Abstract
The thermal decomposition of synthetic serrabrancaite (MnPO4 · H2O) was studied in N2 atmosphere using TG-DTG-DTA. Thermal analysis results indicate that the decomposition occurs in two stages, which are assigned to the dehydration and the reduction processes and the final product is Mn2P2O7. X-ray powder diffraction, FT-IR and FT-Raman techniques were used for identification of the solid decomposition product. The decomposition kinetics analysis of MnPO4 · H2O was performed under non-isothermal condition through isoconversional methods of Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS). The dependences of activation energies on the extent of conversions are observed in the dehydration and the reduction reactions, which could be concluded the “multi-step” processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese phosphates are of considerable industrial interesting properties nowadays because of their wide applications in laser host [1], ceramic [2], dielectric [3], electric [4], magnetic [5], and catalytic [6] processes. Additionally, manganese phosphates are among the widest spread minerals in the class of phosphates [7, 8] such as MnP3O9 [9], MnPO4 · H2O [10] MnPO4 [11], MnHP2O7 [12] and Mn(PO3)3 [13], MnP4O11 [14], Mn2P2O7 [15], β-Mn3(PO4)2 [16] and Mn2P2O7 · 2H2O [17], etc.
Mn2P2O7 has been found to have a wide range of applications such as catalyst and battery cell [6, 7, 15, 17]. In this respect, in the part few years many works have been undertaken a series of research studies on different preparative methods such as the thermal decomposition of Mn2P2O7 · 2H2O, Mn(H2PO2)2 · 2H2O and MnHPO4 · 3H2O precursors [7, 17]. As part of this effort, we are currently undertaking a detailed thermal decomposition kinetics of MnPO4 · H2O, which undergoes dehydration and reduction reactions [18–20]. This material provides synthetic challenges, as Mn3+ is prone to oxidation or reduction when heated or in aqueous solution. In many methods of kinetics estimation, isoconversional method is recommended as trustworthy way of obtaining reliable and consistent kinetic information [21, 22]. It is a ‘model-free method’, which involves measuring the temperatures corresponding to fixed values of the extent of conversion (α) from experiments at different heating rates (β). In the present study, the formation of Mn2P2O7 from MnPO4 · H2O was followed using differential thermal analysis-thermogravimetry (TG-DTG-DTA), X-ray powder diffraction (XRD), Fourier transform-infrared (FT-IR) and Fourier transform-Raman (FT-Raman) techniques. The kinetics analysis of the non-isothermal results for dehydration and reduction steps of MnPO4 · H2O in was carried out using the isoconversional methods of Flynn–Wall–Ozawa (FWO) [23] and Kissinger–Akahira–Sunose (KAS) [24] and was reported for the first time.
Experimental procedures
Synthetic Serrabancaite, MnPO4 · H2O compound was prepared by solution precipitation method and characterized as previously described by the same authors [25]. Thermal analysis measurements (thermogravimetry, TG; differential thermogravimetry, DTG; and differential thermal analysis, DTA) were carried out by a Pyris Diamond Perkin Elmer apparatus by increasing temperature from 373 to 1,073 K with calcined α-Al2O3 powder as the standard reference. The experiments were performed in dynamic air at heating rates of 5, 10, 15, and 20 K min−1. The sample mass was kept of about 8.0 ± 0.3 mg in an alumina pan without pressing. In accordance with thermal analysis results, the synthesized sample was thermally heated in a furnace until the completely dehydrated Mn2P2O7 (pale pink) was obtained. The X-ray powder diffraction patterns of the prepared product and the calcined sample were obtained with a Bruker D8-Advanced model X-ray powder diffractometer with Cu Kα radiation (λ = 0.15406 Å). The room temperature FTIR spectrum of Mn2P2O7 was recorded in the range of 4,000–370 cm−1 with eight scans on a Perkin-Elmer Spectrum GX FT-IR/FT-Raman spectrometer with the resolution of 4 cm−1 using KBr pellets (KBr, Jasco, spectroscopy grade). The FT-Raman spectrum of Mn2P2O7 was recorded with spectrum 2000R NIR FT-Raman system in a Perkin-Elmer Spectrum GX model equipped with a HeNe laser (1,064 nm). The laser power about 500 mW was used for excitation in the range between 4,000 and 100 cm−1 with 32 scans.
