Abstract
The fusion and thermal decomposition of thirty-three diselenide compounds with a urea, thiourea or selenourea group linked with different aliphatic or aromatic substituents have been studied by thermogravimetry, differential scanning calorimetry and mass spectrometry in order to perform comparative thermal stability studies among analogs. A relationship has been found between stability and a series of effects which occur in the compound structures. Analysis of the thermal data indicated that: (a) in general, compounds with a urea or selenourea group are more stable than those with a thiourea group; (b) no difference in stability exists when an aromatic or aliphatic group is linked to the thiourea group but when linked to the urea or selenourea groups, stability does differ; (c) selenourea compounds with aliphatic chain are the most unstable; and (d) the nature of the substituent located on the benzyl ring has no effects on thermal stability. Therefore, criteria for the selection of substituents can be established in order to improve the stability of these drugs. In addition, the mass spectral fragmentation in comparison with thermal analytical data helps in confirming the thermal behavior of the compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal analysis refers to the study of the changes that a physical property of a sample undergoes in terms of time and/or temperature, while the substance is subjected to temperature programing, and it involves a number of techniques [1]. Thermogravimetry (TG) and differential scanning calorimetry (DSC) are very important analytical and quantitative methods for the study of pharmaceutical compounds thermal behavior [2, 3]. There are numerous reviews and studies reporting the use of thermal analysis, in association with other techniques for drug characterization [4, 5], storage and stability of drugs [6]. A number of investigations have been carried out regarding the application of thermal techniques in the study of thermal decomposition, thermal stability [7–10], polymorphism [11–13], solid-state reactions, drug formulations [14–18], purity and other properties of solid compounds used in the pharmaceutical industry [19, 20].
The very useful tools which allow determination of the thermal degradation characteristics of substances during decomposition are TG and DSC analyses. In addition, coupling of the TG or DSC apparatus with a mass spectrometer makes it possible to analyze types of gases released into the atmosphere [21–23]. In addition, a correlation between mass spectral fragmentation and TA degradation of the compounds can helpful when selecting the correct pathway of the fragmentation that can contribute to understanding the properties of the compounds at a molecular level, thereby providing tools for developing novel drug formulations and for determining the active sites of the drugs that are responsible for the chemical, biological and medical reactivity in vivo systems [24–27].
Selenium is an essential trace element for human physiology [28]. Several studies have linked selenium deficiency to increased risk of various diseases such as cancer [29]. Due to its pharmacological potential, the development of novel organoselenium compounds has intensified over the last several years. Among the multiple structures identified, diphenyl diselenides (DPDS) have emerged as new promising candidates for the treatment of a wide range of diseases [30]. In addition, our research team has investigated and developed a large number of diphenyl diselenide derivatives that exert potent cytotoxic effects against different tumor cell lines [31, 32], such as against leishmania parasites [33, 34].
In order to improve the therapeutic potential of these compounds, we have extended these promising findings by synthesizing a panel of novel diphenyl diselenide derivatives that incorporate urea, thiourea and selenourea moieties in order to adjust polarity, solubility and the ability of interaction and the formation of hydrogen bonds. The urea scaffold has been extensively used in the design of new anticancer drugs as an important fragment that generally forms two hydrogen bonds with the kinase ATP binding site [35]. Inspired by the potential inhibitory ability of these compounds, we envisaged the modulation of a urea group by other scaffolds containing thiourea or selenourea entities. Tenovin-1, which contains thiourea template, induces apoptosis by p-53 activation [36]. Related to the structural motif, selenourea has been explored in spite of the fact that there are no studies relating them as biological agents. However, these compounds were prepared in order to study the importance of the increase in the amount of selenium in the biological activity and to determine if this factor acts in tandem with the basic molecular scaffold of the structure. It is not common to find thermochemical studies regarding organoselenium compounds in the solid state [37, 38].
