Abstract
The thermal transformation of Na2C2O4 was studied in N2 atmosphere using thermo gravimetric (TG) analysis and differential thermal analysis (DTA). Na2C2O4 and its decomposed product were characterized using a scanning electron microscope (SEM) and the X-ray diffraction technique (XRD). The non-isothermal kinetic of the decomposition was studied by the mean of Ozawa and Kissinger–Akahira–Sunose (KAS) methods. The activation energies (E α) of Na2C2O4 decomposition were found to be consistent. Decreasing E α at increased decomposition temperature indicated the multi-step nature of the process. The possible conversion function estimated through the Liqing–Donghua method was ‘cylindrical symmetry (R2 or F1/2)’ of the phase boundary mechanism. Thermodynamic functions (ΔH*, ΔG* and ΔS*), calculated by the Activated complex theory and kinetic parameters, indicated that the decomposition step is a high energy pathway and revealed a very hard mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal analysis (TA), e.g., thermogravimetry (TG), differential thermal analysis (DTA), and differential scanning calorimetry (DSC) have been used widely for scientific and practical purposes [1, 2]. These techniques provide important information about physico-chemical parameters, kinetic analysis, polymorphic forms, stability of material, etc., which are reliable and necessary [3]. Thus, the outcomes obtained through this basis can be applied directly in material science for studying thermal behavior, thermal character, and the mechanism and kinetic of solid state reaction. The interpretation of data obtained from these methods, use of various mathematical models and calculation procedures are quite useful. For gaining value of the apparent activation energy, E a, and pre-exponential factor, A, which is the most probable mechanism function g(α) of the reaction, various equations and methods were described such as the Coats and Redfern equation [4], and iterative procedure, i.e., the Ozawa equation [5], Kissinger–Akahira–Sunose (KAS) equation [6], Senum and Yang approximation formulae [7], etc. The mathematical apparatus and calculation procedures used are related to the mathematical analysis of thermogravimetric curves. The calculations, based on multiple rates of thermogravimetric curves, are so-called iso-conversional calculation procedures [3].
Thermal decomposition of metal oxalates has been the subject of many researches for more than a century [8]. Decomposition and its non-isothermal kinetics, belonging to some of the oxalates (Ag2C2O4, NiC2O4, MnC2O4, HgC2O4, PbC2O4, and SrTiO(C2O4)2·4H2O), were reported later [3, 8–10]. The dehydration kinetics of CaC2O4·H2O were deduced from the multiple rate iso-temperature method, and the apparent activation energy, E a, was obtained from the Ozawa and KAS method [6]. The kinetic triplet, the activation energy, E a, the pre-exponential factor, A, and the mechanism functions, f(α), of MgC2O4·2H2O were obtained by analyzing the TG-DTG curves of their thermal decomposition using the Popescu and Flynn–Wall–Ozawa method [11]. Furthermore, the decomposed products, e.g., oxide or metal, which possess pores, lattice imperfections and both characteristics, were determined, and the results are necessary data for their function and further study. Although there has been increasing interest in the study of experimental factors and processing parameters, especially in determining the kinetics of thermal decomposition reactions, many features of oxalate decomposition still remain unclear.
In a previous paper, we presented results on the preparation of lead-free piezoelectric sodium niobate (NaNbO3) powders [12]. The use of Na2C2O4 as starting material (instead of Na2CO3) resulted in a low-temperature solid-state reaction. In general, the sodium oxalate, Na2C2O4, serves as a metal cleaning preparation in the textile, leather and tanning industries; potassium oxalate cleans natural fibers in photography, and both of them are used in analytical and solvent extraction chemistry (sodium oxalate as primary volumetric standard for manganometry and acidimetry) [13]. As starting material for versatile industries, it is very important to determine its thermal decomposition mechanism, kinetics and thermodynamic parameter for advantages in cost and time management for industrial production. Many works on the isothermal kinetic of thermal decomposition of oxalate compounds have been published, but there are no reports on the thermal decomposition kinetic of Na2C2O4 in the literature.
In this work, the thermal decomposition of Na2C2O4 was investigated using non-isothermal thermogravimetry–differential thermal analysis (TG-DTG/DTA), X-ray powder diffraction (XRD), and scanning electron microscopy (SEM). Thus, the non-isothermal kinetics analysis for the decomposition of this compound was carried out, based on the iso-conversional techniques of the Ozawa and Kissinger–Akahira–Sunose (KAS) methods. Possible conversion functions have been estimated by the Liqing–Donghua method [6], combined with 35 algebraic expressions of the conversion functions, g(α). The activation energy, E, and pre-exponential factor, A, were estimated. The transition state thermodynamic functions, ΔH*, ΔG* and ΔS*, were calculated via the activated complex theory.
