Abstract
Microencapsulated ammonium polyphosphate MFAPP or VMFAPP with shell of melamine–formaldehyde or poly (vinyl alcohol)–melamine–formaldehyde resin was prepared by in situ polymerization, respectively. The flame-retardant performance of rigid polyurethane foam (PU) containing MFAPP or VMFAPP was analyzed by limiting oxygen index and UL-94 test. Thermal degradation behaviors of PUAPP, PUMFAPP and PUVMFAPP were studied using TG and TG–FTIR. Above results indicated that VMFAPP and MFAPP have better water resistance and flame retardancy compared with ammonium polyphosphate (APP) in PU composites. Flame-retardant properties of PUMFAPP and PUVMFAPP composites were rarely changed after hot water treatment. Due to better compatibility and the presence of active groups on the surface of APP, microencapsulation demonstrated a positive effect on the mechanical property of PU composites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rigid polyurethane foams (PU) are widely used in building insulation and domestic appliances, due to their superior mechanical properties and low density [1, 2]. However, rigid polyurethane foams are very combustible materials having fast flame spread and high heat release rates [3, 4]. So, it is significant to improve flame-retardant properties of PU.
Intumescent flame-retardant system (IFRs) that has been used in flammable polymers such as PU usually contains three active ingredients, i.e., an acid agent (e.g., ammonium polyphosphate), a carbonization agent (e.g., polyol) and a blowing agent (e.g., melamine) [5, 6]. Ammonium polyphosphate (APP) is a conventional acid source and also a kind of very popular and common inorganic phosphorus/nitrogen flame retardant, which demonstrates a significant phosphorus–nitrogen synergism [7, 8]. However, the flame retardancy of APP is far from satisfactory due to the weak water resistance and poor compatibility in polymers. To solve above problems, microencapsulation is one method [9]. Microencapsulation is a process of enveloping microscopic amounts of matter in a thin film of polymer, which could form a solid wall. This core–shell structure allows isolation of the encapsulated substance from the surroundings and thus protects it from any degrading factors such as water. Even the proper shell could be served as one ingredient such as a carbonization agent or a blowing agent of IFRs [10, 11].
Melamine–formaldehyde (MF) resin is water-resistant,and the flame-retardant synergism of MF resin and APP had been reported [12]. Poly (vinyl alcohol) (PVA), with many O–H groups, can be used as a charring agent in the flame retardancy of polymers [13] and could easily react with low molecular weight compounds [14], which make it is possible to acquire functional polymers poly (vinyl alcohol)–melamine–formaldehyde (VMF). Much work has been reported on flame retardancy of phosphorus/nitrogen flame retardant in PU materials. However, few work focused on effect of microencapsulation on flame retardancy of PU composites.
In this paper, APP was microencapsulated with MF and VMF resin shell by in situ polymerization. The structure of the IFRs has been characterized by Fourier transform infrared (FTIR) and scanning electron microscopy (SEM). The use of APP, MFAPP and VMFAPP as a flame retardant in RPUFs is evaluated by limiting oxygen index (LOI), UL-94 and thermogravimetric analysis (TG), and the results were compared. The water-resistant properties of PU composites containing APP (or MFAPP or VMFAPP) are studied by LOI test and UL-94, SEM and water leaching rate. Also, the residue chars of PU composites containing APP (or MFAPP or VMFAPP) were analyzed by FTIR and SEM. Moreover, the mechanical properties of PU composites that contain different flame retardants (APP, MFAPP and VMFAPP) are studied.
Experimental
Materials
APP (phase II, the degree of polymerization >1,000) was supported by Polyrocks chemical Co. Ltd., China. 4, 4′-diphenylmethane diisocyanate (MDI) was purchased from Aladdin Industrial Corporation (China). Melamine (AR) and formaldehyde was obtained from Tianjing Kemiou Chemical Reagent Co. Ltd., China and Chengdu Kelong Chemical Reagent Factory (Sichuan, China), respectively. PVA (polymerization degree = 500, alcoholysis degree = 88 %) was supplied by Guangzhou Fengbaishun Trading Co. Ltd., China. Polyether polyols (4,110, Hydroxyl value = 410–450 mgKOH/g) was provided by Guangzhou Ruiyin Trading Co. Ltd., China. N-Pentane was supplied by Tianjing Fuchen Chemical Reagent Factory (Tianjin, China). Dibutyltin dilaurate was purchased from Shanghai Lingfeng Chemical Reagent Co. Ltd., China.
