Abstract
In this paper, we aimed to develop a two-step approach for highly efficient synthesis of bright core-shell colored silica submicron particles (core-shell colored SiO2 SMPs) by coupling organic reactive dyes onto silica submicron particle (SiO2 SMPs). C.I. Reactive Blue 21 was selected as model dye to synthesize a core-shell blue silica submicron particle (core-shell blue SiO2 SMPs). 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (3APTMS) and C.I. Reactive Blue 21 were grafted simultaneously on the surface of particles in the aqueous solution to produce bright blue SiO2 SMPs. The effect of the amount of 3APTMS and C.I. Reactive Blue 21 on preparation of blue SiO2 SMPs was studied. To prevent dye on blue SiO2 SMPs from leaking, core-shell blue SiO2 SMPs were prepared in a pre-hydrolyzed Stöber system. Scanning Electronic Microscopy (SEM) and Dynamic Light Scattering (DLS) were used to character the SiO2 SMPs, blue SiO2 SMPs, and core-shell blue SiO2 SMPs. The results indicate that the blue SiO2 SMPs and core-shell blue SiO2 SMPs synthesized under the optimal conditions with regular morphology and good dispersibility. Coupling efficiency of the C.I. Reactive Blue 21 was as high as 89.2% (the quality of dye coupled was about 70 mg/g SiO2). In the same way, six other different colored SiO2 SMPs were also prepared by introducing different color organic reactive dyes on SiO2 SMPs. The bright monodispersed core-shell colored SiO2 SMPs is promising to be used as sensitive and reproducible maker in immunoassay.
Graphical abstract
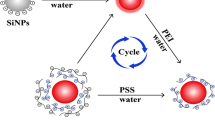
A one-step approach for the high efficiently coupling of reactive dyes onto SiO2 SMPs in an aqueous solution to synthesize monodispersed colored SiO2 SMPs was designed. The hydrolysis and condensation of 3APTMS and dye coupling were preceded at the same time. After modification and coloration, a silica protection layer was grown onto the colored SiO2 SMPs to prevent the dye leakage and to make the colored SiO2 SMPs be pure silica surface. 
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Colored silica nanoparticles (colored SiO2 NPs) were first reported by Winnik et al. in 1990 [1], they were prepared by grafting organic dyes to the surface of derivatized silica. Since the 90s of the 20th century, many studies of colored SiO2 NPs have been reported. In those articles, colored SiO2 NPs were commonly reported as kinds of silica based pigment, due to their rich colors, good stability and don’t fade in the harsh conditions. In recent years, colored SiO2 NPs have attracted research interest due to their bright color, water solubility, low toxicity, and promising applications in biosensors. Our team proved that monodispersed colored SiO2 NPs were good kind of optical label, which can be used in immune sensor regarded as markers to amplify the response signal for detection of pathogenic bacteria [2,3,4,5].
Monodispersed colored SiO2 NPs are conventionally prepared with two step reactions. SiO2 NPs are first prepared and functionalized with silane coupling agent, then organic dyes are grafted onto the surface of amino-modified silica. Winnik et al. added 3-aminopropyltriethoxysilane (APTES) dropwise to stirring SiO2 suspension and refluxed at dry toluene, then colored the amino-functionalized SiO2 NPs in organic reactive dye solution [1]. Giesche et al. dispersed pretreated SiO2 NPs in a mixture of ammonia solution/water/ethanol, and then aminosilane and tetraethyl orthosilicate (TEOS) were added to incorporate aminosilane around the original SiO2 NPs. The dyes were converted into the acid chloride form and coupled on the amino-modified SiO2 NPs [6]. Binkowski et al. prepared amino-modified SiO2 NPs with APTES and N-2-(aminoethyl)-3-aminopropyltrimethoxysilane (U-15) in a medium of methanol and water, then subsequently added into organic dyes aqueous solution to synthesize colored SiO2 NPs [7,8,9]. In former studies, the amino modification of SiO2 NPs was conducted in organic solvent, such as toluene, methanol and ethanol, which was not environmentally friendly and economical. The above preparation methods of colored SiO2 NPs were also divided into two steps, which were time-consuming. Our group has used a one-step inverse microemulsion method to synthesize colored SiO2 NPs by doping C.I. Reactive Blue 14 into SiO2 NPs [3]. The synthesis cost is high due to the use of a large amount of cyclohexane, n-hexyl alcohol, and Triton. Therefore, a new economical, time saving, efficient, and environmental method for preparation of monodispered colored SiO2 NPs was still needed to be investigated.
Some studies showed that it was feasible to modify the SiO2 NPs with APTES in aqueous solution [10,11,12], of which the by-product was more environmentally friendly and easy to be removed. Inspired by this, a novel method was developed for highly efficient covalent coupling of organic reactive dyes onto SiO2 NPs in aqueous solution. In this approach, C.I. Reactive Blue 21 was taken as a model organic dye. First, the original SiO2 submicron particles (SiO2 SMPs) were prepared by Stöber method, and then C.I. Reactive Blue 21 was grafted on the surface of SiO2 SMPs by 3-[2-(2-aminoethylamino)-ethylamino]propyl-trimethoxysilane (3APTMS) in the aqueous solution. To protect the dye molecules coupled on the blue SiO2 SMPs from shedding, Core-shell blue SiO2 SMPs were synthesized by introducing blue SiO2 SMPs into a pre-hydrolyzed Stöber system to grow a further silica protection layer.
