Abstract
Experimental data of excess molar enthalpy (H Em ) of binary liquid mixtures containing (methanol or ethanol or 1-propanol, or 1-butanol) + n-butylamine mixtures have been determined as a function of composition at temperatures 288.15 and 308.15 K, at atmospheric pressure, using a modified 1455 PARR mixture calorimeter. The H Em values are negative for both systems over the whole composition range. The applicability of the ERAS Model to correlate H Em of mixtures studied is tested, and the agreement between experimental and theoretical results is satisfactory. The model results are discussed in terms of the cross-association interactions with temperature variation as well as in terms of the variation of the carbon chain in the alcohols presents in the mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mixtures containing associating components like alcohols and amines exhibit high non-ideal behavior due to the formation of hydrogen bonds between these different species. Experimental studies on excess molar enthalpy (H Em ) or excess molar volume (V Em ) of mixtures containing 1-alkanol + amine have been performed contributing to understand of the molecular interactions that occur between these compounds. Excess molar properties are also used to test the predictive or correlative capability of solutions models involving such polar compounds. Heintz and Papaioannou [1] obtained the experimental excess molar enthalpy data of alcohols + propylamine and alcohols + n-butylamine mixtures at 298.15 K; these data were correlated to the ERAS Model. González et al. [2] studied the experimental behavior of H Em for mixtures containing 1-alkanols + amines, correlating the results DISQUAC model; the results were compared to UNIFAC and ERAS Model. Villa et al. [3, 4] showed the experimental V Em of 1-alkanols + dipropylamine and 1-alkanols + dibutylamine mixtures at 298.15 K and at atmospheric pressure and correlated the results using the ERAS Model. Funke et al. [5] and Reimann and Heintz [6] presented experimental and modeling of H Em and V Em data for 1-alkanol + amine mixtures at various temperatures. Excess molar volume of binary mixtures containing 1-alkanol + (n-di-propylamine or n-di-butylamine) and propylamine + 1-alkanol were reported by Oswal and Desai [7, 8]. Experimental H Em and V Em data for binary mixtures containing alcohols + triethylamine were presented by Kwaterski et al. [9]. Checoni and Francesconi [10] reported the excess molar enthalpy and excess molar heat capacity (Cp Em ) for mixtures containing (1-heptanol or 1-octanol) + (diethylamine + s-butylamine) at 298.15 K and atmospheric pressure; the authors correlated the experimental H Em data to ERAS Model.
In present work, excess molar enthalpy (H Em ) values of mixtures containing alcohols (methanol, ethanol, 1-propanol, and 1-butanol) + n-butylamine have been determined as a function of composition at 288.15 and 308.15 K at atmospheric pressure. The applicability of the ERAS Model [11] to correlate experimental H Em of these mixtures was tested.
Experimental
Methanol, ethanol, 1-propanol, and 1-butanol, both with purity greater than 99.0 mol.%, were supplied by EM Science-Merck (USA). n-Butylamine was dried over molecular sieves and supplied by Across Organics (purity > 99 mol.%). The alcohols were used without previous purification. In order to check the purity of the substances, density and refractive index measurements were performed at 293.15 K using a vibrating-tube densimeter (Anton Paar, DMA 4500, resolution 10−5 g cm−3) and an Abbé type refractometer (Atago, model 3T), respectively. The uncertainties in the refraction index and density measurements are about ±1 × 10−4 and ±1 × 10−4 g cm−3, respectively. Table 1 presents a comparison of the density and refraction index measurements with literature values.
