Abstract
Terazosin (C19H25N5O4, MW = 387) drug is a selective alpha 1 antagonist. It is used for lowering the blood pressure. Also, it used for treatment of symptoms of an enlarged prostate and is therefore a drug of choice for men with hypertension and prostate enlargement. In the present study, mass spectrometry (MS) and thermal analyses (TA) were used to investigate the fragmentation decomposition pathways of terazosin and confirmed by semi-empirical molecular orbital (MO) calculation, using PM3 procedure on the neutral and the positively charged species of the drug. These calculations included, bond length, bond order, partial charge distribution, ionization energy and heats of formation (ΔH f). The mass spectra and TA fragmentation pathways were proposed and compared to each other to select the most suitable scheme representing the correct fragmentation pathway of the drug in both techniques. This selection helps understanding of metabolism of the drug in vivo system. Therefore, comparison between MS and TA helps in selection, the proper pathway representing the fragmentation of this drug. This comparison successfully confirmed by MO calculation.
Graphical Abstract
In the present study, mass spectrometry (MS) and thermal analyses (TA) were used to investigate the fragmentation decomposition pathways of terazosin and confirmed by semi-empirical molecular orbital (MO) calculations. The mass spectra and thermal analyses fragmentation pathways were proposed and compared to each other to select the most suitable scheme representing the correct fragmentation pathway of the drug in both techniques. This selection helps understanding of metabolism of the drug in vivo system. Terazosin hydrochloride (C19H25N5O4) drug (marketed ITRIN), has an IUPAC name; 2-[4-(2-tetrahydrofuranyl) carbonyl]-1 piperazinyl- 6,7-dimethoxy-4-quinazolinamine monohydrochloride dihydrate, and its stereo structure is given below

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terazosin hydrochloride (C19H25N5O4) drug (marketed ITRIN), has an IUPAC name; 2-[4-(2-tetrahydrofuranyl) carbonyl]-1 piperazinyl- 6,7-dimethoxy-4-quinazolinamine monohydrochloride dihydrate, and its stereo structure is given in Fig. 1. It is a highly selective potent α-1 adrenoreceptor antagonist. It is an effective drug for hypertension and prostatic hyperplasia. Terazosin relaxes muscles in the prostate and bladder neck, making it easier to urinate [1].
Mass spectrometry (MS) and thermal analysis (TA) are two physical methods of analysis that are often times used in parallel with each other [2–6]. The complementary nature of these techniques has been demonstrated for some species where the fragmentation and/or subsequent degradation processes began at a similar location within the molecule, i.e., the weaker bond. In MS, the species are ionized, where ions of increased stability is detected. In TA, the species are heated until they decompose yield mass losses until the sample is completely decomposed.
MS has become a power tool for drug metabolism studies [4]. The technique is important because it provides a large amount of structural information with little expenditure of sample. In electron impact (EI) mass spectrum, the fragmentation consists of series of competitive and consecutive unimolecular fragmentation [5]. The fragmentation of ionized molecule depends mainly on their internal energy [6]. At 70 eV, the spectra are very complex; it is difficult to uncover all the competing and consecutive fragmentation reaction. Lowering the energy of the ionized electron beam, it is possible to make the spectra more simple and the high-energy processes are thus suppressed [7].
Thermal analytical techniques can provide important information regarding storage and stability of pharmaceuticals. Thermal analytical methods have thus become important tools for the development of modern medicines [8–12]. These are precise and accurate techniques with low sample requirements and can provide detailed information about new chemical entities even at the very earliest stages of discovery and development of the new compositions and drugs [13–16]. Thermogravimetric TG/DTG analysis used to provide quantitative information on mass losses due to decomposition and/or evaporation of low molecular materials as a function of time and temperature. In conjunction with mass spectrometric analysis [17–19], the nature of the released volatilize may be deduced, thus greatly facilitating the interpretation of thermal degradation processes. On the other hand, computational quantum chemistry can provide additional information about the atoms and bonds, which can be used successfully in an interpretation of experimental results [20]. Application of computational quantum chemistry in addition to experimental results (MS and TA) gives valuable information about the atoms and bonds which helps in the description and prediction of primary fragmentation site of cleavage and subsequent one [6, 21–23].
