Abstract
In this article, we present a new method for the obtaining of ZnCr2O4 and MgCr2O4 embedded in silica matrix. This method consists in the formation of Cr(III), Zn(II) and Cr(III), Mg(II) hydroxycarboxylate/carboxylate compounds, during the redox reaction between the nitrate ion and diol (1,3-propanediol), uniformly dispersed in the pores of hybrid gels. The thermal decomposition of these precursors leads to a mixture of corresponding metal oxides. The gels were synthesized starting from mixtures of Cr(NO3)3·9H2O, Zn(NO3)2·6H2O and Cr(NO3)3·9H2O, Mg(NO3)2·6H2O with tetraethyl orthosilicate and 1,3-propanediol for final compositions 50% ZnCr2O4/50% SiO2 and 50% MgCr2O4/50% SiO2. The obtained gels have been thermally treated at 140 °C, when the redox reaction nitrates-diol took place with formation of the precursors within the xerogels pores. The thermal decomposition of all precursors took place up to 300 °C, with formation of oxides mixtures (Cr2O3 + x and ZnO) and (Cr2O3 + x and MgO), respectively. At 400 °C, Cr2O3 + x turn to Cr2O3 which reacts with ZnO forming ZnCr2O4/SiO2. Starting with 400 °C, Cr2O3 reacts with MgO to an intermediary phase MgCrO4, which decomposes with the formation of MgCr2O4/SiO2. The formation of the precursors inside the silica matrix and the evolution of the crystalline phases were studied by thermal analysis, FT-IR spectrometry, XRD, and TEM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent progress in the field of ceramic technology and theory of sintering has convincingly demonstrated that the possibilities of designing new ceramic materials based on oxides and their compounds are far from exhausted. Nowadays, increased interest has been expressed by researchers in the use of ultradispersed powders in the technology of structural ceramic materials because of their high activity, which makes it possible to reduce the temperatures of their synthesis and sintering [1].
The main method for preparing chromites is based on the interaction between initial oxide components at high temperatures. The formation rate of these compounds depends on many factors, such as the conditions of preliminary treatment of initial components, crystallinity degree, dispersion, and homogeneity of the reaction mixture [2–4].
We present a new method for obtaining of ZnCr2O4 and MgCr2O4 embedded in silica matrix. This method consists in the formation, inside the silica matrix, of some Cr(III), Zn(II) and Cr(III), Mg(II) hydroxycarboxylate/carboxylate compounds, resulted in the redox reaction between nitrate ion and 1,3-propanediol (1,3PG) (OH–CH2–CH2–CH2–OH) and the controlled thermal decomposition of the precursors. The decomposition products have been annealed at different temperatures to obtain ZnCr2O4 and MgCr2O4 embedded in silica matrix.
Experimental
Materials and methods
The reagents used in the synthesis of ZnCr2O4 and MgCr2O4 embedded in silica matrix were: tetraethyl orthosilicate (TEOS), Cr(NO3)3·9H2O, Zn(NO3)2·6H2O, Mg(NO3)2·6H2O, and 1,3-propanediol (1,3PG). All reagents were supplied by Merck and were of analytical purity, >98%. Double distilled water and ethanol (99.2% purity) were used for peptization and solvent, respectively. A schematic representation of the experimental procedure is shown in Fig. 1.
The ethanolic TEOS solution was added drop wise, under magnetic stirring, to the mixture Cr(NO3)3–MII(NO3)2-1,3PG (M = Zn or Mg) (Table 1). The clear solutions obtained after 30 min of stirring on a shaker, were left for gelation at room temperature. The obtained gels were crushed and dried at 40 °C for 2 h. The xerogels were thermally treated in air at 140 °C, when the redox reaction took place between metal nitrates and 1,3-propanediol, with formation of the complex combinations inside the hybrid gels. The gels were thermally treated at 300 °C when the oxidative decomposition of the metal–organic precursors took place with formation of the corresponding metal oxides powders. These powders were annealed at different temperatures to obtain the chromites.