Synthesis of prepolymer MF and MFAPP
Melamine (4 g), 37 % formaldehyde solution (17.9 mL) and distilled water (50 mL) were added into a three-necked bottle with a stirrer. Then 10 % Na2CO3 solution was added into mixture till the pH was 8–9. The mixture was heated slowly up to 80 °C and kept at that temperature for half an hour with stirring. Then the prepolymer MF was obtained.
A total of 60 g APP was dispersed in 150 mL ethanol in a three-necked bottle with a stirrer. Then, 60 g prepolymer MF was put into the bottle, and the pH was adjusted to 2–3 by adding H2SO4. After that, the resulting mixture was kept at 80 °C for 2 h with mechanical stirring. Finally, the slurry was cooled to room temperature, filtered, washed up with distilled water and dried at 80 °C. Finally, the white powder was MFAPP what needed.
Synthesis of prepolymer VMF and VMFAPP
PVA 15 g, melamine (4 g) and distilled water (200 mL) were put into a three-necked bottle with a stirrer. The mixture was adjusted to pH 2–3 with H2SO4, heated to about 90 °C and kept at that temperature for 1.5 h. Then, the pH was adjusted to 8–9 with 10 % NaOH solution, and then 4 g melamine and 10 mL 37 % formaldehyde solution were added into the system. The temperature was kept at 90 °C for 1 h. The prepolymer VMF solution was then prepared, and it was ready for the next step.
A total of 40 g APP was prepared and put into a three-necked bottle with a stirrer along with 100 mL ethanol. Eighty-six grams prepolymer solution obtained from the above step was added to the mixture, and the pH of the mixture was adjusted to pH 3–4 with H2SO4. The mixture was heated at 80 °C with stirring. After 3 h, the mixture was filtered, washed with distilled water and dried at 80 °C. VMFAPP powder was finally obtained.
Preparation of flame-retarded PU composites
The polyether polyols (10.00 g) was mixed with the other ingredients: foaming agent (N-pentane) (1.30 g), catalyst (dibutyltin dilaurate) (0.11 g), flame retardant (APP, MFAPP or VMFAPP), which was stirred well to homogenize and then MDI (10.10) was added into the mixture with continuous stirring. Until the white foam arise, the mixture was poured into the mold. The container was then placed on a flat surface for 6 h. After that, samples were put into specific shapes as per the test requirement, and the foam properties were then measured. Flame-retarded PU composites were prepared according to the formulation given in Table 1.
Measurements
Fourier transform infrared spectroscopy (FTIR)
IR spectra were recorded between 4,000 and 400 cm−1 using a RFX-65A (Analect, USA) FTIR with KBr pellets for solid samples.
Scanning electron microscopy (SEM)
The samples were coated with gold–platinum using a BAL-TEC SCD005 SEM coating system operating at 1.2 kV and 30 mA. SEM examination was performed on a Philips XL30 SEM.
Water leaching rate
About 8 g flame retardants (marked Wa) were packaged with the same filter papers (marked Wc). The specimens were put in about 2L distilled water, and the temperature was kept at different temperature for 36 h. Then removed and dried to constant mass at 80 °C (marked Wt). The water leaching rate of the specimens can be expressed as (Wt − Wc)/(Wa − Wc) × 100 %.
UL-94 Vertical Burning Test
The vertical test was carried out on a CFZ-3-type instrument (Jiangning Analysis Instrument Company, China) according to the UL-94 test standard. The specimens used were of dimensions 120 × 15 × 8 mm3.
Limiting Oxygen Index (LOI)
LOI was measured according to ISO4589. The apparatus used was an oxygen index meter (JF-3, Jiangning Analysis Instrument Company, China). The samples used for the test were of dimensions 100 × 10 × 10 mm3.