This study describes the thermal stability, decomposition and transformation of some new analogous diselenide compounds with urea, thiourea or selenourea groups between the central diselenide core and a variety of substituents, between room temperature and 400 °C. TG, DTG and DSC are used in order to broaden the physicochemical information of these compounds for future use in synthesis or as chemopreventive agents. The results allowed us to gain knowledge concerning these compounds in the solid state, including their thermal stability and thermal decomposition.
Experimental
Materials
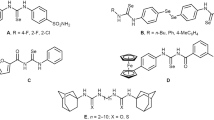
Structures of these 33 newly synthesized compounds are unique, and they had a symmetrical distribution around a central diselenide core. We introduced a urea, thiourea or selenourea group in order to perform comparative studies between analogous (Table 1). Linked to this group, we included different aliphatic or aromatic substituents. The compounds were synthesized by our research group from the reaction between 4,4′-diaminodiphenyldiselenide, obtained in good yield and purity as previously described by us [33], with the corresponding isocyanate, isothiocyanate and isoselenocyanate in dioxane. Isocyanates and isothiocyanates were commercially available, but the corresponding isoselenocyanates were prepared in two steps by formylation of amines followed by the treatment with phosgene and selenium powder in the presence of triethylamine under reflux according to the literature [39] (Scheme 1, results unpublished). All compounds were synthesized with a high grade of purity, as they had been previously evaluated as cytotoxic agents in biological assays. Each product was identified by infrared spectroscopy, 1H-NMR and 13C-NMR spectroscopy, elemental analysis and mass spectrometry.
Methodology
The thermogravimetric studies were carried out with a PerkinElmer TGA-7, and the calorimetric studies were carried out with a PerkinElmer DSC Diamond. The thermobalance was calibrated with alumel and nickel at 10 °C min−1. The calibration of the oven temperatures was carried out automatically. Mass calibration was carried out with a certified mass of 10 mg (ASTM E617). The calorimeter was calibrated with indium and zinc (provided by PerkinElmer and fabricated according to guideline ISO35) at 10 °C min−1 and a nitrogen flow of 20 mL min−1. The gases connected to the equipment were nitrogen and air with a purity of 99.999 %.
Thermogravimetric analyses were performed under nitrogen atmosphere with a gas flow of 40 mL min−1 at 10 °C min−1, using a sample of approximately 3 mg. The T initial, T onset and T max, as well as any associated mass loss, were calculated. All of the experiments were performed at least three times, and the values mean and standard deviation were calculated.
Calorimetric analyses were performed in aluminum capsules for volatiles of 10 μL, at a heating rate of 10 °C min−1, using a sample of approximately 3 mg, in order to establish the T onset, T max and the enthalpy of fusion ∆H f. All of the experiments were performed at least three times, and the values mean and standard deviation were calculated.
The calorimetric analysis starts with the study of the thermal behavior of the compounds before beginning the process of degradation in order to evidence the possible polymorphism of these compounds. For this preliminary study, samples of all the compounds are exposed to successive cycles of heating–cooling. All samples were heated until temperatures 15–20 °C below T onset of degradation process, in order to asses that compounds were not degraded (Table 2). After melting the samples and cooling at room temperature, they were left at room temperature enough time to be able to hypothesize that the compounds recrystallized before successive thermal processes. Additional cycles of heating–cooling are performed if polymorphism is detected.
Mass spectral measurements and instrument: The mass spectra (MS-DIP) have been carried out using a mass spectrometer Agilent 5973A with electron impact ionization at 70 eV. The samples were introduced by means of direct insert probe (DIP). The instrument was calibrated using perfluorotributylamine as standard material.
Results and discussion
Thermal stability in the decomposition
Table 2 shows the degradation temperatures (as T onset) for all the compounds studied, classified as urea, thiourea or selenourea series.
