Abstract
The aim of the present study was to prepare nanocomposites with different CoCr2O4 content (20%, 50%, and 80% CoCr2O4/SiO2 mass%) starting from Co(II) carboxylate and ammonium dichromate using a modified sol–gel method and to evaluate the final product (nature, size and shape) based on the different precursor amount. Thermal analysis was used in order to reveal the formation and decomposition of Co(II) carboxylate to CoO and (NH4)2Cr2O7 to Cr2O3+x, inside the silica gel pores. The oxide mixture interacts at temperatures of ~ 400 °C when Cr2O3+x turn to α-Cr2O3 which leads together with CoO to the formation of CoCr2O4 inside the SiO2 matrix. XRD and FTIR analyses revealed the formation of the single spinel structure in all synthesized samples. The results show that a higher cobalt chromite content inside the silica matrix leads to higher crystallinity. The particle sizes were in nanometer range, and the size variation was small from a sample to another, independent on the concentration (3.9–12.4 nm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
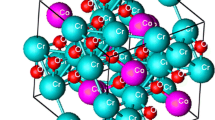
Transition metal chromites having the formula MCr2O4, where A=Co, Ni, Zn, Fe, Cu, Mn, Ca, Mg, etc., have spinel structure with close—packing of oxygen anions. In case of CoCr2O4, the Co2+ ions occupy the tetrahedral (Th) sites and Cr3+ ions the octahedral (Oh) sites, having a normal spinel structure. The spinel structure shows 64 Th sites and 32 Oh sites, from which only 8 Th and 16 Oh sites are occupied by Co2+ and Cr3+ ions. The unit cell is face-centered cubic (fcc) and has a total of 56 atoms (32 oxygen anions and 24 cobalt and chromium cations) as shown in Fig. 1 [1].
CoCr2O4 has attracted interest as it has good catalysis potential for some reactions, such as methane combustion [2], 2-propanol combustion [3], dichloromethane oxidation [4, 5], NOx removal [6,7,8], and CO oxidation [9], phenol alkylation [10], oxidation of trichloroethylene [11]. It can also be used as ceramic pigment [12,13,14] and anode material for lithium ion batteries [15].
CoCr2O4 presents conical spin structure due to the combined effect of the geometrical spin frustration on the Cr sites and the exchange interaction between the Cr and Co spins [16,17,18]. Both Co2+ and Cr3+ are magnetic [19,20,21].
In order to achieve materials with desired properties, many preparation methods were lately research subjects. Besides the classical method involving solid-state reactions of metal oxides at high temperatures, the synthesis methods employed in obtaining cobalt chromite nanoparticles over time were diverse, including co-precipitation [10, 21, 22], the hydrothermal process [23,24,25], sol–gel methods [13, 26, 27], the polymeric precursors route [28, 29] or the sonochemical technique [21, 26]. Each of these preparation methods convey specific features to the resulting particles (particle structure, size, surface area, morphology), features that play a major role in determining the properties of the final material.
Herein we present an alternative route for the preparation of CoCr2O4/SiO2 nanocomposites using a modified sol–gel method. The method is based on the synthesis of the Co(II) carboxylate type precursor in mixture with (NH4)2Cr2O7 inside the silica gel pores, which by decomposition lead to CoO and Cr2O3 oxide formation, uniformly distributed inside the silica matrix, at low temperatures.
Experimental
Materials
The reagents used for the preparation of the CoCr2O4/SiO2 nanocomposites were: Co(NO3)2·6H2O; (NH4)2Cr2O7; 1,3-propandiol (1,3PD); and tetraethylortosilicate (TEOS). All materials were of analytical purity and were purchased from Merck.