Experimental techniques
The thermal decomposition of the formed precursors was studied by thermal analysis using a Diamond Perkin Elmer thermo balance. The experiments have been done in air, in the temperature range 20–500 °C, with a heating rate of 5 °C min−1, using as reference α-Al2O3.
The synthesized powders were characterized by FT-IR spectrometry with a Shimadzu Prestige FT-IR spectrometer, in KBr pellets, in the range 400–4,000 cm−1. The phase composition of the powders was determined by XRD using a Bruker D8 Advance System (monochromatic Mo-Kα radiation) operating at 40 kV and 40 mA. The average crystallite size was calculated based on the XRD patterns using the Scherrer Eq. 1, \( D_{\text{XRD}} = 0. 9\lambda /\beta { \cos }\theta \) [5], where D XRD is the mean crystallite size, λ is the radiation wavelength of Mo-Kα (0.70930 Ǻ), β is the full width at half of the maximum (FWHM) (in radians), and θ is the Bragg angle. TEM images have been recorded on a JEOL JEM 1010 microscope.
Results and discussion
Our previous studies on nanocrystalline metal chromites synthesis, starting from a mixture of Cr(III) nitrate-M(II) nitrate with diol has evidenced that at 70 °C a redox reaction takes place between Cr(NO3)3·9H2O and diol, and at ~130 °C takes place the redox reaction between M(II) nitrate and diol [6, 7]. The oxidation products of the diols (carboxylates) coordinate to the metallic ions Cr3+, Zn2+ forming the precursors for zinc chromite and to Cr3+, Mg2+ with formation of the precursors for magnesium chromite.
The particular feature of the modified sol–gel method reported in this article is the presence of the diol in the system TEOS–Cr(NO3)3–M(NO3)2, as reactant in the redox reaction and its chemical interaction with the hydrolysis products of TEOS leading to hybrid gels [8].
During the heating of the hybrid gels at ~300 °C, at the same time with the oxidative decomposition of the carboxylic compounds, the burning of the chains of the interacted diol takes place, which leads to silica matrices with homogenous mesoporous structure [9].
Obtaining of ZnCr2O4
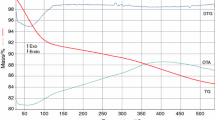
Figure 2 presents the TG, DTG, and DTA curves of the hybrid gel obtained at 140 °C, when the redox reaction was finished, which contains Cr, Zn–hydroxycarboxylate/carboxylate uniformly dispersed in the pores.
The evolution of the TG curve evidenced a thermal decomposition in two steps of the synthesized compound. The first step, that occurs up to 250 °C can be assigned to the loss of uncoordinated and coordinated water molecules and –OH groups resulting from the polycondensation of the gel. The mass loss starting with ~300 °C corresponds to the oxidative decomposition of the complex combination in the pores of the silica gel, confirmed by the exothermic effect registered on DTA curve. Also, a contribution to this mass loss and especially to the exothermic effect has the burning of the organic chains of the diol from the hybrid gel [10]. The result of this decomposition step is a mixture of nonstoichiometric chromium oxide, Cr2O3 + x , and ZnO dispersed inside the silica matrix. Around 400 °C, Cr2O3 + x losses the excedentary oxygen and turns to Cr2O3 [11, 12], which further reacts with the ZnO, forming ZnCr2O4 nuclei in the pores of the silica matrix.
In order to elucidate the thermal evolution of the formed oxidic system, we have characterized the powders obtained at different temperatures by FT-IR spectrometry (Fig. 3) and X-ray diffractometry.
The frequencies of the main IR absorption bands corresponding to the studied samples and the assignment of these bands are shown in Table 2 [8–15].
The powders obtained by thermal treatment of the precursor Cr,Zn-1,3PG/SiO2 at 400, 600, and 1,000 °C and have been studied by X-ray diffractometry. The obtained XRD patterns (Fig. 4) present the evolution of the formation of ZnCr2O4 as crystalline phase starting with 400 °C [14] confirming the results of thermal and FT-IR analysis. The crystallization degree increases with annealing temperature.