Thermogravimetric analysis (TG)
Each sample was examined under air flow (30 mL min−1) on a DTG-60H apparatus (Shimadzu Company) at a heating rate of 10 °C min−1. The mass of all samples was kept within 3–5 mg in an open Al pan.
Thermogravimetry–Fourier transform infrared spectroscopy (TG–FTIR)
The TG–FTIR instrument consists of TG analyzer (TG209 F3 Tar-sus, NETZSCH, Germany) coupled with FTIR spectrometer (TENSOR27, Bruker, Germany) and the transfer line. The investigations were carried out under nitrogen atmosphere at a flow rate of 30.0 mL min−1 for TG, with heating rate of 10 °C min−1.
Compression strength
The compressive strength of the rigid foams prepared was evaluated using SANS-CMT4104 microcomputer control electronic universal testing machine. The cuboid test samples were prepared according to GB/T1041-1992 with a dimension of 50 × 40 × 18 mm. The test was carried out at a cross-head speed of 4 mm min−1 with 2 mm thickness compressed. Three specimens were tested for each sample.
Results and discussion
FTIR spectra of APP, MFAPP and VMFAPP
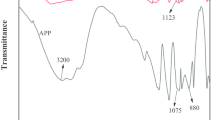
The FTIR spectra of APP, MFAPP and VMFAPP are shown in Fig. 1. The typical absorptions of APP appear at 3,200 (N–H), 1,256 (P=O), 1,066 (P–O symmetric stretching vibration), 880 (P–O asymmetric stretching vibration), 1,018 (symmetric vibration of PO2 and PO3) and 802 (P–O–P) cm−1 [15]. The spectrum of MFAPP shows new absorption peak at 1,651,1,551 and 1,112 cm−1, which are, respectively, corresponding to bending vibration of N–H of primary amine, stretching vibration of C=N and the stretching vibration of symmetric C–O–C– of –CH2–O–CH2– between melamine groups from the MF resin, indicating that the resin exists in the MFAPP.
The spectrum of VMFAPP reveals not only well-defined absorption peaks of APP but also the characteristic bands of MF resin and PVA, which are at 1,433, 1,467, and 1,664 cm−1. The 1,433 and 1,467 cm−1 are attributed to the O–H, C–H bending and –CH2 deformation of PVA, while the 1,664 cm−1 is assigned C=O group in PVA, and the group was likely due to the absorption of the residual acetate group [16], which all convey the information that APP was coated by VMF resin after microencapsulation.
SEM analysis
The surface morphologies of APP, MFAPP and VMFAPP particles are shown in Fig. 2. It is clear that the surface of APP particle is very smooth. After microencapsulation, MFAPP presents a comparably rough surface, which suggests a coating exists on the surface of APP.
VMFAPP also present rough surfaces. A layer of thin film could be seen on the surface clearly, which is inferred to be VMF resin. The result reveals that APP was well coated by VMF resin.
Water leaching rate analysis
Table 2 shows water leaching rate of APP, MFAPP and VMFAPP at different temperature for 36 h. Water leaching rate of APP is 99.0 % at 30 °C, and at higher temperature, which increases to 100.0 %. It makes clear that APP can be easily attacked by moisture or water. However, water leaching rate of microencapsulated APP is less than 6.5 % and changes little as temperature increases. This is because that the shells outside APP are hydrophobic. On the other hand, above results also indicate that the vast majority of APP was microencapsulated well.
Flame retardancy of PU composites
LOI and UL-94 tests are widely used to evaluate the flammability of flame-retardant materials [17]. The results are presented in Fig. 3. Pure PU is highly combustible, and its LOI value is only 16.5 %. With the increase in APP (MFAPP or VMFAPP) content in PU composite, the LOI value of the PU composites increases. The LOI value of the composite with 13.7 mass % APP is 21.5 %, whereas the value of the PUMFAPP composite at the same additive level is 23.0 %. Meanwhile, LOI value of PU composites with 13.7 mass % VMFAPP is 24.0 %. Obviously, PU composites containing microencapsulated APP demonstrate higher LOI values compared with APP. It could be seen that PUMFAPP and PUVMFAPP with 13.7 mass % loading can both obtain a UL-94 rating of V0, while that of PUAPP is V1 at the same loading. For PUMFAPP, this phenomenon might be attributed to the flame-retardant synergism of MF resin and APP. Melamine in MF resin could release gas when burning, which could dilute O2 concentration. Also, because of the existence of PVA as carbonization agent and the MF resin as blowing agent, VMFAPP could form an intumescent system with acid agent (APP), which contributes to higher LOI values and UL-94 rating compared with APP in PU. It can be concluded that core shell structure leads APP better flame-retardant property in PU composites.