Synthesis
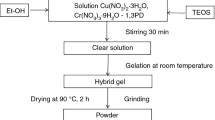
We have prepared three sets of samples for different oxide contents (20%, 50%, and 80 mass% CoCr2O4/SiO2). The reagent quantities used for the synthesis of the precursor gels (G20, G50, G80) yielding 6 g of nanocomposite, are presented in Table 1. Co(NO3)2·6H2O and (NH4)2Cr2O7 have been added to a beaker and have been dissolved, by stirring, in a small water amount (1 mL), until a homogenous mixture was obtained. 1,3PD was added using a 25% excess (to ensure equilibrium displacement) and after homogenization, an ethanolic TEOS solution was added dropwise. Ethanol was used to improve phase miscibility. After 30 min of stirring for homogenization, the clear solutions (sol) were allowed to rest at room temperature for gelation. The resulted gels were dried at 80 °C and the powders obtained after grinding were thermally treated at 170 °C, for 2 h, in order to complete the redox reaction that took place between Co(NO3)2·6H2O and 1,3PD. At this temperature (170 °C), the samples contain Co(II) carboxylate and (NH4)2Cr2O7 embedded in the gel pores. The samples thus obtained were annealed in the temperature range 400–1000 °C, for 3 h. The final product was CoCr2O4/SiO2 (Fig. 2).
Characterization techniques
TG-DTA analysis was performed in air atmosphere up to 500 °C using SETARAM apparatus, LabsysEvo model. Samples were analyzed in dynamic air atmosphere (100 mL min−1), with a heating rate of 5 °C min−1, using as reference α-Al2O3. Phase composition of the powders obtained in the temperature range 400–1000 °C was analyzed using a RigakuUltima IV Diffractometer, with CuKα radiation (λ = 1.5406 Å). Crystallite size and lattice parameters were calculated using WPPF method (whole pattern in fitting profiles), and the instrument influence was subtracted using the diffraction pattern of a Si standard recorder the same conditions. The obtained samples were also characterized by Fourier transformed infrared spectrometry in the range 400–4000 cm−1, using a Shimadzu Prestige 21 FTIR Spectrometer, in KBr pellets. TEM images were attained on a Titan G2 80–200 TEM/STEM (FEI Company, Holland) microscope with image corrector, at 200 kV. The samples were deposited from ethanol solution on 200 mesh copper grids, covered by lacey carbon. The images were recorded on TEM mode using a Digital Micrograph v. 2.12.1579.0. software.
Results and discussion
The modified sol–gel method has been presented in our previous paper [30]. 1,3PD plays the key role in the obtaining of the hybrid gel and also for the formation of Co(II) carboxylate. The precursor of carboxylate type is obtained in the redox reaction between cobalt nitrate and the hydroxyl group of the diol (1,3PD). This reaction occurs inside the gel pores. By heating the mixture of Co(II) carboxylate complex and ammonium dichromate, at higher temperatures, an energetic decomposition takes place resulting in an oxide mixture (CoO + Cr2O3+x) inside the matrix, which subsequently turns into cobalt chromite.
Thermal analysis
According to the thermal analysis study, the gels G20 (Fig. 3a) and G50 (Fig. 3c), heated at 80 °C, have similar thermal behavior. The exothermic effect on DTA at ~ 170 °C corresponds to the decomposition of (NH4)2Cr2O7 [31] and Co(II) carboxylate and to the formation of the oxide mixture.
The exothermic effect from 293 °C is wider and less intense and can be associated with the decomposition of Co(II) carboxylate. The DTA curve of G20 heated at 170 °C (Fig. 3b) shows only the effect from 293 °C which can be attributed to the decomposition of Co(II) carboxylate inside the gel pores.
Due to the presence of SiO2, not all Co(II) carboxylate is decomposed in the first step associated with the decomposition of (NH4)2Cr2O7. The formation of small matrix packages/pockets diminishes the exothermic effect of decomposition and prevents the sample loss as a result of the violent reaction. When the quantity of (NH4)2Cr2O7 is higher, as in G80, the sample behavior is different. The decomposition reaction for this sample is violent and the energetic decomposition reaction takes place at 170 °C with particle spread. Therefore, the sample was difficult to be analyzed by thermal methods.
FTIR analysis
FTIR analysis (Fig. 4) showed the presence of Co(II) carboxylate precursor in mixture with ammonium dichromate, in all samples obtained at low temperature (80 °C). The carboxylate precursor is of malonate type [32], and it was formed in the redox reaction between the \( {\text{NO}}_{3}^{ - } \) ions and the –OH groups of 1,3PD, according to Eq. 1.
The specific carboxylate group vibrations are visible for all three samples: νas(COO−) in the range 1500–1700 cm−1 and νs(COO−) in the range 1300–1400 cm−1. The band assignment [33, 34] for the gels heated at 80 °C and 170 °C for all compositions considered is presented in Table 2.
