Abstract
A modified solid-state reaction was applied to produce lead-free piezoelectric sodium niobate (NaNbO3) powders. The mixture of Na2C2O4 and Nb2O5 was identified by thermo gravimetric analysis (TGA) and differential thermal analysis (DTA). The powders were characterized using a scanning electron microscope (SEM) and the X-ray diffraction technique (XRD). The SEM image suggested that the particle size of the powders obtained ranged from 180 to 360 nm. The XRD pattern showed that the pure perovskite phase of NaNbO3 could be synthesized at the low temperature of 475 °C for 1 h, with an average crystallite size of 31.45 ± 5.28 nm. This temperature was about 300 °C lower than that when using the conventional solid-state method with Na2CO3 as reactant, which resulted in a cost-, energy-, and time-saving method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkaline niobate materials are considered a lead-free candidate for the substitution of widely used commercial lead-based piezoelectric material, based on the aim to avoid highly harmful lead compounds [1–4]. Among several compounds, sodium niobate (NaNbO3) has attracted considerable attention because of its unique properties [3] and high dielectric constant (2000–3000) at Curie temperature [5]. Unlike most oxidic perovskite, NaNbO3 possesses six phase transitions from the ferroelectric phase at low temperature (rhombohedral) to the antiferroelectric room temperature phase (orthorhombic) and non-polar cubic structure at 640 °C [6]. It can form solid solution with other niobate compounds, such as LiNbO3 and KNbO3, to acquire good ferroelectric and piezoelectric properties [7–10]. Traditionally, alkali metal niobate powders have been synthesized via the solid-state reaction of alkali metal carbonates and Nb2O5 [3, 11]. This method requires a high calcination temperature (about 750 °C or more) for a long period of time, possibly causing volatilization of the alkali metal and leading to poor compositional homogeneity [3, 4, 11]. The powders can be agglomerated during heating, which affects their properties [3, 12]. Thereafter, powders with high sinterability and stoichiometric control are necessary for developing NaNbO3-based piezoelectric ceramics. Numerous alternative methods are used to prepare NaNbO3 such as hydrothermal [13], chemical [12], and polymeric precursor processes [14], and the mechanochemical process [3, 15, 16]. Although NaNbO3 was performed by mechanochemical activation after thermal treatment of a stoichiometric Na2CO3/Nb2O5 mixture at 600 °C, this procedure required a long operational period of up to 30 days [3]. Moreover, NaNbO3 was also obtained at a low temperature (450 °C) by the wet-chemical method using a water-soluble malic acid complex [17]. However, most chemical techniques require specialized experimental apparatus and high purity reactant, which are more expensive. Interestingly, sodium tantalate (NaTaO3) powder, with high crystallinity has been successfully synthesized at 600 °C through a simple method called modified solid-state reaction or combustion synthesis, in which urea plays an important role. Unusual starting material (Na2C2O4 instead of Na2CO3/Na2O) has been described. This method was found to produce NaTaO3 as a general route at the lower temperature of ~500–600 °C, when compared with conventional solid-state reaction [18]. On the other hand, urea, which was added as fuel in order to achieve the final product, could cause problems in this method, due to risks if performing on a large scale. Nonetheless, the aim of the present study was to produce NaNbO3 using a simple, rapid, low cost, and environment friendly route, such as a solid-state reaction of Na2C2O4 and Nb2O5 without the addition of any fuel.
Experiment
NaNbO3 was synthesized by a solid-state reaction method. Reagent grade sodium oxalate (Na2C2O4, 99.9%) and niobium oxide (Nb2O5, 99.9%) were employed as raw material. Firstly, starting materials were weighed according to the required stoichiometric ratio that related to the reaction below.
Then, raw materials were mixed together by ball-milling in ethyl alcohol using partially stabilized zirconia balls for 18 h. After drying on a hot plate by regularly stirring at about 80 °C for an approximate period, the thermal behavior during heat treatment was determined by thermo gravimetric analysis (TGA, Perkin Elmer) and differential thermal analysis (DTA, Perkin Elmer). According to TG-DTA results, the mixture was subsequently placed in a closed alumina crucible and calcined for different periods of time in air at various temperatures, ranging from 300 to 600 °C, in order to investigate the formation of NaNbO3.
