Abstract
Density (ρ), viscosity (η), and speed of sound (U) values for the binary mixture systems of methyl benzoate + 2-propanol and ethyl benzoate + 2-propanol including those of pure liquids were measured over the entire mole fraction range at five different temperatures (303.15, 308.15, 313.15, 318.15, and 323.15) K. From these experimentally determined values, various thermo-acoustic parameters such as excess isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), excess molar volume (V E) and excess free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) have been calculated. The excess functions have been fitted to the Redlich–Kister type polynomial equation. The deviations for excess thermo-acoustic parameters have been explained on the basis of the intermolecular interactions present in these binary mixtures. The theoretical values of speed of sound in the mixtures have been evaluated using various theories and have been compared with experimentally determined speed of sound values in order to check the applicability of such theories to the liquid mixture systems under study. Viscosity data have been used to test the applicability of standard viscosity models of Grunberg–Nissan, Hind–Mc Laughlin, Katti–Chaudhary, Heric and Brewer, Frenkel, Tamura and Kurata at various temperatures for the binary liquid systems under study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volumetric, viscometric, and speed of sound investigations of liquids and liquid mixtures are of considerable importance and they play a relevant role in understanding the intermolecular interactions occurring among the component molecules besides finding extensive applications in several industrial and technological processes [1, 2]. Extensive studies have been done to understand these kinds of interactions on different types of binary and ternary mixtures. Several researchers [3–8] have measured the density, viscosity, and speed of sound for a wide range of binary mixtures containing alcohols as one of the components, and these properties were interpreted in terms of specific or nonspecific interactions. The objective of the present study is to understand the influence of non associative molecule on the associative molecule. Alcohols are strongly associated in solution because of dipole–dipole interaction and hydrogen bonding. They are of great importance for their relevant role in chemistry, biology, and studies on hydrogen bonding in liquid mixtures. Alcohols are widely used as solvents. The molecules with –OH group form associative liquids due to hydrogen bonding. The effect shown by the molecules with other functional groups on these molecules plays a relevant role in understanding the behavior of hydrogen bonding. On the other hand alkyl benzoates are non-associated in solution, good hydrogen bonding acceptors. They are widely used in perfumery and pesticides. Also, hydrocarbons are among the most important chemicals used in hydrocarbon processing industries. The investigations regarding the molecular association in liquid mixtures having aromatic group as one of the components is of particular interest, since aromatic group is highly non-polar and can associate with any other group having some degree of polar attractions. Even though considerable work has been reported on alcohols as one of the component in binary and ternary mixtures, the data on binary mixtures of alcohols with alkylbenzoates with temperature variation are scanty. In order to get a better understanding of the nature of molecular interactions based on the temperature dependent ultrasonic studies, the authors have made an attempt to study the thermodynamic and acoustic properties of binary mixtures involving alcohol and alkyl benzoates. So, in our present study we have selected methyl benzoate and ethyl benzoate as non-associative liquids and 2-propanol as associative liquid. In continuation of our earlier reported work on molecular interactions of liquid mixtures [9–12] using excess thermodynamic and acoustic properties, the present study gives further investigations on molecular interactions in the commercially important liquid mixtures of methyl benzoate/ethyl benzoate with 2-propanol over the entire composition range. Here, we report the results of speed of sound, density, and viscosity for the binary liquid mixtures of (methyl benzoate + 2-propanol), (ethyl benzoate + 2-propanol) at temperatures of (303.15, 308.15, 313.15, 318.15, and 323.15) K.