Excess molar enthalpy measurements were performed using a Parr 1455 solution calorimeter with some improvements. A detailed description and performance of the apparatus as well as the experimental procedure are given elsewhere [14]. From pure compounds, the experimental H Em values were determined from the following equation
in which Cpm, Cv, n, ΔT, Cp Em , T r, and T represent the molar heat capacity of the mixture, heat capacity of the calorimeter, amount of mixture (mol), temperature difference due to mixing process, excess molar heat capacity of the mixture, temperature in which the experiment effectively occurs, and reference temperature (288.15–303.15 K), respectively. The quantity Cv is determined by reversal calorimetry, with the actuation of resistance wire (with uncertainty of ±1 × 10−2 W), which supplies a certain amount of heat to pure compounds alcohols (1) and n-butylamine (2), whose mass and heat capacity (Cp i ) are known; so, ΔT 1 and ΔT 2 values are determined; with these values is possible to solve the equations system below and to obtain the Q and Cv values at experiment temperature
Molar heat capacity of the mixture (Cpm) is determined by reversal calorimetry supplying an amount of heat to the mixture. The expression used to calculate Cpm is
in which n s is the molar quantity of the mixture and \( \Updelta T_{\text{Sol}} \) is the temperature variation due to heat supplied to the mixture. The molar heat capacities of ideal solution (\( {\text{Cp}}_{\text{m}}^{\text{Ideal}} \)) is obtained by
wherein x 1 and x 2 are the molar fraction of alcohols and n-butylamine, respectively, and Cp Em is calculated by
For the intermediary mixture compositions, it is not possible to determine H Em directly from pure reagents due to volumetric limitation of apparatus. Hence, a mixture is used, whose \( H_{{{\text{m}}_{0} }}^{\text{E}} \) is previously determined and the value of H Em can be calculated by
wherein n 0 is the amount of mixture in the previous mixing process. Both n 0 and \( H_{{{\text{m}}_{0} }}^{\text{E}} \) values are zero when pure reagent is used. The mixtures were prepared gravimetrically using a Mettler Toledo PB 3002 balance (resolution, ±10−2 g, uncertainty ±5 × 10−3 g). The estimated uncertainty in mole fraction is ±2 × 10−4, and in the excess molar enthalpy measurements is ±1.5 × 10−2 H Em for the central composition range and ±2.5 × 10−2 H Em for the water-rich or alkanediol-rich region.
Results and discussion
Testing systems
The performance of the calorimeter was tested by measuring H Em of the well-investigated systems ethanol + water [15–17] 298.15 K over the whole composition range and atmospheric pressure. A comparison between experimental data with the plentiful literature data taken from Costigan et al. [17] shows an average difference lower than 1.0% over the whole composition range.
Alcohol + n-butylamine systems
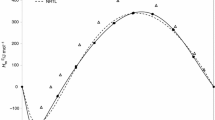
Excess molar enthalpy values are negative over the complete molar fraction range. The thermal effect decreases with increasing the temperature and decreases with the number of the carbon atoms in alcohol increasing. The excess molar enthalpy behavior has a parabolic form for all systems studied with maximum values around the mol fraction 0.42 for mixtures containing methanol and 0.48 for mixtures containing ethanol, 1-propanol, and 1-butanol. Tables 2 and 3 present the experimental H Em values for the alcohols + n-butylamine mixtures at 288.15 and 303.15 K and atmospheric pressure.
The experimental results of H Em were correlated by the Redlich–Kister type equation
The parameters A j were obtained using the method of least squares and are given in Table 4, along with the standard deviations determined from
wherein \( H_{{{\text{m}}_{ \exp } }}^{\text{E}} \)is the excess molar enthalpy obtained experimentally, \( H_{{{\text{m}}_{\text{calc}} }}^{\text{E}} \) is the excess molar enthalpy calculated using the Redlich–Kister equation, N is the number of data points, and k is the number of the adjustable parameters of Eq. 8.
Figures 1, 2, 3 and 4 represent the composition dependence of experimental data of H Em for 1-alkanols + amine mixtures at 288.15 and 308.15 K.
The values of H Em are large and negative over the whole composition range and have a parabolic form. It is observed that the exothermic effect decreases when both the temperature and 1-alkanols chain length increases. Maximum values for H Em are methanol + n-butylamine (−3927 J mol−1 at 288.15 K and −3795 J mol−1 at 308.15 K, both at x butylamine = 0.43), ethanol + n-butylamine (−2974 J mol−1 at 288.15 K and −2925 J mol−1 at 308.15 K, both at x butylamine = 0.46), 1-propanol + n-butylamine (−2917 J mol−1 at 288.15 K and −2890 J mol−1 at 308.15 K, both at x butylamine = 0.48), and methanol + n-butylamine (−2746 J mol−1 at 288.15 K and −2703 J mol−1 at 308.15 K, both at x butylamine = 0.46); these values were obtained using Eq. 8. The magnitude of H Em can be interpreted as the result of different effects which may be divided into physical and chemical contributions. Physical contributions involving non-specific interactions such dipole–dipole and dispersion forces between 1-alkanols and amines contribute positively to H Em . Destruction of the dipole–dipole interactions as well as destruction of intermolecular hydrogen bond in these pure compounds on mixing also gives a positive contribution to H Em . Besides this, there is a chemical contribution due to formation of the unlike intermolecular hydrogen bond between 1-alkanols and amines [5]. This specific interaction contributes negatively to H Em . Due to the large negative values of excess molar enthalpy data, probably the chemical contribution is dominant for the studied mixtures, since the assumption of strong hydrogen bonding effects between the unlike molecules is associated to this contribution.