Water resistance of PU composites
The UL-94 rating and LOI value of PU composites before and after water treatment are shown in Table 3. It could be seen that the LOI value of PUAPP4 decreases 3.0 % after water treatment, which is 0.5 % when that come to PUMFAPP4 and PUVMFAPP4. Moreover, after water treatment, UL94 rating of PUAPP4 falls to V-1, while PUMFAPP4 could keep V-0 rating, so it is with PUVMFAPP4. The reason is that the shells outside APP are hydrophobic, and the comparatively better water resistance of MFAPP and VMFAPP in the PU matrix would prevent the flame retardant from being exuded, which benefits for keeping the flame-retardant properties of PU composites.
Furthermore, the microcosmic morphology of PU composites before and after water treatment was observed by SEM, as shown in Fig. 4. In the case of PUAPP4 after water treatment, some 10- to 20-μm holes could be observed on the surface. It could be speculated that when the composites are exposed to water, the water molecules will absorb on the surface of the material, and some APP grains on the surface will first dissolve in the water, leaving some defects on the surface. On the other hand, after PUMFAPP4 (or PUVMFAPP4) composite was treated with 60 °C water for 36 h, there are still some MFAPP (or VMFAPP) grains left in the composites. The results indicate that microencapsulation has a remarkable effect on the water resistance of APP in PU matrix.
Compression strength
Compression strength of PU composites is presented in Table 4. Compressive strength of PU composites decreases versus APP content, which decreases from 202 to 172 kPa. It indicates that APP shows negative effect on mechanical property in PU due to the poor compatibility. However, because of the better compatibility of MFAPP in PU, compression strength of PUMFAPP decreases by 4 kPa at loading of 7.4 and 13.7 %, and decreases by 8 and 6 kPa at loading of 10.7 and 16.7 % respectively. Meanwhile, it could be seen that compression strength of PUVMFAPP composites is higher than that of PUAPP and PUMFAPP, and it might be attributed to the active group (O–H) of PVA which could react with –NCO of MDI. And then crosslinking network composed of flame-retardant particles and polymer chains in composites is formed. The crosslinking could enhance the mechanical property of PU composites. Above results indicate that microencapsulation has a positive effect on mechanical property of PU.
Thermal analysis
The TG and DTG curves of APP, MFAPP and VMFAPP are shown in Fig. 5. APP has two main decomposition processes, beginning at about 270 °C. The evolved products in the first step are mainly ammonia and water (about 20 % mass loss), and crosslinked polyphosphoric acids (PPAs) are formed simultaneously [18]. The second stage occurs in the range of 500–700 °C, which is the main decomposition process of APP, and its mass loss is about 82 %. Temperatures of maximum mass loss rate (T max) for the two steps are 308 and 572 °C. The residual mass of APP is 11.4 % at 800 °C.
Compared with APP, in the first process, MFAPP decomposes faster because of the decomposition of MF resin. Due to the esterification between APP and PVA, VMFAPP decomposes faster than other two flame retardants below 500 °C [19, 20]. Beyond the temperature of 550 °C, MFAPP and VMFAPP are more stable than APP. The T max for three steps of MFAPP decomposition is 292, 380 and 520 °C and that for VMFAPP is 291, 392, 526 °C, respectively. Moreover, VMFAPP leaves about 23.8 % residue at 800 °C, which is higher than that of APP and MFAPP.
TG and DTG curves of PU composites under air atmosphere are shown in Fig. 6. The thermal oxidative degradation process of pure PU has two stages, and their corresponding T max is 320 and 554 °C, respectively. The initial decomposition temperature at which the mass loss is 5 % of pure PU is 230 °C. PU has almost completely decomposed at 650 °C.