Experimental procedure
Materials and measurement
Sodium oxalate, Na2C2O4 (≥99.0% (RT) 71801, Fluka), was used without further purification. Thermal behavior of Na2C2O4 was investigated using TG-DTA (Perkin Elmer). Initial experiments were conducted with a heating rate of 15, 20, 30 and 40 K min−1 in a temperature range from room temperature to 1,573 K in N2 atmosphere at a rate of 100 cm3 min−1. Then, decomposition of the sample was carried out at 873 K in a furnace for 4 h using a heating/cooling rate of 10 K min−1. Na2C2O4 and its thermal transformation products were investigated further. The phase formation was studied by room temperature X-ray diffraction (XRD, Advance D8), using Ni-filtered CuKα radiation. Sample scanning was done between the angles of 20–80°. Diffraction peaks were analyzed and indexed according to the diffracting planes of different phases. The morphology of samples was examined using a scanning electron microscope (SEM, Hitachi S4700) after gold coating.
Determination of the most probable mechanism function
Since the kinetic parameters depend strongly on the selection of a proper mechanism function for the process, the following equation was used to estimate the most correct reaction mechanism, i.e., g(α) function [6]:
where A (the pre-exponential factor/min−1) and E α (the activation energy/kJ mol−1) are the Arrhenius parameters, and R is the gas constant (8.314 J mol−1 K−1). For determination, the degrees of conversion α (extent of conversion, α = (m i –m t )/(m i –m f ), where m i , m f , and m t are the initial, final, and current sample mass, respectively, at moment t), corresponding to four heating rates (β = 15, 20, 30 and 40 K min−1) taken at the same temperature, were substituted into the left side of Eq. 1, which was combined with 35 types of mechanism functions [14–16]. Potting ln g(α) versus ln β and a linear regression of least square method were conducted. The most probable mechanism function was assumed to be the one for which the slope of the straight line was closest to −1.0000, and the linear correlation coefficient r 2 should be unity.
Calculation of activation energy by iso-conversional procedure
In the kinetic study, the first Ozawa equation [5] was used to calculate the values of the activation energy, E α, of the decomposition reaction of Na2C2O4, as follows:
and Kissinger–Akahira–Sunose (KAS) equation [6]:
The kinetics of such reactions is described by various equations, taking into account the special features of their mechanisms. Data from four TG curves in the decomposition range were used to determine α from experiments at different heating rates (β = 15, 20, 30 and 40 K min−1). The plots of ln β versus 1/T (Eq. 2) and ln (β/T2) versus 1/T (Eq. 3) have provided evidence of apparent activation energy values for decomposition at different values of α. The activation energy, E α, can be estimated from the slope of these plots. This is a model-free method according to the reaction mechanism, and the shape of g(α) function cannot affect this calculation, which was performed without use of the most probable mechanism function.
Calculation of the transition state thermodynamic function
The pre-exponential factor, A, can be estimated from the intercept of the plots from Ozawa (Eq. 2) and KAS (Eq. 3) through the insertion of the most probable function, g(α), and the calculated activation energy, E α. According to the theory of the activated complex (transition state) of Eyring [2, 3, 17], the general equation of A may be written as follows:
where e = 2.7183 is the Neper number; χ is the transition factor, which is unity for monomolecular reactions; k B is the Boltzmann constant; h is Plank’s constant; and T P is the average temperature of the TG curves at different heating rates. Then, the change of entropy may be calculated according to the formula:
Therefore, the changes of the enthalpy, ΔH*, and Gibbs free energy, ΔG*, for the activated complex formation from the reagent can be calculated using the well-known thermodynamical equation:
Results and discussion
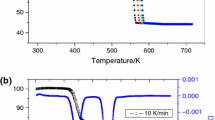
Thermogravimetry–differential thermal analysis curves of the thermal decomposition of Na2C2O4 at a heating rate of 30 K min−1 are illustrated in Fig. 1. The TG curve accordingly revealed a weight loss of ~21%, which occurred during the temperature rise from 800 to 870 K. This observation corresponded to the endothermic peak of the DTA and DTG curve, which centred at 848 and 844 K, respectively. This decomposition step may be related to the decomposition of Na2C2O4 to Na2CO3 and released CO because the overall weight loss of ~21% is close to the theoretical value of 20.9%, which corresponds to the release of 1 mol of CO. The decomposition reaction is suggested to be as the Eq. 8:
This decomposition temperature of the decarbonylation stage was higher than those found in the literature, which lie on the temperature of 773 K [18].