Experimental H Em data were correlated by means of the ERAS Model [11] which combines the real association solution model [18] with a physical term from Flory’s equation of state [19]. The following equations hold for the studied systems
in which
and
A detailed description of calculation procedure as well as terms significance of the Eqs. 11–13 are given in [14]. The values of parameters of the pure components used in the calculations are given in Table 5.
The association constant of pure components (K i ) is calculated using the following expression [20]
wherein s i and h i are the molar entropy of association of pure component and the molar enthalpy of association of pure component (both obtained from [20]), R is the universal gas constant, T is the temperature in which the association constant is obtained. The values of h i and Δh * i in this study are equal due to the similarity in its significance. Cross-association equilibrium constant, K 12, Flory’s parameter, χ 12, molar enthalpy of cross-association, Δh *12 , and molar volume of cross-association, Δv *12 are the adjustable parameters of ERAS Model, whose values are reported in Table 6, along with the average absolute deviation values, calculated using the Eq 9 with the k = 4, and relative deviations calculated using the following expression
in which HERASE is excess molar enthalpy calculated using ERAS Model.
The cross-association parameters were adjusted to excess molar enthalpy experimental data through sequential search technique proposed by Rosenbrock [21] whose description of calculation routine is given in reference [22].
The results obtained from the ERAS Model as well as its physical and chemical contributions are also presented in Figs. 1, 2, 3, and 4. The physical contribution is small and positive in all mixtures; chemical contributions are dominant and take on negative values. Agreement between experimental and calculated data of H Em is good. The adjustable parameters calculated in present work show good agreement with those calculated in the Funke et al. [5] and Reimann-Heintz [6] for mixtures containing 1-alkanols + n-butylamine. According to Funke et al. [2], the ERAS Model is close to the physical reality, and its adjustable parameters have a certain physical meaning, especially in the mixtures containing 1-alkanols + amine; this information is observed in present work, since association constants of 1-alkanols and amines are higher than cross-association constants and strong negative values of H Em requires values for Δh *12 and Δv *12 more negative that those for molar enthalpy association and molar volume association of 1-alkanols and n-butylamine (Table 4). These results indicate that the NH-group is a weaker proton donator than the OH-group [2] as well as suggest that the free electron pair located at the N-atom has a higher polarizability, actuating as a more efficient proton OH···N group than the OH-group itself.
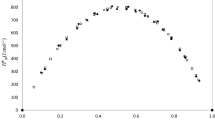
Heintz and Papaioannou [1] present the experimental data for mixtures containing (methanol, ethanol, 1-propanol, 1-butanol) + n-butylamine at 298.15 K and atmospheric pressure. These data were correlated to the ERAS Model by the researchers and K 12, Δv *12 , Δh *12 , and χ 12 are compared to those presented in Table 4. It is possible to verify that their value belongs to parameters interval calculated at 288.15 and 308.15 K. Furthermore, if the experimental data obtained by Duttachoudhury and Mathur [23] are correlated to the ERAS Model, the obtained adjustable parameters are lower than those at 308.15 K, even though the agreement between experimental and calculated H Em to be only qualitatively satisfactory at this temperature (see Table 4; Fig. 5).
Values of H Em as function of the mole fraction of n-butylamine for: a methanol + n-butylamine, b ethanol + n-butylamine, c 1-propanol + n-butylamine and d 1-butanol + n-butylamine at 313.15 K. (■) experimental data [23], (—) ERAS Model, (- - - -) physical contribution and (— —) chemical contribution
Conclusions
Experimental data of excess molar enthalpy for mixtures containing (methanol or ethanol or 1-propanol, or 1-butanol) + n-butylamine were determined at 288.15 and 308.15 K. The ERAS Model allows a good theoretical interpretation of these experimental H Em data. The chemical contribution to the H Em values is negative and dominant in relation to the physical contribution (positive and small) in all mixtures. The negatives values of H Em values for the systems studied may be attributed to structural effects arising from the geometrical fitting of n-butylamine molecules in the hydrogen bonded network of alcohols and to complex formation between alcohols and n-butylamine. Such negative contributions overweigh the positive contributions due to physical interactions (mainly dipole–dipole and dispersion interactions) and to the disruption of the associated alcohols molecules structure by amine.
References
Heintz A, Papaioannou D. Excess enthalpies of alcohol + amine mixtures. Experimental results and theoretical description using the ERAS-Model. Thermochim Acta. 1998;210:69–76.
González IG, de la Fuente JA, Cobos JC. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. Part 4. Application of the DISQUAC model to mixtures of 1-alkanols with primary or secondary linear amines. Comparison with Dortmund UNIFAC and ERAS results. Fluid Phase Equilib. 2000;168:31–58.