Result and discussion
The TG curves of MnPO4 · H2O at four heating rates (5, 10, 15, and 20 K min−1) are shown in Fig. 1a. All curves are approximately in the same shape and indicated that the mass loss is independent of the heating rate. All curves showed that two thermal decomposition steps of MnPO4 · H2O below 873 K All peaks in the DTG and DTA curves (Fig. 1b) closely correspond to the mass loss observed on the TG traces. Additionally, two decomposition stages were shifted toward higher temperatures when the heating rates increase. The mass loss of the first step (at about 482–617 K) at all four heating rates is between 2.43 and 2.56%, which is close to the mass loss of the release of 0.2–0.3 water molecules [26]. This can be ascribed to the dehydration of MnPO4 · H2O due to the water of crystallization.
The second mass loss step of MnPO4 · H2O relates with the consequent release of oxygen and a water molecule, which is referred the reduction reaction (Mn(III) to Mn(II)) [19, 20]. The mass loss of this step (at about 618–833 K) is about 13.02%, which contains 5.15% of O2 and 7.87% of H2O. The mass loss is accompanied by a change of colour from gray–green to pale pink. The DTG and DTA curves at heating rates of 10 K min−1 are shown five peaks at about 481, 505, 551, 765, and 1,001 K and four endothermic effects at about 483, 511, 558, and 765 K, respectively. The synthetic MnPO4 · H2O is a very similar mass loss and transformation reaction, which are also described by Lightfoot et al. [10]. The overall reaction involves the dehydration and reduction processes as shown in Eqs. 1 and 2:
(1 < n < 1.3)
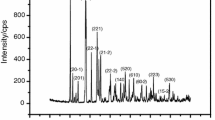
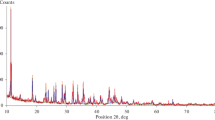
The XRD patterns of synthetic MnPO4 · nH2O and its decomposition product (Mn2P2O7) are shown in Fig. 2. The standard XRD patterns of MnPO4 · H2O (PDF #440071) and Mn2P2O7 (PDF#771243) indicate that both crystal structures are monoclinic system with space group C2/c (Z = 4) and C2/m (Z = 2), respectively. The XRD data has been reported in our previously work [25].
The formation of nanocrystalline Mn2P2O7 sample is further supported by FTIR and FT-Raman spectra in the range of 4,000–370 amd 4,000–100 cm−1, respectively (Fig. 3 a, b). The FTIR and FT-Raman bands are identified in term of the fundamental vibrating units namely P2O7 4− anion, which is very similar to this observed in the literatures [27–29]. The observed bands at 3,424 and 1,633 cm−1 of the FTIR spectra are assigned to the asymmetric stretching (ν3) and bending mode (ν2) of absorbed moisture of KBr pellet. The P–O stretching modes of the [P2O7]4+ anion are known to appear in the 1,250–975 cm−1 region [27, 28]. The symmetric PO2 stretching vibrations (νsym PO2) for the Mn2P2O7 sample are observed in the rage of 1,000–1,100 cm−1, while the asymmetric stretching vibrations (νasym PO2) are located at 1,100 and 1,200 cm−1. The asymmetric (νasym POP) and symmetric stretch (νsym POP) bridge vibrations for this samples are observed in 900–1,000 and 400–700 cm−1, respectively. The PO3 deformation, rocking modes, the POP deformations, the torsional and external modes are found in the 400–230 cm−1 region [29].
Decomposition of crystal hydrates is a solid-state process of the type [30]: A (solid) → B (solid) + C (gas). The kinetics of such reactions is described by various equations taking into account the special features of their mechanisms. This is a model-free method, which involves measuring the temperatures corresponding to fixed values of α from experiments at different heating rates (β). The activation energies (E α) can be calculated according to the isoconventional methods. In kinetic study of MnPO4 · H2O, FWO [23] and KAS [24] equations were used to determine the activation energy of the dehydration reaction.
The equations used for E α calculation are:
Flynn–Wall–Ozawa equation:
KAS equation:
where A (the pre-exponential factor) and E (the activation energy) are the Arrhenius parameters and R is the gas constant (8.314 J mol−1 K−1). The Arrhenius parameters, together with the reaction model, are sometimes called the kinetic triplet. \( g(\alpha ) = \int\limits_{0}^{\alpha } {{\frac{d\alpha }{f(\alpha )}}} \) is the integral form of f(α), which is the reaction model that depends on the reaction mechanism.