Depending on the different aliphatic or aromatic substituents linked to urea, thiourea or selenourea group, the following effects can be observed:
-
In general, the degradation temperature values presented by the compounds containing an aromatic group bonded to urea and selenourea groups are relatively high (Fig. 1), and therefore, these compounds are more stable than thiourea ones (O4–11 ~ Se4–11 ≫ S4–11).
-
A methylene group separating this aromatic substituent has contradictory effects: It confers stability to the thiourea compounds, but there is no evidence of this occurring with urea and selenourea compounds (O4–5, S4–5 and Se4–5 in comparison with O6–8, S6–8 and Se6–8).
-
Compounds with an aromatic substituent without a methylene group have the highest stability and do not depend on the substituent on the aromatic ring (O6–11, S6–11 and Se6–11).
-
Urea and selenourea compounds containing aliphatic substituents are less stable than those with aromatic groups. A minor effect can be observed in thiourea compounds. Selenourea compounds with aliphatic chain (Se1 and Se2) are the most unstable.
-
The selenourea compounds showed very different thermal behavior according the substituent, aliphatic or aromatic, linked to selenourea group:
-
(a)
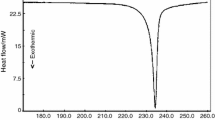
The curve of the selenourea derivatives containing aromatic substituent (e.g., compound Se10, Fig. 2) showed a one-step degradation process. The rate value corresponding to loss of mass of w∞ = 41.0 % was v max = 14.28 % min−1.
-
(b)
However, the curve of selenourea compounds containing an aliphatic substituent showed a multi-step degradation (e.g., compound Se1, Fig. 3). The first degradative step occurs, slowly and gradually, with a mass loss of w∞ = 49 % and a value of rate of mass loss, v max, of 2.56 % min−1 that occurs in a wide thermal interval.
In all the cases, the first step of degradation could be related to the loss of the two R groups bonded to the nitrogen atoms of selenourea groups.
With regard to the type of aliphatic chain bonded to urea, thiourea and selenourea groups, we have observed that those with either a butyl or hexyl group showed diminished thermal stability; the group with a cyclohexyl chain remained thermally very stable.
Thermal stability considerations
In general, and with regard to the functional groups that are present in the molecule, it has been observed that thermal stability depends on the different intensities of the intermolecular bonds and tautomeric balances that the compounds are able to establish. The tautomerism reinforces the electronic delocalization of the structure of the molecule and gives stability to the compounds as well as to the hydrogen intermolecular interactions.
Due to this stabilization, the values corresponding to degradation temperature are comparatively higher in the series in which the urea or selenourea group is present. Compounds O1–11/Se1–11 and S1–11 (oxygen/selenium and sulfur compounds, respectively) showed very different thermal stability due to the type of extremely reinforceable intermolecular attraction that the hydrogen bond between N–H and oxygen or selenium atoms provides.
-
With respect to groups present in the substituent, thermal stabilization is induced by the presence of an aromatic group, possibly due to the potent electronic delocalization which permits coplanarity of the aromatic ring and the urea and selenourea groups. The degradation processes of these molecules are slow, and they will take longer to degrade than the aliphatic compounds.
-
The thermal behavior observed with compounds Se1 and Se2 (aliphatic substituted selenium compounds) was very different than with aromatic substituted compounds or with any of the urea and thiourea compounds (Fig. 4).
-
With regard to the length of the aliphatic chain bonded to the urea, thiourea or selenourea group, we have observed that butyl and hexyl groups diminished thermal stability of compounds (O1–2, S1–2 and Se1–2). The explanation to this could be that when we substituted with less rigid groups, the compound was less packable and sterically hindered. These factors diminished thermal stability of the compounds.
-
With regard to substituents on the aromatic ring, we cannot establish relationships between electron-withdrawing or electron-donating groups and thermal stability of the compounds for any one of the series.