Thermodynamic and transport properties [13, 14] of liquid mixtures provide important information with which to speculate the molecular liquid structure. These properties have been widely used to study the intermolecular interactions between various species present in the liquid mixtures. The study of thermodynamic properties of multicomponent liquid mixtures and data on the analysis in terms of various models are important for industrial and pharmaceutical applications [15]. The excess thermodynamic functions [16] are sensitively dependent not only on the differences in intermolecular forces, but also on the differences in the size of the molecules. Excess functions are thermodynamic properties in excess compared to those of an ideal solution at the same temperature, pressure, and composition. The variation of these properties with temperatures and composition for mixtures containing polar molecules and hydrogen bonded components may be complex due to a decrease or an increase in hydrogen bonding interaction due to mixing, depending upon the nature of the liquids whether they are polar or non-polar, the signs and magnitudes of these excess values can throw light on the strength of interactions. So, from the experimentally determined values of speed of sound density and viscosity, various thermo-acoustic parameters like excess isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), excess molar volume (V E), excess free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) have been calculated. The results of excess values were fitted to the Redlich and Kister [17] polynomial equation. The intermolecular interactions have been estimated in the light of these excess parameters. In the present study, theoretical speed of sound and viscosity values has been evaluated using several empirical relations in the liquid mixtures considering methyl benzoate/ethyl benzoate as one component and 2-propanol as the other component at T = (303.15, 308.15, 313.15, 318.15, and 323.15) K. This kind of evaluation of theoretical speed of sound values proves to be useful to verify the applicability of various postulates of these theories of liquid mixtures and to arrive at some useful inferences regarding the strength of molecular interactions between component liquids in some cases.
Experimental
Materials
The chemicals used in the present study are methyl benzoate, ethyl benzoate, and 2-propanol which are of AR grade obtained from Merck Co. Inc., Germany, with purities of greater than 99 %. All the chemicals were further purified by standard methods [18] and only middle fractions were collected. The density and speed of sound were experimentally determined at a temperature of 303.15 K and compared with the literature [19–25]. Comparison of experimental densities (ρ), viscosity (η), and speed of sound (U) of pure liquids with the literature values are given in Table 1.
Methods
All binary mixtures were prepared gravimetrically in air-tight bottles, and adequate precautions have been taken to minimize evaporation losses. Before use, the chemicals were stored over 0.4 nm molecular sieves approximately for 72 h to remove water content and then degassed. The mass measurements were performed on a digital electronic balance (Mettler Toledo AB 135, Switzerland) with an uncertainty of ±10−8 kg. The binary mixtures were prepared just before use. The uncertainty in mole fraction was estimated to be less than ±0.0001.
The viscosities were measured with Ostwald viscometer. The viscometer was calibrated at each temperature using redistilled water. The uncertainty in viscosity measurement is up to 0.001 mPa s. The flow time has been measured after the attainment of bath temperature by each mixture. The flow measurements were made with an electronic stop watch with a precision of 0.01 s. For all the pure components and mixtures, 3–4 readings were taken, and the average of these values was used in all the calculations.
The densities of the pure compounds and their mixtures were determined accurately using 10 mL specific gravity bottles in digital electronic balance (Mettler Toledo AB 135, Switzerland) with an uncertainty of ±10−8 kg. The average uncertainty in the measured density was ±0.001 kg m−3.
The speed of sound was measured with a single-crystal variable path interferometer (Mittal Enterprises, New Delhi, India) operating at a frequency of 2 MHz that had been calibrated with water and benzene. The uncertainty in the speed of sound was found to be ±0.1 m s−1. In all property measurements, the temperature was controlled within ±0.1 K using a constant temperature bath (M/s Sakti Scientific Instruments Company, India) by circulating water from the thermostat.
Theory
The values of experimentally determined density and speed of sound for the binary mixtures of methyl benzoate and ethyl benzoate with the 2-propanol at 303.15, 308.15, 313.15, 318.15, and 323.15 K over the entire composition range are given in Table 2. It can be observed from Table 2 that the values of speed of sound, density, and viscosity show increase in trend at all the temperatures in the binary systems under study.
Using the experimentally determined values of speed of sound, density, and viscosity, various thermodynamic parameters like excess isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), excess molar volume (V E), excess free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) were calculated.
Kiyohara and Benson [26, 27] stated that the thermodynamic properties of an ideal mixture must be mutually related in the same way as for those of pure substances and real mixtures. Also Douheret et al. [28] suggested that the interpretation of the nature of molecular interactions in mixtures requires a correct calculation of a thermodynamic property of the ideal liquid mixtures by the application of correct ideal mixing rules. In the present work, the authors have calculated the excess values of isentropic compressibility and excess free length values to check the applicability of thermo dynamical ideality (the ideal mixing rules) to the components under study.