At the constant condition of others parameters, the TG curves for dehydration and reduction process of MnPO4 · H2O in N2 atmosphere at various heating rates (5, 10, 15 and 20 °C min−1) are shown in Fig. 1a. According to isoconversional method, the basic data of α and T collected from Fig. 1a are illustrated in Tables 1 and 2, respectively. According to the above-mentioned equations, the plots of ln β versus 1,000 T −1 (FWO) and ln β/T −2 versus 1,000 T −1 (KAS) corresponding to different conversions α can be obtained by a linear regression of least-square method. The activation energies E α can be calculated from the slopes of straight lines with better linear correlation coefficient (r 2). The slopes change depending on the degree of conversion (α) for the dehydration and reduction reaction of MnPO4 · H2O. The α-dependences of the apparent E α values for the dehydration and reduction reactions of MnPO4 · H2O calculated from the slopes of Eqs. 3 and 4 at various α are shown in Tables 3 and 4, respectively. If E α varies with α, the results should be interpreted in terms of multi-step reaction mechanism [21, 22, 30–36]. Unfortunately there is rather all the information available. Considering different conversion functions f(α), a series of values for the pre-exponential factor should be obtained, but this is rather a mathematical exercise but not a kinetic analysis [31–33].
The activation energy values of dehydration process of MnPO4 · H2O were found between 217.53 and 844.12 kJ mol−1 by using FWO and KAS methods, which are also higher than those of the dehydration reactions of some phosphate hydrates (FePO4 · H2O, AlPO4 · H2O and Mn(H2PO4)2 · 2H2O). The activation energy values increase largely in the increasing α (0.2–0.8) (Table 3), so we draw a conclusion that the dehydration of MnPO4 · H2O may be a multi-step reaction, which is different from the single step in some metal phosphates above [22, 37–41].
The activation energy values of reduction step were calculated between 1919.67 and 5687.03 kJ mol−1 by using FWO method and close to the calculated by the KAS method but higher than the other reactions [30–33]. It is clear in Table 4, that the activation energy assumes a value of 1919.67 kJ mol−1 at the beginning of the decomposition reaction and, with increasing mass loss, ups to a value of 5418.94 kJ mol−1 (FWO) method). This increasing dependence of Ε α on α indicates that the overall reaction contains a least two steps, and the kinetics scheme of which corresponds top a reversible reaction followed by an irreversible one [33–35]. The larger activation energy at the beginning of the reaction may be due to the additional destruction of crystal structure, which is related to reduction of Mn3+ to Mn2+ and then the consequent release of oxygen while the smaller activation energy at the end of the reaction due to the weight loss of about one water molecule[18]. The reduction step (2nd) exhibits higher activation energy in comparison with the dehydration step (1st), and this is understandable because this step corresponds to a reduction of Mn3+ to Mn2, in connection with polycondensation reaction [10, 18–20]. This result confirmed that the decomposition product (Mn2P2O7) was obtained. The activation energies calculated in N2 atmosphere for two decomposition of MnPO4 · H2O is higher than those reported previously for the decomposition of MnHPO4 · H2O, FePO4 · 3H2O, Mn(H2PO4)2 · 2H2O under air atmosphere [37–41]. However, the activation energies of the two decomposition reactions of the studied compound are close to those observed in the decomposition of Mn(H2PO2)2 · 2H2O [42] under N2 atmosphere. The activation energies and the multi-step processes of two reactions for the studied compound may be its characteristic; the strong effect of water of crystallization within this structure and metal reduction process before polycondensation reaction. Additionally, these results indicate that the atmosphere for thermal transformation of MnPO4 · H2O have the strong effect on the activation energies. In this respect, these data will be important for further studies of the studied compounds, which include studies under carefully controlled reaction conditions.
Conclusion
The synthetic serrabrancaite (MnPO4 · H2O) decomposes in two steps, which undergoes dehydration and reduction reactions. The dehydration is the elimination of a little amount water, while the reduction step is due to the reduction of Mn3+ to Mn2+ and the consequent release of oxygen and water molecules, respectively. For the dehydration and reduction reactions of synthetic MnPO4 · H2O, the activation energy values calculated by using FWO method are close to those by KAS method, and the respective correlation coefficients are preference, which were reported for the first time. On the basis of correctly established values of the apparent activation energy, certain conclusions can be made concerning the mechanisms and characteristics of the processes. The data of kinetics play an important role in theoretical study, application development and industrial production of a compound as a basis of theoretical. Additionally, various scientific and practical problems involving the participation of solid phases can be solved.