Afterward, calcined powders were subsequently inspected by room temperature X-ray diffraction (XRD, Advance D8), using Ni-filtered Cu Kα radiation, to examine the effect of thermal treatment on phase development and the optimal calcination condition for the formation of crystalline NaNbO3 powders. Powder morphologies and particle size were figured directly using a scanning electron microscope (SEM, LEO1455 VP).
Results and discussion
The TGA and DTA of a powder mixed in the stoichiometric proportions of NaNbO3 are illustrated in Fig. 1. The TG curve accordingly revealed a weight loss of 16.8%, occurring during the temperature rise from 400 to 500 °C. This observation corresponded to the endothermic peak of the DTA curve, which cantered at 484.8 °C. This endotherm may be related to the decomposition of Na2C2O4 to Na2CO3, which lies on the temperature of 450–550 °C, and abruptly to the decomposition of Na2CO3 to Na2O (decomposition temperature in the range of 400 °C) before releasing CO and CO2 molecules, as revealed below [19].
It is interesting to note that there was no weight loss or thermal effect at a temperature of about 100 °C, at which no decomposition occurrence was indicated. The endothermic peak correlates at the range of 100 °C with the release of water molecules. This confirmed that non-absorptive Na2C2O4 raw material contrasts with Na2CO3, because Na2CO3 is the hygroscopic compound which can lead to the erroneous stoichiometric ratio. Therefore, powders with good compositional homogeneity, when comparing with the conventional solid-state method with Na2CO3 as reactant, may possibly be produced via this solid-state reaction.
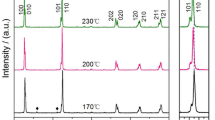
Thus, based on TG-DTA data, the powders were calcined at temperatures ranging from 300 to 600 °C for 4 h in order to investigate the calcination temperature outcome in the development phase. The mixture of starting material was calcined in air using the steady heating/cooling rate of 20 °C at various temperatures, and followed by phase analysis using the XRD technique. Figure 2 shows the XRD pattern of the NaNbO3 powders calcined at different temperatures for 4 h. It can be seen that fine NaNbO3 crystallites were developed at a calcination temperature as low as 400 °C, accompanied by the phase of unreacted Nb2O5 (JCPDS file no. 30-0873) and Na2C2O4 (JCPDS file no. 20-1149). This observation suggests that nucleation of the perovskite NaNbO3 phase did occur. In addition, the minor phase of Nb2O5 and Na2C2O4 was also decreased with escalating calcination temperature, and disappeared completely after the powders were calcined at the calcination temperature of 500 °C for 4 h. Whereas, the intensity of the perovskite NaNbO3 peak was enhanced further and an essentially monophasic NaNbO3 perovskite phase (yield of 100% within the limitations of the XRD technique) was observed. This NaNbO3 phase could be indexed according to an orthorhombic structure with the space group Pbma (no. 57), which was consistent with JCPDS file No. 33-1270. Although the calcination temperature rose at 550 and 600 °C, the monophasic NaNbO3 perovskite phase was also obtained. There was no evidence of the pyrochlore diffraction peak. This result also correlates with the TG-DTA analysis shown above. As the calcination temperature increased, so too did the amount of the NaNbO3 crystallite phase, and this can be seen as intensity of the amplified peak. Since the diffusion coefficient is a temperature dependence parameter, the higher temperature has the most intense effect on the rate of diffusion [20], and can enhance higher atomic mobility [11].
