pomegranate arils dried under vacuum conditions. Int J Food Sci Technol. 2014;49(2):595–605.
Šumić Z, Tepić A, Jokić S, Malbaša R. Optimization of frozen wild blueberry vacuum drying process. Hem Ind. 2014;69(1):77–84.
Méndez-Lagunas L, Rodríguez-Ramírez J, Cruz-Gracida M, Sandoval-Torres S, Barriada-Bernal G. Convective drying kinetics of strawberry (Fragaria ananassa): effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017;230:174–81.
Artık N, Anlı E, Konar N, Vural N. Gıdalarda bulunan fenolik bileşikler. İzmir: Sidas Medya; 2016 (in Turkish).
Larrauri J, Ruperez P, Saura-Calixto F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J Agric Food Chem. 1997;45:1390–3.
Acknowledgements
This study was supported by Scientific Researches Project Unit of University of Mersin (Project Number: 2016-2-TP3-1809).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article declared that they had no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Süfer, Ö., Palazoğlu, T.K. A study on hot-air drying of pomegranate. J Therm Anal Calorim 137, 1981–1990 (2019). https://doi.org/10.1007/s10973-019-08102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08102-1