The excess values of isentropic compressibility \( K_{\text{s}}^{\text{E}} \) were calculated as follows,
where K s represents the calculated value of isentropic compressibility for the mixture
\( K_{\text{s}}^{\text{E}} \) is its excess value, \( K_{\text{s}}^{\text{id}} \) is the ideal isentropic compressibility value, ρ is the density, and U represents the speed of sound. \( K_{\text{s}}^{\text{id}} \) for an ideal mixture was calculated from the relation recommended by Benson and Kiyohara [26, 27] and Douheret et al. [28].
in which \( K_{\text{s,i}}^{\text{o}} \), \( V_{\text{o}}^{\text{i}} \), \( \alpha_{\text{i}}^{\text{o}} \), \( C_{\text{p,i}}^{\text{o}} \) are the isentropic compressibility, molar volume, isobaric thermal expansion coefficient, and molar isobaric heat capacity of pure component i, T represents temperature, ϕ i is the volume fraction, and x i represents the mole fraction of i in the mixture.
The density values have been used to calculate the excess volumes, V E, using the following equation,
where ρ is the density of the mixture and x 1, M 1, and ρ 1 and x 2, M 2, and ρ 2 are the mole fraction, molar mass, and density of pure components 1 and 2, respectively.
The excess values of free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), Gibbs free energy (ΔG *E), and enthalpy (H E) were calculated by using the expressions given in the literature [21, 29] as follows,
where L f represents the calculated value for the mixture and K T represents a temperature dependent constant whose value is K T = (91.368 + 0.3565 T) × 10−8.
Excess Gibbs free energy of activation ΔG *E was calculated as follows,
where R represents gas constant, T is the absolute temperature, η is the viscosity of the mixture and η 1, η 2 are the viscosities of the pure compounds, V is the molar volume of mixture and V 1, V 2 are the molar volumes of the pure compounds,
Excess enthalpy H E was calculated from usual relation.
where H represents the calculated value of enthalpy for the mixture and H 1, H 2 represent enthalpy of pure components 1 and 2, respectively
The excess values for the above parameters were fitted by the method of non-linear least-squares to a Redlich–Kister type polynomial [17].
where \( Y^{E} = K_{\text{s}}^{\text{E}} ,{\mkern 1mu} V^{\text{E}} ,{\mkern 1mu} L_{\text{f}}^{\text{E}} ,{\mkern 1mu} \varDelta G^{{*{\text{E}}}} ,{\mkern 1mu} H^{\text{E}} \). The values of coefficient A i were determined by a regression analysis based on the least-squares method and are reported along with the corresponding standard deviations between the experimental and the calculated values of the respective functions in Table 4.
The standard deviation (σ) was calculated using the relation
where n represents the number of experimental points and m is the number of adjustable parameters.
Results and discussion
The experimental values of speed of sound, density, and viscosity in case of the binary liquid mixtures under study over the entire range of composition and at different temperatures, T = (303.15, 308.15, 313.15, 318.15, and 323.15) K are given in Table 2. From this available data of speed of sound, density, and viscosity, the values of excess isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), excess molar volume (V E), excess free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) were calculated. These excess parameters were plotted against mole fractions of methyl benzoate and ethyl benzoate separately over the entire mole fraction range and at different temperatures. The plots are shown in Figs. 1, 2, 3, 4 and 5. The excess parameters of isentropic compressibility \( \left( {K_{\text{s}}^{\text{E}} } \right) \), molar volume (V E), free length \( \left( {L_{\text{f}}^{\text{E}} } \right) \), excess Gibb’s free energy (ΔG *E), and excess enthalpy (H E) are fitted to the Redlich–Kister type polynomial equation and the coefficients A i evaluated by the method of least-squares, along with standard deviation (σ) are given in Table 3.
The deviations observed in the excess parameters indicate the strength of interactions present between the component molecules of the binary mixtures under study [30]. The variations in these excess parameter values reflect the interactions between the mixing species, depending upon the composition, molecular sizes, and shapes of the components and temperature. The effects which influence the values of excess thermodynamic functions may be the result of physical, chemical, and structural contributions such as:
-
(1)
The chemical effects, like the breaking of molecular association present in the pure liquid which results in the positive values of V E, \( K_{\text{s}}^{\text{E}} \), \( L_{\text{f}}^{\text{E}} \) and negative ΔG *E, on the other hand charge transfer forces, formation of hydrogen bonds, and other complex forming interactions result in the negative values of V E, \( K_{\text{s}}^{\text{E}} \), \( L_{\text{f}}^{\text{E}} \) and positive ΔG *E [29].