With an increase in annealing temperature, the spectrum of each sample changes and the band from 1380 cm−1 (attributed to \( {\text{NO}}_{3}^{ - } \)) disappears completely at temperatures above 400 °C. For the annealed samples, the specific bands for the spinel structure are present in the range 400–800 cm−1 and are clearly highlighted in the FTIR spectra of G80 FTIR. The bands located at ~ 480 cm−1 are attributed to Cr3+ bonding vibrations located in octahedral positions of the spinel structure, while those located at ~ 610 cm−1 are assigned to Co2+ bonding vibrations located in tetrahedral position of the spinel lattice [29, 33].
XRD and TEM analyses
The X-ray analysis (Fig. 5) confirms the crystallization of CoCr2O4 inside the silica matrix as single cubic spinel phase (JCPDS card 00-022-1084) after decomposition (400 °C) and annealing (600–1000 °C). No secondary phases were detected by this technique. The data obtained for CoCr2O4 are similar to those obtained by other researchers [27, 35].
Cell parameters and the crystallite average size obtained for different annealing temperatures are illustrated in Table 3. The increase in annealing temperature does not affect the particle size so dramatically due to the presence of the SiO2 matrix. No particle size measurements were possible for G20 because of the high amorphous SiO2 amount which covers the nanoparticle surface. However, the phase was identified with the same JCPDS file.
The TEM images presented in Fig. 6 were recorded for the samples G20, G50, and G80 annealed at 1000 °C. The tendency of particle aggregation is present in each sample due to the high surface area of the particles, and it is more pronounced in samples with higher oxide content. SiO2 matrix has the role to prevent particle agglomeration [27, 36]. Particles with almost spherical shape are distributed inside the silica matrix in case of G20 (Fig. 6a, b). Figure 6c shows measurements of the interplanar distances for cobalt chromite particles. The value of 2.9 nm represents lattice fringes for 10 interplanar distances, which can be correlated with 220 (d = 2.947 Å) and 311/222 (d = 2.512 Å/2.405 Å) lattice planes from the CoCr2O4 structure (JCPDF 00-022-1084).
For the samples with higher oxide content (Fig. 6d, e) the particle distribution inside the SiO2 matrix is still uniform. Figure 6f presents a particle that exhibits fringes at distances of 2.20 nm corresponding to plane 400 (d = 2.084 Å) from the same XRD file. Figure 6g shows that particles no longer have a regular shape and that the particle growth is no longer inhibited by the matrix. The exposed planes 311 (d = 2.512 Å) and 220 (d = 2.947 Å) are confirmed by the d-space fringe measurements 2.55 nm and 2.77–3.15 nm (Fig. 6h, i).
Conclusions
Nanocomposites with different oxide content (20%, 50%, and 80% CoCr2O4/SiO2) were successfully obtained via modified sol–gel method. The diol 1,3PD played an essential role in the obtaining of the hybrid gel and the formation of Co(II) carboxylate precursor inside the gel pores. The pathway for the preparation of cobalt chromite nanocomposites consisted in heating of a Co(II) carboxylate complex–ammonium dichromate mixture, which energetically decomposed inside the matrix into the oxide mixture CoO + Cr2O3+x followed by the transformation into cobalt chromite. The sample with high (NH4)2Cr2O7 quantity (80% CoCr2O4/SiO2) could not be analyzed by thermal analysis because of the energetic decomposition reaction, which also leads to sample loss.
Fact is that for all three samples with different oxide content, cobalt chromite has been obtained as single phase with no other secondary phases. The influence of the oxide content could be observed in the crystallization of cobalt chromite. Cobalt chromite in the sample with 80% CoCr2O4/SiO2 is well crystallized starting with 400 °C and the nanoparticles have defined shape and measurable particle size (5.4–12.4 nm). In the meantime for sample 20% CoCr2O4/SiO2, no particle size measurements were possible because of the high amorphous SiO2 amount which covers the nanoparticle surface.
As a general conclusion of the present study one can note that the hybrid silica matrix plays an essential role in controlling the decomposition reactions of the Co(II) carboxylate–ammonium dichromate mixture, providing control over particle size and distribution. The desired particle size and tunable properties can be obtained by adjusting temperature and SiO2 concentration. When decreasing the SiO2 content, the decomposition of the mixture is more energetic and ends at higher temperatures (TG/DTA was not registered for G80).