References
Jouini A, Gâcon JC, Ferid M, Trabelsi-Ayadi M. Luminescence and scintillation properties of praseodymium poly and diphosphates. Opt Mater. 2003;24:175–80.
Kitsugi T, Yamamuro T, Nakamura T, Oka M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials. 1995;16:1101–7.
Jian-Jiang B, Dong-Wan K, Kug Sun H. Microwave dielectric properties of Ca2P2O7. J Eur Ceram Soc. 2003;23:2589–92.
Martinelli JR, Sene FF, Gomes L. Synthesis and properties of niobium barium phosphate glasses. J Non-Cryst Solids. 2000;263:299.
Carmen P, Josefina P, Regino SP, Caridad RV, Natalia S. Crystal growth, structure, and magnetic properties of a new polymorph of Fe2P2O7. Chem Mater. 2003;15:3347–51.
Marcu I-C, Sandulescu I, Yves Schuurman Y, Millet J-MM. Mechanism of n-butane oxidative dehydrogenation over tetravalent pyrophosphates catalysts. Appl Catal A. 2008;334;207–16.
Boyle FW Jr, Lindsay WL. Diffraction patterns and solubility products of several divalent manganese phosphate. Soil Sci Soc Am J. 1985;49:761–6.
Boyle FW Jr, Lindsay WL. Manganese phosphate equilibrium relationships in soils. Soil Sci Soc Am J. 1986;50:588–93.
Bagieu-Beucher M. Structure cristalline du polyphosphate de manganèse trivalent Mn(PO3)3. Acta Crystallogr. 1978;B34:1443–6.
Lightfoot P, Cheetham AK, Sleight AW. Structure of manganese(3+) phosphate monohydrate by synchrotron X-ray powder diffraction. Inorg Chem. 1987;26:3544–7.
Nietubyć R, Sobzak E, Attenkofeer KE. X-ray absorption fine structure study of manganese compounds. J Alloys Compd. 2001;328:126–31.
Durif A, Averbuch-Pouchot MT. Structure du diphosphate acide de manganèse(III): MnHP2O7. Acta Crystallogr. 1982;B38:2883–5.
Massa W, Yakubovich OV, Dimitrova OV. A novel modification of manganese orthophosphate Mn3(PO4)2. Solid State Sci. 2005;7:950–6.
Olbertz A, Stachel D, Svoboda I, Fuess H. Redetermination of the crystal structures of nickel cyclotetraphosphate, Ni2P4O12 and of cobalt cyclotetraphosphate, Co2P4O12. Z Kristallogr. 1995;210:241–2.
Stefanidis T, Nord AG. Structure studies of thortveitite-like dimanganese diphosphate, Mn2P2O7. Acta Crystallogr. 1984;C40:1995–9.
El-Bali B, Boukhari A, Glaum R, Gerk M, Maaß K. Contributions on Crystal Structures and Thermal Behaviour of Anhydrous Phosphates. XXIX Preparation and Structure Determination of SrMn2(PO4)2 and Redetermination of SrMn3(PO4)2. Z Anorg Allg Chem. 2000;626:2557–62.
Schneider S, Collin RL. Crystal structure of manganese pyrophosphate dihydrate Mn2P2O7·2H2O. Inorg Chem. 1973;12:2136–9.
Aranda MAG, Attfield JP, Bruque S. Study of manganese phosphate or arsenate hydrates, MnXO4·nH2O (X = P, As), phases and synthesis and structure of the simple, novel salt MnAsO4. Inorg Chem. 1993;32:1925–30.
Aranda MAG, Bruque S. Characterization of manganese(III) orthophosphate hydrate. Inorg Chem. 1990;29:1334–7.
Aranda MAG, Bruque S, Attfield JP. Crystal structures and characterization of a new manganese(III) arsenate, MnAsO4.1·2H2O and manganese(II) pyroarsenate, Mn2As2O7. Inorg Chem. 1991;30:2043–7.
Stojakovic D, Rajic N, Sajic S, Logar NZ, Kaucic V. A kinetic study of the thermal degradation of 3-methylaminopropylamine inside AlPO4-21. J Therm Anal Calorim. 2007;87:337–43.