-
(2)
Physical contributions are from dispersion forces or weak dipole–dipole interactions causing the positive values of V E, \( K_{\text{s}}^{\text{E}} \), \( L_{\text{f}}^{\text{E}} \) and negativeΔG *E.
-
(3)
The structural contribution arising from the geometrical fitting of one component into the other because of the differences in the size and shape of the component molecules resulting in the negative values of V E, \( K_{\text{s}}^{\text{E}} \), \( L_{\text{f}}^{\text{E}} \) and positive ΔG *E.
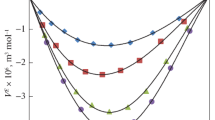
Figures 1a, b show the excess isentropic compressibility \( (K_{\text{s}}^{\text{E}} ) \) for the binary liquid mixtures of methyl benzoate and ethyl benzoate with 2-propanol, respectively, over the entire mole fraction range and at different temperatures T = (303.15, 308.15, 313.15, 318.15, 323.15) K. It is clear from Fig. 1a, b that the \( K_{\text{s}}^{\text{E}} \) values are negative over the entire mole fraction range for the systems under study and at investigated temperatures, this indicates the presence of strong interactions in these mixtures. As the temperature increases, it has been observed that the negative \( K_{\text{s}}^{\text{E}} \) values are found to increase in both the systems, and the changes in \( K_{\text{s}}^{\text{E}} \) values with respect to temperature are small in these mixtures. Also with the increase in temperature, the solute–solvent interactions get weaker causing the excess values to decrease at higher temperature. The negative values of \( K_{\text{s}}^{\text{E}} \) are of the order (ethyl benzoate + 2-propanol) > (methyl benzoate + 2-propanol). The sign of excess isentropic compressibility plays a relevant role in assessing the compactness due to molecular interaction in liquid mixtures through charge transfer, dipole–dipole interactions, and dipole-induced dipole interactions interstitial accommodation, and orientational ordering leading to more compact structure making, which enhances excess isentropic compressibility to have negative values. Fort and Moore [31] suggested that the liquids having different molecular sizes and shapes mix well there by reducing the volume which causes the values of \( K_{\text{s}}^{\text{E}} \) to be negative. It also suggests that the liquids are less compressible when compared to their ideal mixtures signifying the chemical effects including charge transfer forces, formation of hydrogen bond, and other complex forming interactions. It can also be said that the molecular interactions are strong in these binary liquid mixtures and that the medium is highly packed. Similar results were obtained by earlier workers [32, 33].
The variation of excess molar volume (V E), with respect to mole fraction, x 1, is given in Fig. 2a, b over the entire composition range and at different T = (303.15, 308.15, 313.15, 318.15, and 323.15) K. The strength of the intermolecular interactions in binary liquid mixtures can be explained using the sign and magnitude of the V E values. The factors that are mainly responsible for the contraction of volume causing the V E values to be negative are due to strong specific interactions like the association of component molecules through hydrogen bonds, due to dipole–dipole interactions, or it may be due to the induced dipole–dipole interactions, whereas the expansion of volumes leading to positive V E values is due to breaking of one or both of the components in a solution, the geometry of molecular structure which does not allow the fitting of one component molecules into the voids created by the molecules of other component, and the steric hinderance of the molecules. In our present study, the V E values are mostly negative in both the cases. So this kind of behavior of V E can attributed to the formation of hydrogen bond, disruption of alcohol self-associations, and the structural characteristics like geometrical fitting of one component into the other as a result of the increase in difference of size and shape of the component molecules. As the temperature increases, it has been observed that, the negative values of V E are found to decrease indicating the decrease of interactions between the unlike molecules. It is clear from Fig. 2a, b that the negative values of V E are in the following order, (ethyl benzoate + 2-propanol) > (methyl benzoate + 2-propanol). The expansion in molar volume can be attributed to the presence of weak intermolecular forces of attraction [34]. Similar results were reported by García et al. [35]. The negative values of V E indicate that there is more compact packing of the molecules which implies that the molecular interactions are strong, whereas the positive values indicate a loose packing of molecules in the binary mixture compared to those in the pure component. Similar results were observed by earlier workers [35, 36].