Some analytical methods have been previously reported for determination of terazosin in biological fluids and pharmaceutical preparations. Terazosin was determined by spectroscopic method [2], fluorimetry [3], high-performance liquid chromatography (HPLC) with fluorescence detection [4], HPLC [5], x-ray fluorescence spectrometry based on the formation of ion-pair associates with zinc thiocyanate [6].
The aim of the present work is focusing on further application of our previously work on various drugs [6, 21–23]. This work includes a correlation between mass spectral fragmentation and TA degradation of the terazosin drug and comparing these experimental data with the theoretical MO calculation to identify the weakest bonds ruptured during both mass and thermal studies. Consequently, the choice of the correct pathway of such fragmentation knowing this structural session of bonds can be used to decide the active sites of this drug responsible for its chemical, biological and medical reactivity in vivo system.
Experimental
Molecular orbital calculations (MOCS)
The MOCS were performed using semi-empirical molecular orbital calculation. The method used in these computations is the parametric method (PM-3) described by Stewart [24]. The default criteria for terminating all optimizations were increased by a factor of 100 (keyword PRECISE). All the molecular orbital calculations were carried out at the Restricted Hartree–Fock level for the neutral molecule of terazosin, while the unrestricted Hartree–Fock level were carried out for its cation by using PM3 method followed by full optimization of all geometrical variables (bond lengths and bond orders), without any symmetry constraint. All structures were optimized to a gradient norm 0.01–0.05, using the eigenvector following (EF) routine [25]. All the semi-empirical MO calculations were performed with the MOPAC2000 software package [26] implemented on an Intel Pentium IV 3.0 GHz computer.
Thermal analyses (TA)
The thermal analyses (TA) of terazosin drug were made using conventional thermal analyzer/Shimadzu system of DTA-50 and 30 series TG-50. The mass losses of 5 mg sample and heat reopens of the change of the sample were measured from room temperature up to 600 °C in inert argon atmosphere at heating rate = 10 °C min−1. These instruments were calibrated using indium metal as a thermal stable material. The reproducibility of the instrument reading was determined by repeating each experiment more than twice.
Mass spectrometry (MS)
Electron ionization (EI) mass spectrum of Terazosin is obtained using Thermo Finnegan TRACE DSQ quadruple mass spectrometer with electron multiplier detector equipped with GC–MS data system. The direct probe (DP) for solid material was used in this study. The sample was put into a glass sample micro-vial, by a needle (≈1 µg max), the vial installed on the tip of the DP containing heating cable and inserted into the evacuated ion source. The sample was ionized by electron beam emitted from the filament, the generated ions being effectively introduced into the analyzer by the focusing and extractor lenses system. The MS was continuously scanned, and the obtained spectra were stored. Electron ionization mass spectra were obtained at ionizing energy value of 70 and 12 eV, ionization current of 60 µA and vacuum is better than 10−6 torr.
Results and discussion
It is of great interest to study the chemistry and reactivity of terazosin drug (Fig. 1) because of its importance in medicine [27].
Knowledge obtained from thermal decomposition mechanisms of the neutral drug is very important to understand the chemical process that shared in biological systems [28]. It is difficult to establish the exact major fragmentation pathway in EI using conventional MS. With combining the above two techniques and the data obtained from the MO calculation, it is possible to understand the following topics:
-
1.
Stability of the drug under thermal degradation in solid state and mass spectral fragmentation in gas phase.
-
2.
Prediction of the primary site of fragmentation and subsequent bond cleavage.
-
3.
The correct pathway in both techniques.
-
4.
Understanding what actually happened in biodegradation of the drug or its derivatives in vivo system and metabolites.
-
5.
Thermal stability of the drug was required information for handling, stronger and shelf life.