Thermal stability of fusion process
None of the compounds studied showed polymorphic behavior. During the first thermal scan of each case, one of two things happened: Either there was only an endothermic process, typical of a melting process, or a degradation exotherm occurred, corresponding to the joint process of fusion and degradation of the compound. After a first heating–cooling scan of the sample, the compounds solidify into an amorphous form.
Analysis of calorimetric data displayed for these compounds showed that compounds containing aliphatic substituents present lower values than those with aromatic’s ones, as occurs in the degradation process; this was true for all the series tested. The T onset and enthalpy of fusion values for compounds of urea, thiourea and selenourea series are shown in Table 3.
The fusion temperature values presented by the compounds which present a urea or selenourea group between the principal chain and the substituent are relatively high and, therefore, these series of compounds generally have greater thermal stability during the fusion than the compounds with a thiourea group.
The functional group present in the substituent is not a determining factor for the urea and thiourea compounds, and no significant differences are observed among the compounds.
However, when we substituted a butyl or hexyl group in compounds with a selenourea group, the T onset diminished with respect to a cyclohexyl group or aromatic substitution (Se1–2 and Se3–11) due to the fact that the order is not as rigid and also because the packaging presents other possibilities.
Thermal stability considerations
When the molecules are structurally analyzed, it is observed that the urea, thiourea or selenourea groups can establish hydrogen bonds that are a determining factor in the stability; stronger hydrogen intermolecular interactions give more stability to the crystal.
The selenourea and urea compounds have higher values of T onset than their analogous sulfur compounds. In some compounds, the hydrogen bonds were so strong that the decomposition occurred without previous fusion.
An increase in physical stability is observed with aromaticity, due to the electronic delocalization in the π orbitals of the aromatic ring that causes and additional intermolecular interaction.
Mass spectrometry
The mass spectral fragmentation in comparison with thermal decomposition can be used to confirm and to elucidate the general and thermal behavior of the compounds analyzed [40].
The compounds studied present a urea, thiourea or selenourea groups, and they must fragment in α position (nitrogen–carbon fragmentation) with high relative abundance in MS of resulting fragments. The pattern of fragmentation has been established by mass spectrometry (Fig. 5).
In addition, only thiourea compounds fragment in β position; then, a double Mc Lafferty rearrangement is observed (Fig. 6) reporting, for all these compounds, a fragment with a m/z 340 (fragment F). The fragment at m/z 428 (and isotope distribution corresponding of two Se atoms), fragment G (C14H10N2Se2S2)+·, corresponds to a double fragmentation in α position with elimination of two (R–NH·) radical groups.
The occurrence of the most important fragments in the mass spectrum of each compound is shown in Table 4. From the aforementioned data, it can be concluded that, in the mass spectrum of urea and selenourea compounds, almost no fragmentation occurs and low abundance (total ions of each fragment generated) values are detected (Fig. 7). This fact reveals that these urea and selenourea compounds, probably due to their structural and thermal stability, may have difficulty to suffer fragmentation.
However, in the mass spectrum of thiourea compounds, that are less thermal stable compounds, an increase in the number and abundance, in terms of total ions, of the most important fragments which are characteristic of their pattern of fragmentation is detected (Fig. 8).
The compounds show different mass spectral behaviors. It can be suggested that there is a possible correlation between thermal stability and difficulties that the urea and selenourea compounds show during their fragmentation process.
Conclusions
A relationship is found among thermal analytical data and structure of the different series of urea, thiourea and selenourea diselenide compounds.
Analysis of the thermal data indicated that, in general, compounds with a urea and selenourea group are more stable than those with a thiourea group. In most cases, there are no significant differences between urea and selenourea compounds.
No difference in stability exists when an aromatic or aliphatic group is linked to the thiourea group but when linked to the urea or selenourea groups, stability does differ.
The thermal behavior observed for aliphatic selenourea derivatives is very different to the rest of the analyzed compounds. These selenourea structures are the most unstable.
The nature of the substituent located on the benzyl ring has no effects on thermal stability. It has not been possible to establish relationships between electron-withdrawing or electron-donating groups and thermal stability of the compounds for any one of the series.