The XRD pattern (Fig. 5) of the hybrid gel containing only the Cr(III)-hydroxycarboxylate annealed at 400 °C, evidences the crystallization of the simple oxide, Cr2O3, within the amorphous silica matrix. In case of the sample Cr,Zn-1,3PG/SiO2 annealed at 400 °C, the lines of Cr2O3 are not evidenced. Thus, we can assume that Cr2O3 reacts with ZnO forming ZnCr2O4 nuclei. The mean nanoparticles diameter was calculated using the Scherrer formula and the results were 3.5 nm for the sample annealed at 600 °C and 6 nm for the sample annealed at 1,000 °C, respectively.
Obtaining of MgCr2O4
The complex combination Cr,Mg-1,3PG/SiO2 obtained at 140 °C, has been studied by thermal analysis at heating, in air, up to 500 °C (Fig. 6).
The mass loss up to 200 °C corresponds to the elimination of the coordinated water. The mass loss in the range 200–300 °C is attributed to the same processes as in Fig. 2; subsequently, at 300 °C we obtain a mixture of Cr2O3 + x and MgO. Up to 400 °C, take places the transition of Cr2O3 + x to Cr2O3 which reacts with MgO with formation of the intermediary phase MgCrO4, which at ~600 °C decomposes to MgCr2O4 [16].
The presence in the silica matrix of the complex combination and the behavior during the thermal treatment, have been studied by FT-IR spectrometry. The FT-IR spectra are shown in Fig. 7.
The FT-IR spectra of the initial synthesized compound Cr,Mg-1,3PG/SiO2 exhibits similar bands with the ones presented in Table 2.
The FT-IR spectra of the decomposition product obtained at 380 °C exhibits two band located at ~940 cm−1 characteristic to CrVI–O bonds vibrations, from Cr2O3 + x or probably from MgCrO4. The FT-IR spectra of the residue obtained at 580 °C present the bands at 940 cm−1 assigned to the vibrations of CrVI–O bonds from MgCrO4, sustaining the hypothesis of the formation of magnesium chromate as intermediary phase, resulted from the thermal analysis. There are also present the bands characteristic to MgCr2O4 at 654 and 589 cm−1 [17].
In order to obtain magnesium chromite dispersed in SiO2, the powders obtained at 140 °C, were annealed for 2 h at 400, 600, and 1,000 °C. The obtained XRD patterns of the obtained compounds are presented in Fig. 8.
For Cr,Mg-1,3PG/SiO2 annealed at 400 °C, the XRD patterns does not evidence crystalline phases, presenting an amorphous aspect. The XRD patterns of the samples annealed at 600 and 1,000 °C, evidence MgCr2O4 as crystalline phase starting with 600 °C, inside the amorphous silica [18].
The samples Cr,Zn-1,3PG/SiO2 and Cr,Mg-1,3PG/SiO2 annealed at 1,000 °C for 2 h were studied by TEM microscopy to see the distribution of the nanoparticles ZnCr2O4 and MgCr2O4 inside the silica matrix (Fig. 9).
Both chromites ZnCr2O4 and MgCr2O4 nanoparticles are homogenously dispersed within the silica matrix, having diameters lower than 50 nm.
Conclusions
The proposed sol–gel method, is based on the formation of the complex combinations of carboxylate type in the pores of the hybrid gels, in the redox reaction between metal nitrates and 1,3-propanediol. By thermal decomposition of the precursors formed, a mixture of corresponding metal oxide is obtained, which by appropriate thermal treatments leads to the obtaining of chromites at low temperatures, ~400 °C in case of ZnCr2O4 and ~600 °C in case of MgCr2O4. TEM images of the powders obtained at 1,000 °C evidenced a homogeneous distribution of the nanoparticles of ZnCr2O4 and MgCr2O4 inside the silica matrix.
The synthesis method, ‘‘modified sol–gel method’’, used is an original one and excellent to obtain the chromites embedded in SiO2 matrix.
Abbreviations
- 1,3PG:
-
1,3-Propanediol
- TEOS:
-
Tetraethyl orthosilicate
References
Morozova LV, Popov VP. Synthesis and investigation of magnesium chromium spinel. Glass Phys Chem. 2010;36(1):86–91.