Computational molecular orbital calculations (MOCS)
Molecular orbital (MO) calculation gives valuable information about the structure and reactivity of the molecules, which actually be used to support the experimental evidence. The much important parameters calculated using MO calculation which includes bond orders, bond length, charge distribution, and heat of formation and ionization energy. In the present work, the calculations have been carried out on terazosin neutral molecule (related to TA decomposition) and charged molecular ion (related to MS fragmentation) which is used for prediction of weakest bond rupture to follow the fragmentation pathways in both techniques. Figure 2 shows the numbering system of terazosin skeleton that helps in ordering the calculated parameters. Table 1 represents the values of bond length/Å and bond order. One can conclude the following from Table 1.
-
1.
Small differences in bond length in terazosin system upon ionization, indicating that no appreciable change in the geometries during this variation.
-
2.
The lowest bond order (important for prediction of primary site of cleavage) observed at bond C17–C21 for both neutral (0.919) and positive species (0.932).
-
3.
Upon ionization of terazosin the stability of the molecule decreased by −39.47 kJ mol−1 = (ΔH f (−25.08) − ΔH +f (14.39)).
The charge distribution on different atoms (C, N and O) and heats of formation; ΔH f/k J mol−1 for neutral and ionic terazosin species and the numbering system of the drug are summarized in Fig. 2a, b, respectively. Significant changes in the electron distribution with given system often takes place during the ionization. The MOCS data of neutral and ionized forms of terazosin are shown in Table 1 and represent the values of bond length and bond order.
Thermal analyses and MOCS of neutral tera molecule
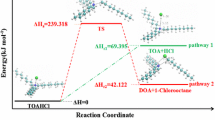
The TA of terazosin are illustrated in Fig. 3 in which this drug decomposed completely within the temperature range of 80–400 °C (mass loss = 99.0 %). Figure 3a shows the TG of the drug; it refers to its thermal decomposition in three steps. The first one occurs in the temperature range 72.18–139.14 °C practical mass loss = 6.58 %. It may be related to the loss of two water molecules (estimated mass loss = 7.83 %). The second on occurs at 204.28–358.33 °C of practical mass loss = 29.94 %; it may be attributed to the loss C7H13NO2 of estimated mass loss = 30.66 % as a result of rupture of two weak bonds C3–N4 and C5–N4. These two mass losses are confirmed by the appearance of two endothermic peaks in DTA (Fig. 3b) at temperature 117.97 and 273.62 °C, respectively. The third mass loss occurs in the temperature range of 358.33–640.45 °C. It may be attributed to the loss of C10H11N3O2.HCl (practical mass loss = 54.65 %, estimated = 53 %) as a result of rupture of the weak bond C7–N4. It appears as two exothermic peaks in DTA at 345.98 and 558.66 °C, respectively. These peaks may be attributed to chemical fragmentation and rearrangements to give new possible final forms. Therefore, these thermal decomposition endothermic peaks and chemical exothermic rearrangements of thermal decomposition of Terazosin can be represented by the Scheme 1. In this scheme, the ruptured bonds are those thermally unstable as detected by MOCS. These bonds are C3–N4 (1.489, 0.972) < N4–C5 (1.490, 0.968) < C7–N4 (1.423, 1.103), which ordered from the weakest to the strongest depending upon the values of bond length/Å and bond order of the neutral form of tera (Fig. 2a). This order of thermal bond rupture is also correlated with the charge values on atoms of these bonds as given by: C3–N4 (−0.1090, 0.0616) < N4–C5 (0.0616, −0.1079) < C7–N4 (0.0522, 0.0616). The charge values on bond atoms are actually affected both bond length or bond order as a result of repulsive or attractive forces between these atoms [28]. These results may help to identify some of possible metabolites of this drug in vivo system.
Mass spectral (MS) fragmentation and MOCS of terazosin ionized drug molecule
The scope of this investigation is restricted to a search for prediction of the first and subsequent bond ruptures during the course of fragmentation of terazosin drug in MS technique. The subsequent fragmentation in MS is determined to large extent by the initial bond rupture of molecular ion [6, 17–23]. A number of mass spectrometric techniques have utilized helping in rationalized the correct pathways of the molecules, among which are: threshold measurement [29] and metastable abundance ratios [30]. On the other hand, computational can provide important information which can be used successfully in description of primary site of cleavage. These theoretical data can particularly, valuable for MS because they study in gas phase species, which can be handled much more easily by quantum chemistry [24–27].