Thermal decomposition of the PUAPP4 composites includes three steps. Initial thermal decomposition temperature is 242 °C. The first step of mass loss is the main decomposition of the composite, and T max for this step is 281 °C. Because the polyphosphoric acids that released from APP accelerate the decomposition of PUAPP4, PUAPP4 decomposes faster than pure PU at first stage. T max values for last two processes are 537 and 728 °C, respectively, PUAPP4 leave about 9.8 % residue at 800 °C.
For PUMFAPP4, its initial thermal decomposition temperature is 238 °C, and T max of first step is 291 °C. It could be seen that PUMFAPP4 decomposes slowly compared with PUAPP. It is because MF resin shell could prevent APP reacting with PU in first process. Due to the decomposition of MF shell occurs at this stage, mass loss of PUMFAPP4 is 3.9 % higher than that of PUMFAPP4 and PUVMFAPP4, which is consistent with the content of MF resin in PUMFAPP4, and leads to a lower residue left (7.8 %) after decomposition at 800 °C.
Thermal decomposition behavior of PUVMFAPP4 also includes three steps. Initial thermal decomposition temperature is 248 °C, which is higher than PUAPP4 and PUMFAPP4. From 248 to 300 °C, PUVMFAPP4 decomposes slowly compared with PUAPP4 and PUMFAPP4. The DTG curve demonstrates that Tmax of PUVMFAPP4 (292 °C) is higher than that of PUAPP4 (281 °C). The result indicates that VMFAPP enhances the thermal stability of PU at initial thermal degradation process compared with APP. This might be attributed to the presence of active groups (–OH) on surface of VMFAPP, which results in forming crosslinking network structure composed of flame-retardant particles and polymer chains. This crosslinking effect may delay the thermal decomposition of PU matrix at low temperature [21]. It could be seen that the third decomposition process of PUVMFAPP is from 612 to 760 °C, which is mainly the decomposition of charred residue. Mass loss of PUVMFAPP4 is 14.1 %, which is less than that of PUAPP4 (17.3 %) and PUMFAPP4 (14.6 %). It indicates that the formational charred residue of PUVMFAPP4 is more stable compared with PUAPP4 and PUMFAPP4. Residual mass of PUVMFAPP4 is 10.1 % at 800 °C.
TG–FTIR analysis
TG–FTIR is an effective method for analysis of the gaseous products during thermal decomposition, which is used here to study the thermal degradation behavior of PU. Figure 7 shows the 3D TG–FTIR spectra of gas phase in the thermal degradation of PU and PU composites. In Fig. 7a, peaks are in the regions of around 3,500–3,700 cm−1 around 2,200–2,400 cm−1 and around 1,400–1,670 cm−1, and the spectrum fit well to the reported FTIR features of gas products such as H2O (3,500–3,700 cm−1), CO2 (2,300–2,400 cm−1) and HNC=O deformation (1,530 cm−1) [22–26]. It could be seen that the main products of PU are CO2 (2,349 cm−1), H2O (3,400–4,000 cm−1), compounds containing aromatic ring (1,631 cm−1) and compounds containing HNC=O (1,530 cm−1). PU without any additive decomposes dramatically when heated and produces lots of compounds containing aromatic ring and HNC=O in the process of depolymerization and the main-chain break (Fig. 8).
There is a significant difference in pyrolysis products of PUAPP4. The decomposition behavior of PUAPP4 is delayed compared with PU, and it exhibits characteristic bands of compounds containing P–O–P (1,090, 802 cm−1) and NH3 (933 cm−1), which appears at 300 °C. Due to the decomposition of MF resin, for PUMFAPP4, CH3OH (O–H deformation, 660 cm−1), CH2O (1,746 cm−1) and H2O (3,783 cm−1) are detected below 300 °C, which is corresponding to the TG of MFAPP. Pyrolysis products from APP containing P–O–P (1,090, 802 cm−1) and NH3 (933 cm−1) appear at 350 °C. PUVMFAPP4 displays the similar spectra with PUMFAPP4 at 1,090, 802 and 933 cm−1 as temperature increasing to 350 °C.