Mančić L, Marinković ZV, Vulić P, Milošević O. The synthesis-structure relationship in the ZnO-Cr2O3 system. Sci Sinter. 2004;36:189–96.
Szczygiel I, Winiarska K. Low-temperature synthesis and characterization of the Mn–Zn ferrite. J Therm Anal Calorim. 2011;104:577–83.
Souaya ER, Ismail EH, Mohamed AA, Milad NE. Preparation, characterization and thermal studies of some transition metal ternary complexes. J Therm Anal Calorim. 2009;95:253–8.
Suryanarayana C, Grant Norton M. X-ray diffraction. A practical approach. New York: Plenum Press; 1998.
Stefanescu M, Sasca V, Birzescu M. Thermal behaviour of the homopolynuclear glyoxylate complex combinations with Cu(II) and Cr(III). J Therm Anal Calorim. 2003;72(2):515–24.
Stefanescu M, Sasca V, Birzescu M. Studies on the thermal decompositions of heteropolynuclear glyoxylates of Cr(III) and Cu(II). J Therm Anal Calorim. 1999;56(2):569–78.
Stefanescu M, Stoia M, Stefanescu O, Popa A, Simon M, Ionescu C. The interaction between teos and some polyols. Thermal analysis and FT-IR. J Therm Anal Calorim. 2007;88:19–26.
Stefanescu M, Stoia M, Stefanescu O. Thermal and FT-IR study of the hybrid ethylene–glycol–silica matrix. J Sol Gel Sci Techn. 2007;41:71–8.
Brinker CJ, Scherrer GW. Sol–gel science: the physics and chemistry of sol-gel Processing. New York: Academic Press; 1990.
Stefanescu M. Consideration on the formation of the mixed oxides starting from substances with high reactivity. PhD Thesis, University of Timisoara, Romania, 1993.
Barbu M, Stoia M, Stefanescu O, Stefanescu M. Thermal and FT-IR studies on the interaction between Cr(NO3)3·9H2O and some diols. Chem Bull. 2010;55(69):180–5.
Bernazzani P, Sanchez RF. Structural and thermal behavior of polystyrene thin films using ATR–FTIR–NanoDSC measurements. J Therm Anal Calorim. 2009;96:727–32.
Purnendu P, Manivannan V. Microwave metathetic approach for the synthesis and characterization of ZnCr2O4. J Eur Ceram Soc. 2008;28:1665–70.
Pocol V, Patron L, Carp O, Brezeanu M, Segal E, Stanica N. Some polynuclear coordination compounds precursor of chromites synthesis, physicochemical characterization and thermal stability. J Therm Anal Calorim. 1999;55:143–54.
Bielanski A, Deren J, Duczyminska E. Elektrische Leitfahigkeit von MgO–Cr2O3 Gemischen verschiedener Zusammensetzung und verschiedenen Sinterungsgrades. Z Anorg Allg Chim. 1962;316(1–2):75–88.
Rida K, Benabbas A, Bouremmad F, Pen MA, Martinez-Arias A. Influence of the synthesis method on structural properties and catalytic activity for oxidation of CO and C3H6 of pirochromite MgCr2O4. Appl Catal A. 2010;375:101–6.
Joint Committee on Powder Diffraction Standards-International Center for Diffraction Data. Swarthmore, 1993.
Aknowledgements
This study was partially supported by the strategic grant POSDRU/88/1.5/S/50783, Project ID50783 (2009), co-financed by the European Social Fund—Investing in People, within the Sectoral Operational Programme Human Resources Development 2007-2013 and by the strategic grant POSDRU/21/1.5/G/13798 inside POSDRU Romania 2007-2013, co-financed by the European Social Fund—Investing in People.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbu, M., Stefanescu, M., Stoia, M. et al. New synthesis method for M(II) chromites/silica nanocomposites by thermal decomposition of some precursors formed inside the silica gels. J Therm Anal Calorim 108, 1059–1066 (2012). https://doi.org/10.1007/s10973-011-1933-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1933-7