Therefore, in order to improve the stability of compounds, criteria for the selection of substituents can be established.
A possible correlation between thermal stability and the difficulties that the urea and selenourea compounds showed during their fragmentation process can confirm their thermal behavior.
References
Lever T, Hains P, Rouquerol J, Charsley EL, Eckeren PV, Burlett DJ. ICTAC nomenclature of thermal analysis (IUPAC) recommendations. Pure Appl Chem. 2014;86:545–53.
Craig Duncan QM, Reading M. Thermal analysis of pharmaceuticals. Boca Raton: CRC Press; 2007.
Yoshida MI, Oliveira MA, Gomez ECL, Mussel WN, Castro WV, Soares CDV. Thermal characterization of lovastatin in pharmaceutical formulations. J Therm Anal Calorim. 2011;106:657–64.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Amorim PHO, Ferreira APG, Machado LCM, Cervini P, Cavalheiro ETG. Investigation on the thermal behavior of beta-blockers antihypertensives atenolol and nadolol using TG/DTG, DTA, DSC, and TG–FTIR. J Therm Anal Calorim. 2015;120:1035–42.
Neto HS, Novák C, Matos J. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97(1):367–74.
Bannach G, Cervini P, Cavalheiro ETG, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behavior of epinephrine. Thermochim Acta. 2010;499:123–7.
Bannach G, Arcaro R, Ferroni DC, Siqueira AB, Treu-Filho O, Ionashiro M, Schnitzler E. Thermoanalytical study of some antiinflammatory analgesic agents. J Therm Anal Calorim. 2010;102:163–70.
Ambrozini B, Cervini P, Cavalheiro ETG. Thermal behavior of the beta-blocker propranolol. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-5118-7.
Silva ACM, Galico DA, Guerra RB, Legendre AO, Rinaldo D, Galhiane MS, Bannach G. Study of some volatile compounds evolved from the thermal decomposition of atenolol. J Therm Anal Calorim. 2014;115:2517–20.
Correa JCR, Perissinato AG, Serra CHD, Trevisan MG, Salgado HRN. Polymorphic stability of darunavir and its formulation. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4984-3.
Owusu-Ware SK, Cherry AJ, Baltus CB, Spencer J, Antonijevic MD. Thermal analysis of novel biphenylamide derivatives. J Therm Anal Calorim. 2015;121:437–52.
Silva PSP, Castro RAE, Melro E, Silva MR, Maria TMR, Canotilho J, Eusebio MES. Structural evidence of polymorphism and conformational isomorphism of a somewhat flexible molecule: m-anisic acid. J Therm Anal Calorim. 2015;120:667–77.
Neto HS, Novak C, Matos JR. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97:367–74.
Kumar N, Goindi S, Saini B, Bansal G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2014;115:2375–83.
Lira AM, Araujo AAS, Basilio IDJ, Santos BLL, Santana DP, Macedo RO. Compatibility studies of lapachol with pharmaceutical excipients for the development of topical formulations. Thermochim Acta. 2007;457:1–6.
de Souza SMM, Franco PIBEM, Leles MIG, da Conceicao EC. Evaluation of thermal stability of enalapril maleate tablets using thermogravimetry and differential scanning calorimetry. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4648-3.
da Silva EP, Pereira MAV, Lima IPD, Lima NGPB, Barbosa EG, Aragao CFS, Gomes APB. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-015-5077-z.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68:335–57.
Giron D, Goldbronn C. Use of DSC and TG for identification and quantification of the dosage form. J Therm Anal Calorim. 1997;48:473–83.
Etienne S, Becker C, Ruch D, Germain A, Calberg C. Synergetic effect of poly(vinyl butyral) and calcium carbonate on thermal stability of poly(vinyl chloride) nanocomposites investigated by TG–FTIR–MS. J Thermal Anal Calorim. 2010;100:667–77.