From above analysis, it can concluded that the main evolved gas products for PU are CO2, H2O, compounds containing aromatic ring and compounds containing HNC=O. Due to the additive of APP, pyrolysis products containing P–O–P and NH3 are produced. PUMFAPP4 releases CH3OH, CH2O and H2O because of the thermal degradation of MF shell below 300 °C. However, these gas products (CH3OH, CH2O and H2O) are not detected for PUVMFAPP below 300 °C, which indicates that the VMF shell is more stable compared with MF shell at low temperature because of the reaction between VMF shell and PU.
Conclusions
In this work, APP was microencapsulated with MF or VMF resin by in situ polymerization method. Microencapsulated APP (MFAPP or VMFAPP) decreases its water absorption and increases its water resistance in PU matrix. LOI values of PUMFAPP4 increased by 1.5 % and those of PUVMFAPP4 composites increased by 2.5 % compared with PUAPP4. At the additive level of 13.7 %, APP used alone in PU does not reach the UL 94V-0 rating, while MFAPP or VMFAPP used alone in PU can reach V-0. Moreover, after water treatment at 60 °C for 36 h, composites containing microencapsulated APP could still maintain good flame-retardant properties. These results show that microencapsulation leads APP better water resistance and flame retardancy in PU. In TG study, VMFAPP and MFAPP are more stable than APP at high temperature. However, the corresponding composite shows better thermal performance at low temperature. Due to better compatibility and active groups (–OH) that could form crosslinking network between flame retardant and polymer chains, PUVMFAPP shows better mechanical property compared with PUAPP.
References
Tang Z, Maroto-Valer MM, Andrésen JM, Miller JW, Listemann ML, McDaniel PL, et al. Thermal degradation behavior of rigid polyurethane foams prepared with different fire retardant concentrations and blowing agents. Polymer. 2002;43(24):6471–9.
Hatakeyama H, Matsumura H, Hatakeyama T. Glass transition and thermal degradation of rigid polyurethane foams derived from castor oil-molasses polyols. J Therm Anal Calorim. 2013;111(2):1545–52. doi:10.1007/s10973-012-2501-5.
Wang G, Zang Q, Han W. The potential fire risk of rigid polyurethane foam. Fire Sci. Technol. 2013;32(5):469–75.
Usta N. Investigation of fire behavior of rigid polyurethane foams containing fly ash and intumescent flame retardant by using a cone calorimeter. J Appl Polym Sci. 2012;124:372–3382. doi:10.1002/app.3535210.1002/app.
Awad WH, Wilkie CA. Further study on the flammability of polyurea: the effect of intumescent coating and additive flame retardants. Polym Adv Technol. 2011;22(8):1297–304. doi:10.1002/pat.1963.
Wu K, Zhang Y, Hu W, Lian J, Hu Y. Influence of ammonium polyphosphate microencapsulation on flame retardancy, thermal degradation and crystal structure of polypropylene composite. Compos Sci Technol. 2013;81:17–23. doi:10.1016/j.compscitech.2013.03.018.
Chen L, Wang Y-Z. A review on flame retardant technology in China. Part I: development of flame retardants. Polym Adv Technol. 2009;21:1–26. doi:10.1002/pat.1550.
Chen XL, Jiang YF, Jiao CM. Synergistic effects between hollow glass microsphere and ammonium polyphosphate on flame-retardant thermoplastic polyurethane. J Therm Anal Calorim. 2014;117(2):857–66. doi:10.1007/s10973-014-3831-2.
Wu K, Song L, Wang Z, Hu Y. Microencapsulation of ammonium polyphosphate with PVA-melamine-formaldehyde resin and its flame retardance in polypropylene. Polym Adv Technol. 2008;19(12):1914–21. doi:10.1002/pat.1231.
Tang Q, Wang B, Shi Y, Song L, Hu Y. Microencapsulated ammonium polyphosphate with glycidyl methacrylate shell: application to flame retardant epoxy resin. Ind Eng Chem Res. 2013;52(16):5640–7. doi:10.1021/ie302591r.