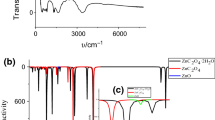
The XRD patterns of sodium oxalate (Na2C2O4) powder and its calcined product (at 873 K) are illustrated in Fig. 2. The diffraction pattern of Na2C2O4 powder could correspond to the monoclinic sodium oxalate (JCPDS file no. 49-1816 (▼), space group P21/a (14)). After calcination at 873 K for 4 h, the diffraction pattern suggests an appearance of the monoclinic, γ-Na2CO3 [JCPDS no. 72-0628, space group C2/m (12)], accompanied by the cubic NaO2 (JCPDS file no. 77-0207, space group Fm3m) as a minority phase. This result could be correlated to TGA-DTG/DTA analysis, which suggested that the decomposition of Na2C2O4 to Na2CO3 was in the region of this temperature.
The scanning electron micrographs of Na2C2O4 powder and its calcined product (at 873 K) are illustrated in Fig. 3a, b, respectively. Na2C2O4 powder was found to have uniform morphological features, with a polyhedral shape and obvious edges. The particle was in the range of micron size. On the contrary, the micrograph of the particle’s calcined product (at 873 K) consisted of non-uniform grain. The macropores and agglomeration, which could result from the thermal decomposition process, were found.
Figure 4 shows the TG, DTG, and DTA curves in the decomposition range of Na2C2O4, with four heating rates of 15, 20, 30, and 40 K min−1. Data of α and T collected from the TG curves in the decomposition range of 0.1 < α < 0.9 at various heating rates are illustrated in Table 1, and used to determine the kinetic parameters of the process in all calculation procedures. According to Eq. 3 combined with 35 conversion functions, ln g(α) calculated different α values at the same temperature, and four heating rates on ln β, must give rise to straight lines, so the slope and linear correlation coefficient, r 2, can be determined. Table 2 lists the results for all of the 35 types of mechanism functions. The slope determined from function no. 18 was found to be the closest to −1.0000 and the correlation coefficient, r 2, was better than others. This function was considered to be the most probable reaction mechanism for the description of Na2C2O4 decarbonylation. Therefore, it can be stated that the mechanism function for the decomposition of Na2C2O4 (splitting of carbon monoxide) is the mechanism of phase boundary reaction (cylindrical symmetry, R2 or F1/2 model) with integral form g(α) = 1 – (1 – α)1/2 and differential form f(α) = 2(1 – α)1/2.
Figure 5a, b illustrate the plots of ln β versus 1/T (Eq. 1) and ln (β/T 2) versus 1/T (Eq. 2) for the decomposition process of Na2C2O4, based on the Ozawa and KAS analysis, respectively. The activation energy, E α, of the decomposition reaction of Na2C2O4, which was calculated from the slope of these straight lines and their correlation coefficient, r 2, are tabulated in Table 3a (Ozawa method) and b (KAS method). The calculated activation energies obtained from different equations, in which the values obtained by the KAS method were generally higher, were found to be consistent. It can be seen that the values of E α tend to decrease with the increase of conversion α. It can be noted also that the E α values are dependent on α, and the decomposition reaction should be interpreted in terms of a multi-step reaction mechanism [19]. As the dependence can disclose the complexity of a process and identify its kinetic scheme, the shape of the decreasing dependence of E α on α has been identified from model data [20]. This study reported that the decomposition reaction was complicated by diffusion. This process is met widely in solids decomposed in the following way: solid → solid + gas [21]. In addition, values of the correlation coefficient, r 2, for all cases of calculation were greater than 0.9949. It can be seen that the values of E α obtained from the Ozawa and KAS methods (Eqs. 2, 3) are reliable.
On the basis of TG curves at four heating rates and using Eqs. 1–3, values of the apparent activation energy, E α, were calculated, and the most probable reaction mechanism function of the studied reaction was determined. Based on these results, the pre-exponential factor, A, can be estimated from intercept of the plots from the Ozawa (Eq. 2) and KAS (Eq. 3) methods. The related transition state thermodynamic functions (ΔS*, ΔH* and ΔG*) also can be calculated according to Eqs. 5–7. The corresponding values are shown in Table 3a (Ozawa method) and b (KAS method). As seen from Table 3a (Ozawa method) and b (KAS method), the change of the entropy, ΔS*, for the decomposition of Na2C2O4 is positive and can be described as corresponding activated complexes that have a lower degree of arrangement (higher entropy) than the initial state. Regarding the fundamentals of the activated complex theory (transition theory) [2, 3, 13, 14], a positive value of ΔS* indicates a malleable activated complex that leads to very many degrees of rotation and vibration freedom, which results in a “fast” stage reaction. On the other hand, a negative value of ΔS* suggests that the degree of structural complexity (arrangement, organization) of the activated complex is higher than that in the non activated complex, and may be indicated as “slow” stage [19, 22–25]. The positive value of the activation enthalpy, ΔH*, showed that the decomposition stage is connected to the introduction of heat, and agrees well with the endothermic peak in the DTA result. The high ΔH* value indicated that this decomposition step needs high energy. The positive value of Gibbs energy, ΔG*, suggested that this is a non-spontaneous process. These results indicated that the decomposition step of Na2C2O4 is a high energy pathway and revealed a very hard mechanism.