Villa S, Riesco N, de la Fuente IG, González JA, Cobos JC. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. Part 5. Excess molar volumes at 298.15 K for 1-alkanols + dipropylamine in terms of the ERAS model. Fluid Phase Equilib. 2001;190:113–25.
Villa S, Riesco N, de la Fuente IG, González JA, Cobos JC. Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. Part 6. Excess molar volumes at 298.15 K for 1-alkanols + dibutylamine systems. Characterization in terms of the ERAS model. Fluid Phase Equilib. 2002;198:313–29.
Funke H, Wetzel M, Heintz A. New applications of the ERAS model. Thermodynamics of amine + alkane and alcohol + amine mixtures. Pure Appl Chem. 1989;61(8):1429–39.
Reimann R, Heintz A. Thermodynamic excess properties of alkanol + amine mixtures and application of the ERAS Model. J Sol Chem. 1991;20:29–37.
Oswal SL, Desai HS. Studies of viscosity and excess molar volume of binary mixtures: 1. Propylamine + 1-alkanol mixtures at 303.15 and 313.15 K. Fluid Phase Equilib. 1998;149:359–76.
Oswal SL, Desai HS. Studies of viscosity and excess molar volume of binary mixtures: 3. 1-Alkanol + di-n-propylamine, and di-n-butylamine mixtures at 303.15 and 313.15 K. Fluid Phase Equilib. 2001;186:81–102.
Kwaterski M, Rezanova EN, Lichtenthaler RN. Excess molar volumes and excess molar enthalpies of binary and ternary mixtures of (ethanol or 1-butanol), triethylamine and n-hexane. Fluid Phase Equilib. 2005;25:170–85.
Checoni RF, Francesconi AZ. Measurements of excess molar enthalpy and excess molar heat capacity of (1-heptanol or 1-octanol) + (diethylamine or s-butylamine) mixtures at 298.15 K and 0.1 MPa. J Therm Anal Calorim. 2009;97(2):747–53.
Heintz A. A new theoretical approach for predicting excess properties of alkanol/alkane mixtures. Ber Bunsenges Phys Chem. 1985;89:172–81.
Torres RB, Francesconi AZ, Volpe PLO. Experimental study and modelling using the ERAS-Model of the excess molar volume of acetonitrile-alkanol mixtures at different temperatures and atmospheric pressure. Fluid Phase Equilib. 2003;210:287–306.
Lide DR (ed.). CRC handbook of chemistry and physics. 85th ed. Boca Raton: CRC Press; 2004/2005.
Checoni RF, Francesconi AZ. Measurement and correlation of excess molar enthalpy at various temperatures: acetonitrile + diethylamine or s-butylamine mixtures. J Therm Anal Calorim. 2005;80:295–301.
Lama RF, Lu BCY. Excess thermodynamic properties of aqueous alcohol solutions. J Chem Eng Data. 1965;10:216–9.
Boyne JA, Williamson AG. Enthalpies of mixing of ethanol and water at 25 °C. J Chem Eng Data. 1967;12:318–9.
Costigan MJ, Hodges LJ, Marsh KN, Stokes RH, Tuxford CW. The isothermal displacement calorimeter: design. Modifications for measuring exothermic enthalpies of mixing. Aust J Chem. 1980;33:2103–19.
Kretschmer CB, Wiebe R. Thermodynamics of alcohol-hydrocarbon mixtures. J Chem Phys. 1954;22:1697–701.
Orwoll RA, Flory RA, Vrij A. Statistical thermodynamics of chain molecule liquids. I. An equation of state for normal paraffin hydrocarbons. J Am Chem Soc. 1964;86:3507–14.
Nath A, Bender E. On the thermodynamics of associated solutions. I. Na analytical method for determining the enthalpy and entropy of association and equilibrium constant for pure liquid substances. Fluid Phase Equilib. 1981;7:275–87.
Rosenbrock HH. An automatic method for finding the greatest or least value of a function. Comput J. 1960;3:175–84.
Kuester JL, Mize JH. Optimization techniques. NY: McGraw-Hill; 1973.
Duttachoudhury HB, Mathur HB. Heats of mixing of n-butylamine/water and n-butylamine/alcohol systems. J Chem Eng Data. 1974;19:145–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Checoni, R.F. Excess molar enthalpy for methanol, ethanol, 1-propanol, 1-butanol + n-butylamine mixtures at 288.15 and 308.15 K at atmospheric pressure. J Therm Anal Calorim 101, 349–357 (2010). https://doi.org/10.1007/s10973-009-0624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0624-0