References
Sickafus KE, Wills JM. Spinel coumpounds: structure and property relations, structure of spinel. J Am Ceram Soc. 1999;82(12):3279–92.
Hu J, Zhao W, Hu R, Chang G, Li C, Wang L. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion. Mater Res Bull. 2014;57:268–73.
Hosseini SA, Alvarez-Galvan MC, Fierro JLG, Niaei A, Salari D. MCr2O4 (M¼Co, Cu, and Zn) nanospinels for 2-propanol combustion: correlation of structural properties with catalytic performance and stability. Ceram Int. 2013;39:9253–61.
Wang Y, Jia AP, Luo MF, Lu JQ. Highly active spinel type CoCr2O4 catalysts for dichloromethane oxidation. ApplCatal B-Environ. 2015;165:477–86.
Zhang T-T, Song J-D, Chen J-X, Jia A-P, Luo M-F, Lu J-Q. Catalytic combustion of dichloromethane over supported CoCr2O4/TUD-1 catalysts: the effect of CoCr2O4 particle size on the modification of surface properties and the catalytic performance. Electrochim Acta. 2017;247:1–11.
TeraokaY KagawaS. Simultaneous catalytic removal of NOx and diesel soot particulates. Catal Surv Jpn. 1998;2:155–64.
Fino D, Russo N, Saracco G, Specchia V. Catalytic removal of NOx and diesel soot over nanostructured spinel-type oxides. J Catal. 2006;242:38–47.
Fino D, Russo N, Saracco G, Specchia V. Removal of NOx and diesel soot over catalytic traps based on spinel-type oxides. Powder Technol. 2008;180:74–8.
Ruszel M, Grzybowska B, Samson K, Gressel I, Klisinska A. MIICr2O4-spinels as supports for Au nanoparticles in oxidation of CO. Catal Today. 2006;112:126–9.
Wang Y, Zhou Z, Jia M, Zhu X, Zhang W, Jiang D. Spinel-Type cobalt chromites as novel and highly ortho-selective catalysts for phenol alkylation. Catal Lett. 2005;104:67–71. https://doi.org/10.1007/s10562-005-7438-x.
Kim D-C, Ihm S-K. Application of spinel-type cobalt chromite as a novel catalyst for combustion of chlorinated organic pollutants. Environ Sci Technol. 2001;35:222–6.
Li Z, Du Y, Chen Z, Sun D, Zhu C. Synthesis and characterization of cobalt doped green ceramic pigment from tannery sludge. Ceram Int. 2015;41:12693–9.
Grazenaite E, Pinkas J, Beganskiene A, Kareiva A. Sol-gel and sonochemically derived transition metal (Co, Ni, Cu, and Zn) chromites as pigments: a comparative study. Ceram Int. 2016;42:9402–12.
Hedayati HR, Sabbagh Alvani AA, Sameie H, Salimi R, Moosakhani S, Tabatabaee F, Amiri Zarandi A. Synthesis and characterization of Co1-xZnxCr2-yAlyO4 as a near-infrared reflective color tunable nano-pigment. Dyes Pigments. 2015;113:588–95.
Li Z, Huang X, Hu J, Stein A, Tang B. Synthesis and electrochemical performance of three-dimensionally ordered macroporous CoCr2O4 as an anode material for lithium ion batteries. Electrochim Acta. 2017;247:1–11.
Bao H, Yang S, Ren X. Magnetodielectric effect in CoCr2-XFeXO4. J Phys Conf Ser. 2011;266:1–6.
Singh K, Maignan A, Simon C, Martin C. FeCr2O4 and CoCr2O4 spinels: multiferroicity in the collinear magnetic state. Appl Phys Lett. 2011;99:172903. https://doi.org/10.1063/1.3656711.
Tokura Y. Multiferroics—toward strong coupling between magnetization and polarization in a solid. J Magn Magn Mater. 2007;310:1145–50.
Menyuk N, Dwight K, Wold A. Ferrimagnetic spiral configurations in cobalt chromite. Le J de Phys. 1964;25(5):528–36.
Dutta DP, Manjanna J, Tyagi AK. Magnetic properties of sonochemically synthesized CoCr2O4 nanoparticles. J Appl Phys. 2009;106:043915. https://doi.org/10.1063/1.3204659.