Mass spectral fragmentation of terazosin drug using EI (Fig. 4a, b) mass spectra and chemical ionization (CI) (Fig. 4c) was recorded and investigated. Typical mass spectra (bar-graph) of the drug at different energy values are shown in Fig. 4. Mass spectrum of terazosin reveals (Scheme 2) three competitive and consecutive fragmentation pathways.
The EI spectra of terazosin at 70 eV (Fig. 4a) consist of a wealthy competitive and consecutive fragment ions ranging from m/z = 43 up to molecular ion at m/z = 387. The main fragmentation pathways following EI of terazosin at 70 eV are given by the proposed Scheme 2, in which the signal appeared at m/z = 387(RI = 33.20 %) at 70 eV is related to the molecular ion [M]+ of general formula [C19H25N5O4]+. This molecular ion represents 33.20 % of the base peak at m/z = 233(RI = 100 %). Pathway (1) refers to the formation of fragment ions of m/z = 316 (RI = 30.05 %) and 288 (RI = 9.26 %) of the formulae [C15H13N5O3]+ and [C14H13N5O2]+ obtained as a result of rupture of bonds C21–C24 and C21–N1 bonds, respectively. Pathway (2) refers to the formation of fragment ions of m/z = 233 as a base peak (RI = 100 %), m/z = 205 (RI = 27.91 %) of the formulae [C11H13N4O2]+ and [C10H10N2O2]+ as a result of rupture of bonds C2–C3 and N4–C5, respectively. Pathway 3 refers to fragment ions of m/z = 71 (RI = 25.09 %) and m/z = 43 (RI = 12.11 %) of the formulae [C4H7O]+ and [C2H3O]+ obtained as a result of rupture of bonds C21–C24 and C24–O28, respectively. These fragments are obtained as result of consecutive ruptures of weak bonds as obtained from MOCS (Table 1). In Scheme 2; the ruptured bonds are those unstable as detected by MOCS. These bonds are C21–C24 (1.540, 0.898) < C2–C3 (1.534, 0.964) < C3–N4 (1.488, 0.964) < N4–C5 (1.488, 0.962) < C21–N1 (1.452, 0.991) < C7–N4 (1.366, 1.376) < C24–O28 (1.426, 0.898), respectively, which ordered from the weakest to the strongest depending upon the values of bond length and bond order of the cation form of tera (Fig. 2b). This order of bond rupture is also correlated with the charge values on atoms of these bonds as given by: C21–C24 (0.2393, 0.0646) < C2–C3 (-0.0846, −0.731) < C3−N4 (−0.731, 0.3980) < N4−C5 (0.3980, −0.1673) < C21−N1 (0.2393, −0.0825) < C7–N4 (−0.0305, 0.3980) < C24–O28 (0.0646, −0.2800), respectively. The charge values on bond atoms are actually affected both bond length and bond order as a result of repulsive or attractive forces between these atoms. These results may help to identify most of the possible metabolites of this drug in vivo system.
On measurement of mass spectra of terazosin by EI at lower power of 15 eV (Fig. 4b) and using CI technique (Fig. 4c) refers to change in nature of the previously obtained main and fragment ions using EI at 70 eV. At 15 eV, the base peak becomes m/z = 387 (RI = 100 %), and on using CI, it appears at m/z = 388 (RI = 100 %) of [M]+ of the drug main molecule and the fragment ion of m/z = 233 (RI = 40 %) which completely disappear on using CI technique. This means that, lowering of energy of the power source increases RI of the drug main molecular ion and makes lot of fragment ions obtained at 70 eV of low RI and/or completely disappeared.