It can be observed from Fig. 3a, b that the \( L_{\text{f}}^{\text{E}} \) values have a negative trend similar to what we have observed in case of the \( K_{\text{s}}^{\text{E}} \) at all the temperatures under study. The negative values of \( L_{\text{f}}^{\text{E}} \) suggest that specific interactions are present between unlike molecules in these binary systems [37] and that the molecules may form multimers through hydrogen bonding between the hydroxyl group (–OH) of 2-propanol and –CH group of benzoates.
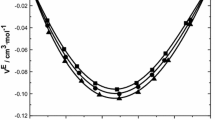
Figure 4a, b represent the excess Gibb’s free energy of activation (ΔG *E) with respect to mole fraction x 1, over the entire composition range and at T = (303.15, 308.15, 313.15, 318.51, and 323.15) K. It can be seen from Fig. 4a, b that the ΔG *E values are positive at all temperatures and over the entire range of mole fraction. These positive values indicate the existence of strong intermolecular interaction through hydrogen bonding between the component molecules of the liquid mixtures under study. The maximum deviation is observed for (ethyl benzoate + 2-propanol) system indicating the strength of bond formation in this system is more compared to that of (methyl benzoate + 2-propanol) system. Similar results were observed by earlier workers [38].
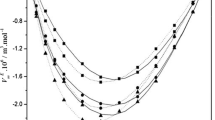
From Fig. 5a, b it is clear that the excess values of enthalpy (H E) are negative with respect to the mole fraction, x 1, over the entire composition range and at T = (303.15, 308.15, 313.15, 318.15, and 323.15) K. The negative values of H E tend to decrease with increase in temperature, this insist the fact that there are strong specific interactions between unlike molecules in these liquid mixtures [39]. The negative H E values also suggest the existence of inter molecular hydrogen bond and the breaking of associated structures in both cases.
The variations in these above excess parameters with mole fraction and temperature predict the presence of hydrogen bonding between the compounds in both the binary mixtures. The strength of bond formation between the compounds in ethyl benzoate + 2-propanol system is more than that in methyl benzoate + 2-propanol system, this is because of the increased chain length and larger effective radius of the rotating unit of pure ethyl benzoate when compared to that of pure methyl benzoate. Also, the excess parameters calculated in the present study correlate with one another and at the same time each parameter supports the formation of hydrogen bonding in these binary liquid mixtures.
In the present study, theoretical speed of sound values has been evaluated in the binary mixtures considering methyl/ethyl benzoate as one component and 2-propanol as the other component at all investigated temperatures. This kind of evaluation of theoretical speed of sound values proves to be useful to verify the applicability of various postulates of these theories of liquid mixtures and to arrive at some useful inferences regarding the strength of molecular interactions between component liquids in some cases. The theories due to Nomoto (U NOM) [40], Impedance relation (U IMP) [41], Van Dael and Vangeel (U VDV) [42], Junjie’s (U JM) [43, 44], free length theory (U FLT) [45], and Rao’s (U R) [46] are employed and the Average percentage error along with the χ 2 fit values described elsewhere [4] for the binary mixture and at all investigated temperatures are compiled in Table 4. The average percentage error values are small. On comparison, the Nomoto’s relation and free length theory relation are found to give some valuable estimate of the experimental values of speed of sound values in these binary mixtures at all the temperatures.
The dynamic viscosities of the liquid mixtures have been correlated with composition using several empirical relations due to Grunberg–Nissan [47], Hind et al. [48], Katti and Chaudari [49], Heric and Brewer [50], Frenkel [51], and Tamura and Kurata [52]. The experimental and theoretical values of viscosity with their corresponding interaction terms and standard deviation values for the liquid mixtures at T = (303.15, 308.15, 313.15, 318.15, and 323.15) K are compiled in Table 5. All the empirical models gave a reasonable fit, but there is good agreement between the theoretical and experimental values in case of (ethyl benzoate + 1-propanol) + benzene mixture. The estimated standard deviations are smaller in all cases indicating that the present mixture viscosities are well-correlated by these viscosity models.
Further, the hydrogen bond between the component systems has also been confirmed from the FT-IR [53]. The shifts observed are caused by the strong interaction between the high electro-negative charge of oxygen in 2-propanol and hydrogen of the benzoates.