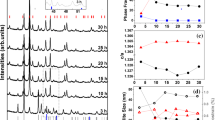
As the finest calcination temperature was established at 500 °C, a dwell time ranging from 15 min to 4 h was applied at 475 °C (instead of 500 °C). This temperature was preferred because of the rapid diffraction peak change of the powder calcined at 450 and 500 °C. The XRD pattern of the NaNbO3 powders, which were calcined at 475 °C with different dwell times, is shown in Fig. 3. It was found that the single-phase of NaNbO3 powder was also successfully synthesized at the calcination temperature of 475 °C, with a dwell time of 60 min or more applied. The increase in crystallinity of the NaNbO3 phase was seen to relate with the escalation of dwell time. Although the calcination temperature of 475 °C was higher than the nucleation temperature of the powder obtained by a polymeric precursor [14], this was a single-step and low-cost starting material method that could save time, energy, and cost. Likewise, this temperature was much lower than the conventional solid-state reaction process with Na2CO3 as reactant, which was in the range of 750 °C [3]. This observation indicated that calcination temperature and dwell time might play an important role in evolution of the pure phase product and also be consistent with other systems [21].
The volume fraction of the perovskite phase formation was considered at various calcination temperatures and dwell times. These relative amounts of perovskite, Na2C2O4 and Nb2O5 phases, were approximated by calculating the ratio of the main X-ray peak intensities of perovskite NaNbO3, Na2C2O4, and Nb2O5 phase using the following equation [22]:
where I perov , \( I_{{{\text{Na}}_{2} {\text{C}}_{2} {\text{O}}_{4} }} \), and \( I_{{{\text{Nb}}_{2} {\text{O}}_{5} }} \) stand for the intensities belonging to the strongest reflection peak of (002) perovskite, (111) Na2C2O4, and (180) Nb2O5, respectively. A volume fraction increase of the perovskite NaNbO3 phase formation of the calcined powders, resulting from the calcinations process at various temperatures and dwell times, is shown in Table 1. As the calcination temperature rose, the yield of the perovskite phase increased significantly until the temperature reached 500 °C, and a pure phase of NaNbO3 was established. Likewise, in observing powders calcined at 475 °C for different dwell times, the NaNbO3 phase was enlarged as the dwell time increased up to 60 min, and the monophasic phase of NaNbO3 was seen to form.
Accordingly, the Johnson–Mehl–Avrami, or JMA equation was used in the present study. This equation was found appropriate for describing a wide variety of isothermal solid-state transformations [23, 24]. It was used to study the kinetic of the reaction and mechanism involving nucleation and growth, and has the general form of:
where x is the volume fraction of the perovskite phase formed, k the reaction rate constant, t the calcination time, and n the Avrami exponent. For analyzing the results, the relation of ln [ln 1/(1 − x)] versus ln t was plotted. Figure 4 shows a good linear fit of the Avrami plot for NaNbO3 powders calcined at 475 °C. This shows that the isothermal formation of the perovskite phase can be described accurately by the theory of phase transformations. The constant n, which can be calculated from the slope of this Avrami plot, was found to be 1.79. This indicated that the reaction of solid solution formation is diffusion controlled (n is less than 2.5) [25]. The beginning stage of transformation is a fixed number of perovskite nuclei [26].
The average crystallite size of the powders obtained can be determined from the XRD pattern according to Scherrer’s equation [27]:
where D is the average crystallite size, k a constant equal to 0.89, λ the wavelength of X-ray radiation, β the full width at half maximum (FWHM), and θ B the diffraction angle. The average crystallite size of powders calcined at 475 °C for 15 min to 4 h was found to be from 21.52 to 35.56 nm, and that of powders calcined at the optimum condition (475 °C for 60 min) was about 31.45 nm. The increase in crystallinity of the NaNbO3 phase was affected by increasing dwell time. This may confirm that the dwell time also plays an important role in development of the pure phase creation.
SEM micrographs of the powder calcined at 475 °C for 60 min are given in Fig. 5. The particle size, which can be estimated from these micrographs, was found to be in the range of 180 to 360 nm. This value is greater than the average crystallite size calculated from XRD patterns. The inconsistency value could point out the agglomeration of the calcined powders. No evidence of difference phase or pyrochlore phase was found. This outcome relates to the XRD result, in which the monophasic perovskite phase of NaNbO3 can be established after calcination at 475 °C for 60 min.