Correlation between TA and MS
It is important to make a discussion between results of TA and MS of terazosin, to see the behavior of the drug in both techniques. In TA, some bonds are thermally unstable in neutral form (Fig. 2a); and consequently decomposed in TG as consecutive pathway. These bonds are C3–N4 (1.489, 0.972) < N4–C5 (1.490, 0.968) < C7–N4 (1.423, 1.103), which ordered from the weakest to the strongest depending upon the values of bond length and bond order of the neutral form of tera (Fig. 2a) coming from MOCS and as represented in Scheme 1. In EI, MS at 70 eV high powers energy leads to more bond ruptures including those ruptured in TA. These bonds are C21–C24 (1.540, 0.898) < C2–C3 (1.534, 0.964) < C3–N4 (1.488, 0.964) < N4–C5 (1.488, 0.962) < C21–N1 (1.452, 0.991) < C7–N4 (1.366, 1.376) < C24–O28 (1.426, 0.898), respectively, which ordered from the weakest to the strongest depending upon the values of bond length and bond order of the cation form of tera (Fig. 2b). This comparison shows the agreement to some extent between TA and EI Mass in the proposed fragmentation pathways. Consequently, the effect of such fragmentation on the drug behavior in human body can be expected and also its metabolites can easily be identified. The obtained thermal fragments and mass fragment ions obtained in vitro system are found to be very similar to metabolites obtained in vivo systems [31–33]. This conclusion reveals the importance of TA and MS vitro systems before going to search for metabolites in vivo system.
Conclusions
Terazosin drug has great medical importance; it acts as alpha-1-selective adrenoceptor blocking agent. It is used to treat hypertension (high blood pressure) and benign prostatic hyperplasia (enlarged prostate). It causes the blood vessels (veins and arteries) to relax and expand, improving blood flow. Terazosin also relaxes muscles in the prostate and bladder neck, making it easier to urinate. Due to this importance, in the present study, MS and TA were used to investigate the fragmentation decomposition pathways of terazosin and confirmed by semi-empirical molecular orbital (MO) calculation, using PM3 procedure on the neutral and the positively charged species of the drug. These calculations included, bond length, bond order, partial charge distribution, ionization energy and heats of formation (ΔH f). The mass spectra and TA fragmentation pathways were proposed and compared to each other to select the most suitable scheme representing the correct fragmentation pathway of the drug in both techniques. This selection helps understanding of metabolism of the drug in vivo system. Therefore, the successful comparison between MS and TA helps in selection the proper pathway representing the fragmentation of this drug. This comparison successfully confirmed by MO calculation.
References
Ganjali MR, Faridbod F, Larijani B, Riahi S, Hosseini M, Esfahani EN, Norouzi P. Terazosin potentiometric sensor for quantitative analysis of terazosin hydrochloride in pharmaceutical formulation based on computational study. Int J Electrochem Sci. 2010;5:200–14.
Shrivastava A, Gupta VB. Stability-indicating RP-HPLC method for the simultaneous determination of prazosin, terazosin, and doxazosin in pharmaceutical formulations. Sci Pharm. 2012;80(3):619–31.
Larsen BS, Ewen CNM. Mass spectrometry of biological materials. New York: Marcel Dekker; 1998.
Levsen K. Fundamental aspects of organic mass spectrometry. Weiheim: Verlag Chemie; 1978.
Zayed MA, Fahmey MA, Hawash MF. Investigation of diazepam drug using thermal analyses, mass spectra and semi-empirical MO calculations. Spectrochim Acta A. 2005;61:799–805.
Das KG, James EP. Organic mass spectrometry. New Delhi: Oxford and IB 11 Publishing Co; 1976.
Barbas R, Prohens R, Puigjaner C. A new polymorph of norfloxacin complete characterization and relative stability of its trimorphic system. J Therm Anal Calorim. 2007;89(3):687–92.
Zayed MA, Hawash MF, Fahmey MA. Structure investigation of codeine drug using mass spectra, thermal analyses and semiempirical MO calculations. Spectrochim Acta A. 2005;64:363–71.
Sovizi MR. Investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102:285–9.
Santos AFO, Basílio ID Jr, de Souza FS, Medeiros AFD, Pinto MF, de Santana DP, Macêdo RO. J Application of thermal analysis in study of binary mixtures with metformin. Therm Anal Calorim. 2008;93(2):361–4.
Michalik K, Drzazga Z, Michnik A. Calorimetric characterization of 2′,3′-dideoxyinosine water solution. J Therm Anal Calorim. 2008;93(2):521–6.