Roth C, Mohanty P. Magnetic phase transitions in cobalt chromite nanoparticles. J Supercond Nov Magn. 2011;24:629–33.
Kumar L, Mohanty P, Shripathi T, Rath C. Appearance of superparamagnetic phase below curie temperature in cobalt chromite nanoparticles. Nanosci Nanotechnol Lett. 2009;1:199–203.
Zakutna D, Repko A, Matulkova I, Niznansky D, Ardu A, Cannas C, Mantlıkova A, Vejpravova J. Hydrothermal synthesis, characterization, and magnetic properties of cobalt chromite nanoparticles. J Nanopart Res. 2014;16:2251. https://doi.org/10.1007/s11051-014-2251-3.
Abbasi A, Hamadanian M, Salavati-Niasari M, Mazhari MP. Hydrothermal synthesis, characterization and photodegradation of organic pollutants of CoCr2O4/Ag nanostructure and thermal stability of epoxy acrylate nanocomposite. Adv Powder Technol. 2017;28:2756–65.
Ptak M, Maczka M, Hermanowicz K, Pikul A, Hanuza J. Particle size effects on the magnetic and phonon properties of multiferroic CoCr2O4. J Solid State Chem. 2013;199:295–304.
Plocek J, Holec P, Kubickova S, Pacakova B, Matulkova I, Mantlikova A, Nemec I, Niznansky D, Vejpravova J. Stabilization of transition metal chromite nanoparticles insilica matrix. Int J ChemMolecNucl Mater Metall Eng. 2014;8(11):1219–28.
Kamran M, Ullah A, Mehmood Y, Nadeem K, Krenn H. Role of SiO2 coating in multiferroic CoCr2O4 nanoparticles. AIP Adv. 2017;7:025011. https://doi.org/10.1063/1.4973732.
Eliziário SA, Andrade JM, Lima SJG, Paskocimas CA, Soledade LEB, Hamer P, Longo E, Souza AG, Santos IMG. Black and green pigments based on chromium–cobalt spinels. Mat Chem Phys. 2011;129:619–24.
Gingasu D, Mindru I, Culita DC, Patron L, Calderon-Moreno JM, Osiceanu P, Preda S, Oprea O, Parvulescu V, Teodorescu V, Walsh JPS. Structural, magnetic and catalytic properties of cobalt chromite obtained through precursor method. Mater Res Bull. 2015;62:52–64.
Berei E, Stefanescu O, Muntean C, Vlase T, Taranu BO, Dabici A, Stefanescu M. A novel route for the preparation of CoCr2O4/SiO2 nanocomposite starting from Co(II)–Cr(III) carboxylate complex combinations. J Mat Sci. 2018;53(6):4159–72.
Lima MD, Bonadimann R, Andrade MJ, Toniolo JC, Bergmann CP. Nanocrystalline Cr2O3 and amorphous CrO3 produced by solution combustion synthesis. J Eur Ceram Soc. 2006;26:1213–20.
Stefanescu O, Stefanescu M. New Fe(III) malonate type complex combination for development of magnetic nanosized γ-Fe2O3. J Organomet Chem. 2013;740:50–5.
Tanisan B, Dondi M. Cobalt chromite nano pigments synthesis through microwaveassistedpolyol route. J Sol–Gel Sci Technol. 2017;83(3):590–5. https://doi.org/10.1007/s10971-017-4449-1.
Nakamoto K. Infrared spectra of inorganic and coordination compounds. New York: Wiley; 1970.
Cui H, Zayat M, Levy D. Sol–Gel synthesis of nanoscaled spinels using propylene oxide as a gelation agent. J Sol–Gel Sci Technol. 2005;35:175–81.
Xiao SH, Xu HJ, Hu J, Li LY, Li XJ. Dependence of structural and magnetic properties of CoFe2O4/SiO2nanocomposites on annealing temperature and component ratio. Phys E. 2008;40:3064–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berei, E., Ştefănescu, O., Muntean, C. et al. Study on the formation of CoCr2O4/SiO2 nanocomposite obtained from Co(II) carboxylate and ammonium dichromate. J Therm Anal Calorim 138, 1863–1870 (2019). https://doi.org/10.1007/s10973-019-08783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08783-8