Wiecinska P. Thermal degradation of organic additives used in colloidal shaping of ceramics investigated by the coupled DTA/TG/MS analysis. J Thermal Anal Calorim. 2015;. doi:10.1007/s10973-015-5075-1.
Izato Y, Miyake A. Thermal decomposition mechanism of ammonium nitrate and potassium chloride mixtures. J Therm Anal Calorim. 2015;121:287–94.
Zayed MA, Fahmey MA, El-Desawy M, Farrag YS. Structure characterization of terazosin drug using mass spectrometry and thermal analyses techniques in comparison with semi-empirical molecular orbital (MO) calculations. J Therm Anal Calorim. 2015;120:1061–9.
Yılmaz N, Oz S, Atakol A, Svoboda I, Aydiner B, Akay MA, Atakol O. An experimental and theoretical study toward the synthesis, structure and thermal decomposition of some phenyl tetrazoles. J Therm Anal Calorim. 2015;119:2321–8.
Zayed MA, El-Dien FAN, Hawash MF, Fahmey MA. Mass spectra of gliclazide drug at various ion sources temperature. J Therm Anal Calorim. 2010;102:305–12.
Lizarraga E, Zabaleta C, Palop JA. Mechanism of thermal decomposition of thiourea derivatives. J Therm Anal Calorim. 2008;93:887–98.
Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–77.
Fernandes AP, Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim Biophys Acta. 2015;1850(8):1642–60.
Nedel F, Campos VF, Alves D, McBride AJ, Dellagostin OA, Collares T, Savegnago L, Seixas FK. Substituted diaryl diselenides: cytotoxic and apoptotic effect in human colon adenocarcinoma cells. Life Sci. 2012;91(9–10):345–52.
Plano D, Baquedano Y, Ibáñez E, Jiménez I, Palop JA, Spallholz JE, Sanmartín C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules. 2010;15(10):7292–312.
Romano B, Plano D, Encío I, Palop JA, Sanmartín C. In vitro radical scavenging and cytotoxic activities of novel hybrid selenocarbamates. Bioorg Med Chem. 2015;23(8):1716–27.
Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jiménez-Ruiz A, Palop JA, Sanmartín C. Selenocyanates and diselenides: a new class of potent antileishmanial agents. Eur J Med Chem. 2011;46(8):3315–23.
Baquedano Y, Moreno E, Espuelas S, Nguewa P, Font M, Gutiérrez KJ, Jiménez-Ruiz A, Palop JA, Sanmartín C. Novel hybrid selenosulfonamides as potent antileishmanial agents. Eur J Med Chem. 2014;3(74):116–23.
Chen JN, Wang XF, Li T, Wu DW, Fu XB, Zhang GJ, Shen XC. Wang; HS Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem. 2016;1(107):12–25.
Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100.
Liebman JF, Slayden SW. Thermochemistry of organoselenium and organotellurium compounds. In: Rappoport Z, editor. The chemistry of organic selenium and tellurium compounds, vol. 3. New York: Wiley; 2012. p. 139–65.
Patial BS, Thakur N, Tripathi SK. Crystallization study of Sn additive Se–Te chalcogenide alloys. J Therm Anal Calorim. 2011;106:845–52.
Attanassov PK. Synthesis of 4-(phenylamino)quinazoline-2(1H)-selenoles and diselenides from isoselenocyanates. Helv Chim Acta. 2004;87:1873–87.
Fahmey MA, Zayed MA, El-Shobak HG. Study of some phenolic-iodine redox polymeric products by thermal analyses and mass spectrometry. J Therm Anal Calorim. 2005;82:137–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz, M., Palop, J.A., Sanmartín, C. et al. Thermal stability and decomposition of urea, thiourea and selenourea analogous diselenide derivatives. J Therm Anal Calorim 127, 1663–1674 (2017). https://doi.org/10.1007/s10973-016-5645-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5645-x