Pourmortazavi SM, Hajimirsadeghi SS, Hosseini SG. Characterization of the aluminium/potassium chlorate mixtures by simultaneous TG-DTA. J Therm Anal Calorim. 2006;84:557–61.
Lever SD, Papadaki M. Study of condition-dependent decomposition reactions; part I. The thermal behavior and decomposition of 2-nitrobenzoyl chloride. J Hazard Mater. 2004;115(1–3):91–100.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Tech. 2008;180:2093–102.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;141:322–6.
Fahmy MA, Zayed MA, Keshk YH. Comparative study on the fragmentation of some simple phenolic compounds using mass spectrometry and thermal analyses. Thermochim Acta. 2001;366:183–8.
Fahmey MA, Zayed MA. Phenolic-iodine redox products. Mass spectrometry, thermal analysis and other physicochemical methods of analyses. J Therm Anal Calorim. 2002;67:163–75.
Fahmey MA, Zayed MA, El-Shobaky HG. Study of some phenolic-iodine redox polymeric products by thermal analyses and mass spectrometry. J Therm Anal Calor. 2005;82:137–42.
Somogyi Á, Harrison AG, Paizs B. Using gas-phase guest-host chemistry to probe the structures of b ions of peptides. J Am Soc Mass Spectrom. 2012;23:2055–8.
Zayed MA, Hawash MF, Fahmey MA, El-Gizouli AMA. Investigation of ibuprofen drug using mass spectrometry, thermal analyses and semi-empirical molecular orbital calculation. J Therm Anal Calorim. 2012;108:315–22.
Zayed MA, Mohamed GG, Fahmey MA. Thermal and mass spectral characterization of novel azo dyes of p-acetoamidophenol in comparison with Hammett substituent effects and molecular orbital calculations. J Therm Anal Calorim. 2012;107:763–76.
Frag EYZ, Zayed MA, Omar MM, Elashery SEA, Mohamed GG. Spectrophotometric determination of carbamazepine and mosapride citrate in pure and pharmaceutical preparations. Arabian J Chem. 2012;5:375–82.
Stewart JJP. Optimization of parameters for semiempirical methods I. J Comput Chem. 2004;10(2):209–20.
Baker J. An algorithm for the location of transition states. J Comput Chem. 2004;7(4):385–95.
Stewart JJP. Software package MOPAC 2000. Tokyo: Fujitsu Limited; 1999.
Stanley SM. Equine metabolism of buspirone studied by high-performance liquid chromatography/mass spectrometry. J Mass Spectrom. 2000;35:402–7.
Zayed EM, Zayed MA, Hindy AMM. Thermal and spectroscopic investigation of novel Schiff base, its metal complexes, and their biological activities. J Therm Anal Calorim. 2014;116:391–400.
Loew G, Chadwick M, Smith D. Applications of molecular orbital theory in organic molecules. Org Mass Spectrom. 1973;7:1241.
Cooks RG, Beynon JH, Caprioli RM, Laster GR. Metastable Ions. Amsterdam: Elsevier; 1973.
Ey O, Base SK, Kwon JW, You M, Lee DC III, Lee MG. Pharmacokinetic and pharmacodynamics consequences of inhibition of terazosin metabolism via CYP3A1 and/or 3A2 by DA-8159, an erectogenic, in rats. Br J Pharmacol. 2007;151:24–34.
Cheah PY, Yuen KH, Liong ML. Improved high-performance liquid chromatographic analysis of terazosin in human plasma. J Chromatogr B Biomed Sci Appl. 2000;745(2):439–43.
Vincent J, Dachman W, Blaschke TF, Hoffman BB. Pharmacological tolerance to alpha 1-adrenergic receptor antagonism mediated by terazosin in humans. J Clin Investig. 1992;90(5):1763–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zayed, M.A., Fahmey, M.A., El-Desawy, M. et al. Structure characterization of terazosin drug using mass spectrometry and thermal analyses techniques in comparison with semi-empirical molecular orbital (MO) calculations. J Therm Anal Calorim 120, 1061–1069 (2015). https://doi.org/10.1007/s10973-015-4462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4462-y