Conclusions
The excess parameters like \( K_{\text{s}}^{\text{E}} \), V E, \( L_{\text{f}}^{\text{E}} \), ΔG *E, and H E were calculated from the experimentally determined speed of sound, density, and viscosity values. The formation of hydrogen bond between the mixtures is identified by studying the variations in these parameters. The values of excess isentropic compressibility, excess free length, and excess enthalpy are found to be negative, excess Gibb’s free energy of activation is positive over the entire range of composition at all temperatures for the liquid mixture systems considered in the present study. The excess molar volume values changed from negative to positive for the binary systems over the entire range of composition and at all the temperatures considered in the present study. This is a clear indication for the presence of hydrogen bonding between the component molecules. The difference in molar masses of the liquid molecules is also responsible for the existing specific interactions between the molecules of the component liquids. Besides, the computed speed of sound values from different theories has been correlated with the experimentally measured values. Speed of sound values obtained from Nomoto’s and free length theory relations is in good agreement with the experimental values. The experimental viscosity values are compared with the viscosity values obtained from different empirical relations and these are in good agreement with the experimental values.
References
Oswal SL, Oswal P, Shalak RP. Speed of sound, isentropic compressibilities and excess molar volumes of binary mixtures containing p-dioxane. J Solut Chem. 1998;27:507–20.
Kumar H, Kaur M, Gaba R, Kaur K. Thermodynamics of binary liquid mixtures of cyclopentane with 2-propanol, 1-butanol and 2-butanol at different temperatures. J Therm Anal Calorim. 2011;105:1071–80.
Zorebski E, Waligora A. Densities, excess molar volumes, and isobaric thermal expansibilities for 1,2-ethanediol + 1-butanol, or 1-hexanol, or 1-octanol in the temperature range from (293.15 to 313.15) K. J Chem Eng Data. 2008;53:591–5.
Boruń A, Żurada M, Bald A. Densities and excess molar volumes for mixtures of methanol with other alcohols at temperatures (288.15–313.15 K). J Therm Anal Calorim. 2010;100:707–15.
Dash SK, Pradhan SK, Dalai B, Moharana L, Swain BB. Studies on molecular interaction in binary mixtures of diethyl ether with some alkanols—an acoustic approach. Phys Chem Liq. 2012;50:735–49.
Checoni RF. Excess molar enthalpy for methanol, ethanol, 1-propanol, 1-butanol + n-butylamine mixtures at 288.15 and 308.15 K at atmospheric pressure. J Therm Anal Calorim. 2010;101:349–57.
Sreenivasulu K, Govinda V, Venkateswarlu P, Sivakumar K. Thermodynamic properties of non-electrolyte solutions. J Therm Anal Calorim. 2013; doi: 10.1007/s10973-013-3395-6.
Savaroglu G, Aral E. Speeds of sound and isentropic compressibilities in binary mixtures of 2-propanol with several 1-alkanols at 298.15 K. Int J Thermophys. 2005;26(5):1525–35.
Sastry SS, Babu S, Vishwam T, Parvateesam K, Tiong HS. Excess parameters for binary mixtures of ethyl benzoate with 1-propanol, 1-butanol and 1-pentanol at T = 303, 308, 313, 318, and 323 K. Phys B. 2013;420:40–8.
Babu S, Sastry SVK, Tiong HS, Sastry SS. Experimental and theoretical studies of ultrasonic velocity in binary liquid mixtures of ethyl benzoate. J Chem. 2012;9(4):2309–14.
Sastry SVK, Babu S, Tiong HS, Sastry SS. Molecular interaction studies in ternary mixture of ethyl hydroxy benzoate by ultrasonic velocity measurements. Res J Pharm Biol Chem Sci. 2012;3(2):500–5.
Sastry SVK, Babu S, Tiong HS, Sastry SS. Ultrasonic investigation of molecular interactions in ternary mixtures at 303 K. J Chem Pharm Res. 2012;4(4):2122–5.
Glinski J, Chavepeyer G, Platten JK. Surface properties of diluted solutions of n-heptane, n-octanol and n-octanoic acid in nitromethane. Chem Phys. 2001;272:119–26.
Salgado DG, Tovar CA, Cerdeirina CA, Carballo E, Romani L. Second-order excess derivatives for the 1,3-dichloropropane + n-dodecane system. Fluid Phase Equilib. 2002;199:121–34.