Qiu XL, Lu LX, Zhang ZX, Tang GY, Song GL. Preparation, thermal property, and thermal stability of microencapsulated n-octadecane with poly(stearyl methacrylate) as shell. J Therm Anal Calorim. 2014;118(3):1441–9. doi:10.1007/s10973-014-4040-8.
Wu K, Zhang Y-K, Zhang K, Shen M-M, Hu Y. Effect of microencapsulation on thermal properties and flammability performance of epoxy composite. J Anal Appl Pyrolysis. 2012;94:196–201. doi:10.1016/j.jaap.2011.12.009.
Saihi D, Vroman I, Giraud S, Bourbigot S. Microencapsulation of ammonium phosphate with a polyurethane shell part I: coacervation technique. React Funct Polym. 2005;64(3):127–38. doi:10.1016/j.reactfunctpolym.2005.05.004.
Nakamura N. Study on ketalization reaction of poly(viny1 alcohol) by ketones. VII. Reaction between poly(viny1 alcohol) and aromatic ketones and behavior of poly(viny1 ketal) in water. J Appl Polym Sci. 1995;57:1145–53.
Kandelbauer A, Despres A, Pizzi A, Taudes I. Testing by fourier transform infrared species variation during melamine–urea–formaldehyde resin preparation. J Appl Polym Sci. 2007;106(4):2192–7. doi:10.1002/app.26757.
El-Zaher NA, Osiris WG. Thermal and structural properties of poly(vinyl alcohol) doped with hydroxypropyl cellulose. J Appl Polym Sci. 2005;96(5):1914–23. doi:10.1002/app.21628.
Li Z, Qu B. Flammability characterization and synergistic effects of expandable graphite with magnesium hydroxide in halogen-free flame-retardant EVA blends. Polym Degrad Stab. 2003;81(3):401–8. doi:10.1016/s0141-3910(03)00123-x.
Camino G, Grassie N, McNeill IC. Influence of the fire retardant, ammonium polyphosphate, on the thermal degradation of poly(methyl methacrylate). J Polym Sci Polym Chem Ed. 1978;16:95–106.
Broadbent J, Hirschler M. Red phosphorus as a flame retardant for a thermoplastic nitrogen-containing polymer. Eur Polym J. 1984;20(11):1087–93.
Kay M, Price A, Lavery I. Review of intumescent materials, with emphasis on melamine formulations. J Fire Retard Chem. 1979;6(2):69–91.
Qin H, Zhang S, Zhao C, Hu G, Yang M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer. 2005;46(19):8386–95.
Chuang F-S. Analysis of thermal degradation of diacetylene-containing polyurethane copolymers. Polym Degrad Stab. 2007;92(7):1393–407.
Chen X, Huo L, Jiao C, Li S. TG–FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J Anal Appl Pyrolysis. 2013;100:186–91. doi:10.1016/j.jaap.2012.12.017.
Wu K, Hu Y, Song L, Lu H, Wang Z. Flame retardancy and thermal degradation of intumescent flame retardant starch-based biodegradable composites. Ind Eng Chem Res. 2009;48(6):3150–7.
Wu K, Song L, Hu Y, Lu H, Kandola BK, Kandare E. Synthesis and characterization of a functional polyhedral oligomeric silsesquioxane and its flame retardancy in epoxy resin. Prog Org Coat. 2009;65(4):490–7. doi:10.1016/j.porgcoat.2009.04.008.
Jellinek H, Takada K. Toxic gas evolution from polymers: evolution of hydrogen cyanide from polyurethanes. J Polym Sci Polym Chem Ed. 1977;15(9):2269–88.
Acknowledgements
The financial supports from National Natural Science Foundation of China (No. 51003123, No. 51303004), Zhujiang Science & Technology New-star Program of Guangzhou, China (No. 2013J2200016), Integration of Industry, Education and Research of Guangdong Province Project, (2011A091000007) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, F., Wu, K., Lu, M. et al. Thermal degradation and flame retardancy of microencapsulated ammonium polyphosphate in rigid polyurethane foam. J Therm Anal Calorim 120, 1327–1335 (2015). https://doi.org/10.1007/s10973-015-4425-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4425-3