Boonchom B, Youngme S, Srithanratana T, Danvirutai C. Synthesis of AlPO4 and kinetics of thermal decomposition of AlPO4·H2O-H4 precursor. J Therm Anal Calorim. 2007;91:511–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction Kinetics in Differential Thermal Analysis. J Anal Chem. 1957;29:1702–6.
Boonchom B, Youngme S, Maensiri S, Danvirutai C. Nanocrystalline serrabrancaite (MnPO4·H2O) prepared by a simple precipitation route at low temperature. J Alloys Compd. 2006;454:78–82.
Witzke T, Wegner R, Doering T, PÖllmann H, Schuckmann W. Serrabrancaite, MnPO4·H2O, a new mineral from the Alto Serra Branca pegmatite, Pedra Lavrada, Paraiba, Brazil. Amer Mineral. 2000;85:847–9.
Baril M, Assaaoudi H, Butler IS. Pressure-tuning Raman microspectroscopic study of cobalt(II), manganese(II), zinc(II) and magnesium(II) yrophosphate dihydrates. J Mol Struc. 2005;751:168–71.
Harcharras M, Ennaciri A, Rulmont A, Gilbert B. Vibrational spectra and structures of double diphosphates M2CdP2O7 (M = Li, Na, K, Rb, Cs). Spectrochim Acta. 1997;A53:345–52.
Brouzi K, Ennaciri A, Harcharras M, Capitelli F. Structure and vibrational spectra of a new trihydrate diphosphate, MnNH4NaP2O7·3H2O. J Raman Spectrosc. 2004;35:41–6.
Vlaev LT, Nikolova MM, Gospodinov GG. Non-isothermal kinetics of dehydration of some selenite hexahydrates. J Solid State Chem. 2004;177:2663–9.
Vlase T, Vlase G, Brita N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Calorim. 2007;88:805–12.
Budrugeac P. The Kissinger law and the IKP method for evaluating the non-isothermal kinetic parameters. J Therm Anal Calorim. 2007;89:143–51.
Singh BK, Sharma RK, Garg BS. Kinetics and molecular modeling of biologically active glutathione complexes with lead(II) ions. J Therm Anal Calorim. 2006;84:593–600.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Pyrolysis. 2008;81:253–62.
Zhang K, Hong J, Cao G, Zhan D, Tao Y, Cong C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Thermochim Acta. 2005;437:145–9.
Boonchom B, Maensiri S, Danvirutai C. Soft solution synthesis, non-isothermal decomposition kinetics and characterization of manganese dihydrogen phosphate dihydrate Mn(H2PO4)2·2H2O and its thermal transformation products. Mater Chem Phys. 2008;109:404–10.
Boonchom B. Kinetics and thermodynamic properties of the thermal decomposition of manganese dihydrogenphosphate dihydrate. J Chem Eng Data. 2008;53:1553–8.
Boonchom B, Danvirutai C. A simple synthesis and thermal decomposition kinetics of MnHPO4·3H2O rod-like microparticles obtained by spontaneous precipitation route. J Optoelectron Adv Mater. 2008;10:492–9.
Boonchom B, Danvirutai C. Thermal decomposition kinetics of FePO4·3H2O precursor to synthetize spherical nanoparticles FePO4. Ind Eng Chem Res. 2007;46:9071–6.
Boonchom B, Danvirutai C. Synthesis of MnNiP2O7 and nonisothermal decomposition kinetics of a new binary Mn0.5Ni0.5HPO4·H2O precursor obtained from a rapid coprecipitation at ambient temperature. Ind Eng Chem Res. 2008;47:5976–81.
Noisong P, Danvirutai C, Srithanratana T, Boonchom B. Synthesis, characterization and non-isothermal decomposition kinetics of manganese hypophosphite monohydrate. Solid State Sci. 2008;10:1598–604.
Acknowledgments
The authors would like to thank the Chemistry Department, Khon Kaen University for providing research facilities. This work is financially supported by the Thailand Research Fund (TRF) and the Commission on Higher Education (CHE): Research Grant for New Scholar (MRG5280073), Ministry of Science and Technology, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonchom, B., Danvirutai, C. & Thongkam, M. Non-isothermal decomposition kinetics of synthetic serrabrancaite (MnPO4 · H2O) precursor in N2 atmosphere. J Therm Anal Calorim 99, 357–362 (2010). https://doi.org/10.1007/s10973-009-0096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0096-2