Abstract
Samples of flexible PU foam were prepared from a polyol (Elastoflex W 5516/115) and an isocyanate prepolymer (Iso 145/8), both commercial Elastogram products. For the thermooxidative stabilization, two phenolic compounds were used, separately or in mixture. These compounds were: 2,6-di-t-butyl-4-methyl-phenol (non-reactive) (AO-1), 3,5-di-t-butyl-4-hydroxy-benzyl alcohol (reactive, AO-2), used in total mass% of 0.3/1.5. The TG/DTG/DTA curves were drawn up in dynamic air, with a heating rate of 10 °C min−1, until 500 °C. For the unstabilized sample a single thermodegradative TG step, with a maximum rate at 268–270 °C was observed, whereas for the stabilized samples, supplementary steps at higher temperature were observed. The changes in the TG/DTG/DTA parameters are not in a single relationship with the mass% of the stabilizator, due to the following: AO-1 is easily migrated out from PV, especially, at higher concentrations. AO-2 had positive effect at all studied concentrations. But the most remarkable effect is the synergetic effect of a 1:1 mixture of AO-1 and AO-2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polyurethanes have a special place among the industrial polymers, due to a large variety of materials. So, flexible foams with very good physico-mechanical properties can be obtained from aromatic isocyanates and polyetheric polyols. But a major disadvantage of these polymers is a rapid degradation due to self-oxidation [1–3]. The example of methyliden-diphenyl-diisocyanate (MDI) is significant: 
Therefore, the antioxidants are compulsory additives in each polyurethane composition. There are two types: reactive (which are inserted in the polymeric chain during the synthesis) and non-reactive antioxidants. The last one has the advantage of no influence on the designed properties of the polyurethane but also the major disadvantage of migration to the polymer surface due to the huge difference of the molecular mass.
In some recent paper [4–6] the kinetic analysis of thermoanalytical data (TG and/or DSC) prove its utility in estimation of the antioxidant efficiency.
The aim of the present paper is to perform a study on the thermal behaviour of an MDI type polyurethane stabilized with reactive and/or non-reactive additives. The effect of the concentration of added stabilizator, as well as a possible synergism by using a mixture of reactive/non-reactive stabilizators were the main objectives.
Experimental
Polyurethane synthesis
The raw materials were:
-
MDI isocyanate prepolymer (ISO 145/8 from Elastogran, Hungary)
-
Polyesteric polyol (Elastoflex W 5516/115 from Elastogran, Hungary
-
Catalyst 1,4-diazabiciclo [2,2,2] octan (Dabco from Air Products and Chemicals, USA)
-
Thermooxidative stabilizators: a non-reactive type 2,6-di-t-butyl-4-methyl-phenol (AO-1) and a reactive type 3,5-di-t-butyl-4-hydroxy benzyl alcohol (AO-2), both from Fluka, Switzerland. The structural formulas are presented in Scheme 1.
The reaction components were added, under intense stirring, into a polyethylene vessel, and the reaction mass was allowed to expand for 90 min. In all samples the volume ratio of isocyanate to polyol was ½, with 0.03 mass% of catalyst. The stabilizator content of the prepared samples is presented in Table 1.
Thermal analysis
The TG/DTG/DTA curves were drawn up on a Perkin Elmer DIAMOND devices, in dynamic air atmosphere (100 mL min−1), with a heating rate of 10 °C min−1 until 500 °C.
The evolved gas analysis (EGA) was carried out by a coupled TG/FT-IR technique, using a Perkin Elmer SPECTRUM 100 devices with an IR gas chamber connected to the exit of the DIAMOND furnace. The same air flow of 100 mL min−1 and a heating rate of 20 °C min−1 were used. The FT-IR spectra were processed by the Sadtler Gas Vapor Library. The thermal degradation was discussed on the basis of these data and the FT-IR-UATR spectra of the initial samples.
Results and discussions
In Fig. 1 some typical thermoanalytical curves are presented.
In all samples, the thermodegradation due to an exothermic process (thermooxidation) and the maximum of the reaction rate corresponds to the maximum of the evolved reaction heat. In comparison with the unstabilized polymer, all the other samples present a more complicated thermodegradation process, first of all observed by the appearance of two or more DTG/DTA maximums.
A better estimation of stabilizator’s effect was possible on a comparative mass loss versus temperature diagram. For example, in Fig. 2 such diagrams for a total stabilizator concentration of 0.6 mass% are presented.