Conclusions
In conclusion, kinetic parameters of Na2C2O4 (decarbonylation reaction) decomposition can be determined on the basis of thermogravimetric data. The kinetics of thermal decomposition of Na2C2O4 under non-isothermal heating was studied using the Ozawa and KAS methods. The results obtained from these two different calculation procedures were found to correlate with each other. Values of the apparent activation energy and pre-exponential factor, and the change of entropy, enthalpy, and Gibbs free energy, the most probable mechanisms and characteristics of the process were reported. These could be the important data for further studies and synthesis of the materials involved.
References
Wendlandt WW. Thermal methods of analysis. New York: John Wiley & Sons Inc; 1974.
Šesták J. Thermodynamical properties of solids. Prague: Academia; 1984.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolysis. 2008;81:253–62.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Ozawa TA. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Liqing L, Donghua C. Application of iso-temperature method of multiple rate to kinetic analysis. J Therm Anal Calorim. 2004;78:283–93.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal Calorim. 1977;11:445–9.
L’vov BV. Kinetics and mechanism of thermal decomposition of nickel, manganese, silver, mercury and lead oxalates. Thermochim Acta. 2000;364:99–109.
Galwey AK, Brown ME. Thermal decomposition of ionic solids. Amsterdam: Elsevier; 1999.
Patra BS, Otta S, Bhattamisra SD. A kinetic and mechanistic study of thermal decomposition of strontium titanyl oxalate. Thermochim Acta. 2006;441:84–8.
Zhang JJ, Ren N, Bai JH. Non-isothermal decomposition reaction kinetics of the magnesium oxalate dihydrate. Chin J Chem. 2006;24:360–4.
Chaiyo N, Boonchom B, Vittayakorn N. Solid-state reaction synthesis of sodium niobate (NaNbO3) powder at low temperature. J Mater Sci. 2010;45:1443–7.
Budavari S, O’Neil M, Smith A, editors. The Merck index; An encyclopedia of chemicals, drugs and biologicals. 11th ed. Rahway: Merck Co. Inc.; 1989.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Heide K, Höland W, Gölker H, Seyfarth K, Müller B, Sauer R. Die bestimmung kinetischer parameter endothermer zersetzungsreaktionen unter nicht-isothermen bedingungen. Thermochim Acta. 1975;13:365–78.
Zhang JJ, Ge LG, Zha XL, Dai YJ, Chen HL, Mo LP. Thermal decomposition kinetics of the Zn(II) complex with Norfloxacin in static air atmosphere. J Therm Anal Calorim. 1999;58:269–78.
Bamford CH, Tipper CFH. Comprehensive chemical kinetics. Amsterdam: Elsevier; 1980.
McAlexander LH, Beck CM, Burdeniuc JJ, Crabtree RH. Fluoroalkane aromatization over hot sodium oxalate. J Fluorine Chem. 1999;99:67–72.
Boonchom B, Vittayakorn N. Dehydration behavior of synthetic Al0.5Fe0.5PO4·2.5H2O. J Chem Eng Data. 2010;55:3307–11.
Zhang K, Hong J, Cao G, Zhan D, Tao Y, Cong C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Thermochim Acta. 2005;437:145–9.
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. Int J Chem Kinet. 1996;28:95–101.
Vlaev LT, Gospodinov GG. Study on the kinetics of the isothermal decomposition of selenites from IIIB group of the periodic system. Thermochim Acta. 2001;370:15–9.
Boonchom B, Thongkam M. Kinetics and thermodynamics of the formation of MnFeP4O12. J Chem Eng Data. 2010;55:211–6.
Boonchom B, Kongtaweelert S. Study of kinetics and thermodynamics of the dehydration reaction of AlPO4∙H2O. J Therm Anal Calorim. 2010;99:531–8.
Boonchom B, Danvirutai C, Thongkam M. Non-isothermal decomposition kinetics of synthetic serrabrancaite (MnPO4∙H2O) precursor in N2 atmosphere. J Therm Anal Calorim. 2010;99:357–62.
Acknowledgements
This work was supported by the Thailand Research Fund (TRF), Thailand Graduate Institute of Science and Technology (TGIST), and the National Nanotechnology Center (NANOTEC) NSTDA, Ministry of Science and Technology, Thailand through its “Center of Excellence Network” Program.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaiyo, N., Muanghlua, R., Niemcharoen, S. et al. Non-isothermal kinetics of the thermal decomposition of sodium oxalate Na2C2O4 . J Therm Anal Calorim 107, 1023–1029 (2012). https://doi.org/10.1007/s10973-011-1675-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1675-6