Conclusion
Crystalline powders of sodium niobate NaNbO3 were synthesized from a modified solid-state reaction of Na2C2O4 and Nb2O5. This method is an excellent, simple and cost effective way to prepare stoichiometric, homogeneous, and fine powders. The perovskite phase of NaNbO3 was successfully synthesized at the low temperature of 475 °C for 1 h, with an average crystallite size of 31.45 ± 5.28 nm. This temperature is about 275 °C lower than that used in the conventional method, which lies in the 750 °C range. As dwell time increased, XRD peaks became narrower, and a pattern similar to that expected for orthorhombic NaNbO3 was achieved, as indicated by the separate peaks. The resulting NaNbO3 powders comprised agglomerated particles of 180 to 360 nm in size.
References
Matsubara M, Yamaguchi T, Sakamoto W, Kikuta K, Yogo T, Hirano S-I (2005) J Am Chem Soc 88:1190
Zhaoa P, Zhanga B-P, Lib J-F (2008) Scripta Mater 58:429
Hungría T, Pardo L, Moure A, Castro A (2005) J Alloys Compd 395:166
Ke T-Y, Chen H-A, Sheu H-S, Yeh J-W, Lin H-N, Lee C-Y, Chiu H-T (2008) J Phys Chem C 112:8827
Jona F, Shirane G (1993) Ferroelectric crystals. Dover Publications, New York
Wang X-B, Shen Z, Hu Z-P, Qin L, Tang SH, Kuok MH (1996) J Mol Struct 385:1
Du H, Zhou W, Luo F, Zhu D, Qu S, Li Y, Pei Z (2008) J Appl Phys 104:034104-7
Chang Y, Yang Z-P, Ma D, Liu Z, Wang Z (2008) J Appl Phys 104:024109-8
Wu J, Xiao D, Wang Y, Wu W, Zhang B, Zhu J (2008) J Appl Phys 104:024102-4
Lei C, Ye Z-G (2008) Appl Phys Lett 93:042901-3
Hsiao Y-J, Chang Y-H, Chang Y-S, Fang T-H, Chai Y-L, Chen G-J, Huang T-W (2007) Mater Sci Eng B 136:129
Lanfredi S, Dessemond L, Rodrigue ACM (2000) J Eur Ceram Soc 20:983
Goh GKL, Lange FF, Haile SM, Levi CG (2003) J Mater Res 18:338
de Lima Nobre MA, Longo E, Leite ER, Varela JA (1996) Mater Lett 28:215
Moure A, Hungría T, Castro A, Pardo L (2009) J Eur Ceram Soc 29:2297
Rojac T, Kosec M, Malic B, Holc J (2005) Mater Res Bull 40:341
Camargo ER, Popa M, Kakihana M (2002) Chem Mater 14:2365
Xu J, Xue D, Yan C (2005) Mater Lett 59:2920
McAlexander LH, Beck CM, Burdeniuc JJ, Crabtree RH (1999) J Fluorine Chem 99:67
Callister WD (2007) Materials science and engineering: an introduction. Wiley, New York
Chaiyo N, Vittayakorn N (2008) J Ceram Process Res 9:381
Feng G, Rongzi H, Jiaji L, Zhen L, Lihong C, Changsheng T (2009) J Eur Ceram Soc 29:1687
Sheibani S, Ataie A, Heshmati-Manesh S (2008) J Alloys Compd 455:447
Yan Z-J, Dang S-E, Wang X-H, Lian P-X (2008) Trans Nonferrous Met Soc China 18:138
Jang HM, Cho SR, Lee K-M (1995) J Am Ceram Soc 78:297
Shen Y, Hng HH, Oh JT (2004) Mater Lett 58:2824
Klug HP, Alexander LE (1974) X-ray diffraction procedure of polycrystalline and amorphous materials. Wiley, New York
Acknowledgements
This work was supported by the Thailand Research Fund (TRF), Thailand Graduate Institute of Science and Technology (TGIST), National Research Council of Thailand (NRCT), and the National Nanotechnology Center (NANOTEC) NSTDA, Ministry of Science and Technology, Thailand through its “Center of Excellence Network” Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiyo, N., Boonchom, B. & Vittayakorn, N. Solid-state reaction synthesis of sodium niobate (NaNbO3) powder at low temperature. J Mater Sci 45, 1443–1447 (2010). https://doi.org/10.1007/s10853-009-4098-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-4098-z