Some observations are noticeable:
-
(i)
By all sample the degradation began near 250 °C
-
(ii)
The differences in thermal stability can be estimated by:
-
the mass loss at a certain temperature: the smaller the mass loss is, the more stable the sample is; according to Fig. 2, the mass loss was in the following order:
$$ {\text{MS}} > 3{\text{S}} \approx 2{\text{S}} > 4{\text{S}} $$(1) -
the slope at a certain temperature \( \partial m/\partial T \)is a measure of the thermo-degradation rate: also the smaller it is, the more stable the sample is; the order is as follows:
$$ {\text{MS}} > 3{\text{S}} \approx 2{\text{S}} > 4{\text{S}} $$(2)
-
-
(iii)
Both Eqs. 1 and 2 indicate the MS as the less stable sample and 4S the most stable one. By comparison with 2S and 3S, it is obvious that a synergism occurs by using a mixture of reactive and non-reactive stabilizator. The effect of concentration will be discussed. According to Fig. 3, the non-reactive stabilizer had a positive effect only at concentrations lower than 0.6%, especially in the range of 250 to 300 °C. At higher temperatures, even if the concentration is higher, these are favourable conditions for stabilizator migration out from the polymeric mass.
In the reactive stabilizator a different behaviour is observed. According to Fig. 4, AO-2 had a positive effect at all studied concentrations. The concentration dependence of this effect is rather insignificant. However the best behaviour in the range of 250 to 320 °C was obtained at concentrations of 1.2–1.5 mass%.
The thermal behaviour of the polyurethane stabilized with a 1/1 mixture of AO-1 and AO-2 proved the synergism, especially at low concentrations (see Fig. 5). Probably the decreasing of the stabilization effect at higher concentrations is in connection with the mentioned tendency of AO-1 for migration out from the polymeric mass.
Spectroscopic analysis
EGA
The FT-IR spectra (see Fig. 6 and Table 2) confirmed that more than 80% of the evolved gases are the same by the non-stabilized as well as by the stabilized samples. This is an argument that the stabilizators are included in the polymer structure playing the role of stabilization and not of chain extender.
UATR
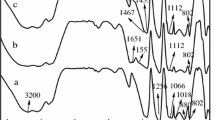
The UATR-FT-IR spectra of the initial samples (Fig. 7.) confirmed (see Table 3) that the polymer was obtained from a polyesteric polyol and aromatic diisocyanate using an aminic catalyst. It was confirmed that the polyurethane contains –OH as the end chain.
Bands presented in Tables 4 and 5 show the differences between AO-2 bifunctional (contains one phenolic –OH blocked non-reactive, implied in process of stabilization, and one reactive alcoholic –OH binding with polyurethane in reactive stabilization process) and AO-1 that contain only phenolic –OH blocked and non-reactive. For mixed stabilization all characteristic bands appear.
Conclusions
A versatile way for obtaining and processing data regarding the thermooxidative stabilization of polyurethane foams was suggested.
The best results were obtained by the mixed reactive non-reactive stabilization, which allowed low concentrations of stabilizator. This low quantity avoided a significant migration (by the non-reactive) or an important influence on the polymeric chain.
The obtained results will be useful by designing practical recipes for thermostable elastic polyurethane foams.
References
Berta M, Lindsay C, Pans G, Camino G. Effect of chemical structure on combustion and thermal behaviour of polyurethane elastomer layered silicate nanocomposites. Polym Degrad Stab. 2006;91:1179–91.
Hatchett DW, Kodippili G, Kinyanjui JM, Benincasa F, Sapochak L. FTIR analysis of thermally processed PU foam. Polym Degrad Stab. 2005;87:555–61.
Duquesne S, LeBras M, Bourbigot S, Delobel R, Camino G, Eling B, et al. Thermal degradation of polyurethane and polyurethane/expandable graphite coatings. Polym Degrad Stab. 2001;74:493–9.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data. Comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80:59–64.
Šimon P, Fratricova M, Schwarzer P, Wilde H-W. Evaluation of the residual stability of polyurethane automotive coatings by DSC. J Therm Anal Calorim. 2006;84:679.
Vlase T, Vlase G, Doca N, Bolcu C, Borcan F. Kinetics of non-isothermal decomposition of three IRGANOX-type antioxidants. J Therm Anal Calorim. 2008;92(1):15–8.
Acknowledgements
This work was supported from Romanian Ministry of Education, Research and Innovation between Grant 574/2009, PN II, ID_2376.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlase, T., Bolcu, C., Vlase, G. et al. Thermooxidative stabilization of a MDI. J Therm Anal Calorim 99, 973–979 (2010). https://doi.org/10.1007/s10973-009-0536-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0536-z