Resa JM, Gonzalez C, Goenaga JM, Iglesias M. Influence of temperature on ultrasonic velocity measurements of ethanol + water + 1-propanol mixtures. J Therm Anal Calorim. 2007;87:237–45.
Sharma S, Jasmin B, Ramani J, Patel R. Density, excess molar volumes and refractive indices of β-pinene with o, m, p-xylene and toluene at 303.15, 308.15 and 313.15 K. Phys Chem Liq. 2011;49:765–76.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Vogel AI. Text book of organic chemistry. 5th ed. New York: Wiley; 1989.
Vasudha K, Kumari DV, Yuvaraja G, Krishnaiah A. Excess volumes and viscosities for the binary systems of 2-propanol with alkyl acetates at 303.15 K. J Chem Pharm Res. 2011;3(5):108–15.
Mutalik V, Manjeshwar LS, Sairam M, Aminabhavi TM. Excess molar volumes, deviations in viscosity and refractive index of the binary mixtures of mesitylene with ethanol, propan-1-ol, propan-2-ol, butan-1-ol, pentan-1-ol, and 3-methylbutan-1-ol at 298.15, 303.15, and 308.15 K. J Mol Liq. 2006;129:147–54.
Sreekanth K, Kondaiah M, Kumar DS, Rao DK. Influence of temperature on thermodynamic properties of acid–base liquid mixtures. J Therm Anal Calorim. 2012;110:1341–52.
Mohan TM, Sastry SS, Murthy VRK. Thermodynamic, dielectric and conformational studies on hydrogen bonded binary mixtures of propan-1-ol with methyl benzoate and ethyl benzoate. J Solut Chem. 2011;40:131–46.
Riddick JA, Bunger WB, Sakano TK. Techniques of chemistry. Organic solvents. 4th ed. New York: Wiley; 1986.
Aminabhavi TM, Phayde TSM, Khinnavar SR, Gopalakrishna B, Keith CH. Densities, refractive indices, speeds of sound, and shear viscosities of diethylene glycol dimethyl ether with ethyl acetate, methyl benzoate, ethyl benzoate, and diethyl succinate in the temperature range from 298.15 to 318.15 K. J Chem Eng Data. 1994;39:251–60.
Chueh CF, Swanson AC. Estimated group contribution method. In: Reid RC, Prausnitz JM, Poling BE. The properties of gases and liquids. 4th ed. New York: McGraw Hill; 1987. pp 138
Kiyohara O, Benson GC. Ultrasonic speeds and isentropic compressibilities of n-alkanol + n-heptane mixtures at 298.15 K. J Chem Thermodyn. 1979;11:861–73.
Benson GC, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem Thermodyn. 1979;11:1061–4.
Douheret G, Pal A, Davis MI. Ultrasonic speeds and isentropic functions of (a 2-alkoxyethanol + water) at 298.15 K. J Chem Thermodyn. 1990;22:99–108.
Narendra K, Srinivasu Ch, Kalpana Ch, Narayanamurthy P. Excess thermo dynamical parameters of binary mixtures of toluene and mesitylene with anisaldehyde using ultrasonic technique at different temperatures. J Therm Anal Calorim. 2012;107:25–30.
Pandey JD, Rai RD, Shukla RK, Shukla AK, Mishra N. Ultrasonic and thermodynamic properties of quaternary liquid system at 298.15 K. Indian J Pure Appl Phys. 1993;31:84–90.
Fort RJ, Moore WR. Adiabatic compressibilities in binary liquid mixtures. Trans Faraday Soc. 1965;61:2102–10.
Gupta M, Vibhu I, Shukla JP. Ultrasonic velocity, viscosity and excess properties of binary mixture of tetrahydrofuran with 1-propanol and 2-propanol. Fluid Phase Equilib. 2006;244:26–32.
Iloukhani H, Zoorasna N, Sloeimani R. Excess molar volumes and speeds of sound of tetrahydrofuran with chloroethanes or chloroethenes at 298.15 K. Phys Chem Liq. 2005;43:391–401.
Bhatia SC, Rani R, Bhatia R, Anand H. Volumetric and ultrasonic behaviour of binary mixtures of 1-nonanol with o-cresol, m-cresol, p-cresol and anisole at T = (293.15 and 313.15) K. J Chem Thermodyn. 2011;43:479–86.
García B, Aparicio S, Navarro AM, Alcalde R, Leal JM. Measurements and modeling of thermophysical behavior of (C1–C4) alkylbenzoate/(C1–C11) alkan-1-ol mixed solvents. J Phys Chem B. 2004;108:15841–50.
Narendra K, Srinivasu Ch, Fakruddin Sk, Narayanamurthy P. Excess parameters of binary mixtures of anisaldehyde with o-cresol, m-cresol and p-cresol at T = (303.15, 308.15, 313.15, and 318.15) K. J Chem Thermodyn. 2011;43:1604–11.
Oswal SL, Pandiyan V, Kumar BK, Vasantharani P. Thermodynamic and acoustic properties of binary mixtures of oxolane with aniline and substituted anilines at 303.15, 313.15 and 323.15 K. Thermochim Acta. 2010;507:27–34.
Subha MCS, Swamy GN, Bal ME, Rao KSKV. Excess volume and viscosity of ethoxy ethanol with n-butylamine, sec-butylamine, tert-butylamine, n-hexylamine, n-octylamine and cyclohexylamine. Indian J Chem A. 2004;43:1876–81.
Narendra K, Srinivasu Ch, Narayanamurthy P. Excess properties of binary mixtures of o-xylene, m-xylene and p-xylene with anisaldehyde at different temperatures. J Appl Sci. 2012;12(2):136–44.
Nomoto O. Empirical formula for sound velocity in binary liquid mixtures. J Phys Soc Jpn. 1958;13:1528–32.
Baluja S, Parrania PH. Acoustical properties of 3-α-furyl acrylic acid in protic and aprotic solvents. Asian J Chem. 1995;7:417–23.
Van Dael W, Vangeel E. Theory of ultrasound. In: Van Deal W. Thermodynamic properties and velocity of sound, London: Butterworth; 1975, Chap. 5.
Junjie Z. Junjie’s theory of ultrasound. In: Savaroglu G, Aral E. Densities, speeds of sound and isentropic compressibilities of the ternary mixture of 2-propanol + acetone + cyclohexane and the constituent binary mixtures at 298.15 K and 303.15 K. Fluid Phase Equilib. 2004;215:253–62.
Junjie Z. J. China. Univ. Sci. Technol. 1984;14:298–300.
Jacobson B. Ultrasonic velocity in liquids and liquid mixtures. J Chem Phys. 1952;20:927–8.
Rao GVR, Sarma AVV, Krishna JS, Rambabu C. Theoretical evaluation of ultrasonic velocities in binary liquid mixtures of o-chlorophenol at different temperatures. Indian J Pure Appl Phys. 2005;43:345–54.
Grunberg L, Nissan AH. Mixture law for viscosity. Nature. 1949;164:799–800.
Hind RK, Mc Laughlin E, Ubbelohde AR. Structure and viscosity of liquids camphor + pyrene mixtures. Trans Faraday Soc. 1960;56:328–30.
Katti PK, Chaudhari MM. Viscosities of binary mixtures of benzyl acetate with dioxane, aniline and m-cresol. J Chem Eng Data. 1964;9:442–3.
Heric EL, Brewer JC. On the viscosity of ternary mixtures. J Chem Eng Data. 1966;11:66–8.
Frenkel YI. Theory of the viscosity of liquid mixtures. Petroleum. 1946;9:27.
Tamura M, Kurata M. On the viscosity of binary mixture of liquids. Bull Chem Soc Jpn. 1952;25:32–8.
Mohan TM, Sastry SS, Murthy VRK. Conformational and dielectric relaxation studies on hydrogen bonded binary mixture of isopropyl alcohol in methyl benzoate and ethyl benzoate. J Mol Struct. 2010;973:157–62.
Acknowledgements
The authors gratefully acknowledge the Project No.: ERIP/ER/0703688/M/01/1134, dated 31-03-2010 of DRDO and UGC DRS LEVEL III program No. F.530/1/DRS/2009 (SAP-I), dated 09-02-2009 New Delhi, to the department of Physics, ANU for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sastry, S.S., Babu, S., Vishwam, T. et al. Excess parameters for binary mixtures of alkyl benzoates with 2-propanol at different temperatures. J Therm Anal Calorim 116, 923–935 (2014). https://doi.org/10.1007/s10973-013-3570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3570-9