2 Experimental
2.1 Chemicals and apparatus
TEOS and (2-(N-morpholino)ethanesulfonic acid (MES) were obtained from Aladdin Industrial Inc. (Shanghai, China). Ethanol and ammonia (25–28 wt%) were purchased from Xilong Sientific Co., Ltd (Shanghai, China). 3APTMS was purchased from Tianjin Heowns Biochemical Technology Co., Ltd. (Tianjin, China). The dyes used in this study were supplied by Zhejiang Shunlong Chemical Co., Ltd (Zhejiang, China) and listed in supplementary information. All reagents were used as received without further purification; the water used in this work was pure water.
Hitachi SU8010 scanning electron microscopy (SEM) was purchased from Hitachi Inc. (Tokyo, Japan); Malvern Nano ZS potential laser particle analyzer was provided by Malvern Instruments Co., Ltd. (Worcestershire, UK); UV 2600 UV–vis spectrophotometer was purchased from Shimadzu Co., Ltd (Shanghai, China); Multiskan spectrum was purchased from Thermo Fisher Scientific Inc. (Waltham, USA).
2.2 Synthesis of monodisperse SiO2 SMPs
Monodispersed SiO2 SMPs were prepared by Stöber method with slight modification [13, 14]. In a typical reaction process, 2.7 mL of TEOS in 27.3 mL of ethanol was rapidly added into a mixture containing 10 mL of ethanol, 18 mL of H2O, and 1.7 mL of ammonia. The reaction mixture was kept at room temperature for 5 h under magnetic stirring. The resulting submicron particles were isolated by centrifugation at 10,000 rpm for 8 min to collect deposits, washed twice with ethanol and three times with water, respectively, and dispersed in water for further usage.
2.3 Coloration of unmodified SiO2 SMPs
To prepare C.I. Reactive Blue 21 solution, accurate weight of 1.0 g of C.I. Reactive Blue 21 was dissolved in 10 mL of water. The C.I. Reactive Blue 21 solution was stirred at room temperature overnight and filtered by 0.22 μm pore-size membranes to remove any undissolved material. A portion of 0.1 g of SiO2 SMPs was ultrasonic dispersed in 15 mL of pure water; variation of 3APTMS (0.5, 1, 5, 10, 20, 40, 60, and 80 μL) and 80 μL of C.I. Reactive Blue 21 solution were introduced into water dispersion of the unmodified SiO2 SMPs. After reacting at room temperature for 3 h under magnetic stirring, the resulting blue SiO2 SMPs were isolated by centrifugation. The resulting submicron particles were washed once with water and twice with ethanol then dispersed in 2.5 mL ethanol.
2.4 Synthesis of the core-shell blue SiO2 SMPs
A Stöber system containing 10 mL ethanol, 0.8 mL H2O, 0.2 mL TEOS, and 0.1 mL NH3·H2O (25–28%) was hydrolyzed for 30 min. A volume of 2.5 mL of the above prepared blue SiO2 SMPs ethanol dispersion solution was added into the pre-hydrolyzed Stöber system and reacted for 12 h at room temperature under magnetic stirring [15]. The resulting core-shell blue SiO2 SMPs with protective layer were isolated by centrifugation and washed several times with ethanol and water.
2.5 Characterizations
The size and morphology of original SiO2 SMPs, blue SiO2 SMPs, and core-shell blue SiO2 SMPs were characterized by SEM. The samples were prepared by depositing a drop of dispersion of particles onto carbon grids and operated at 3.0 kV. Particle size distributions were recorded by measuring the diameters of few particles in each sample by Malvern Nano ZS potential laser particle analyzer. The maximum absorption wavelength of C.I. Reactive Blue 21 was determined by UV–vis spectrophotometer. The coupling efficiencies of the C.I. Reactive Blue 21 were determined by multiskan spectrum. All experiments were performed at room temperature (25 ± 1 °C).
3 Results and discussions
The procedure to prepare the core-shell blue SiO2 SMPs is illustrated in Scheme 1, a complete such study is carried out with C.I. Reactive Blue 21. Based on Stöber method, uniform, spherical SiO2 SMPs are obtained with an average diameter of about 195 nm, which is presented by SEM observation in Fig. 1. 3APTMS was selected as bridge to link dye molecules and the SiO2 SMPs. 3APTMS and C.I. Reactive Blue 21 solution were added simultaneously into the SiO2-water mixture to graft the C.I. Reactive Blue 21 onto SiO2 SMPs by one step. The hydrolysis and condensation of 3APTMS and dye coupling were preceded at the same time, as hydrolysis of 3APTMS was a quick procedure in the presence of water. The dye coupled SiO2 SMPs were introduced into a pre-hydrolyzed Stöber system to carry out the growth of silica layer to prevent the dye shedding. To establish the optimum conditions for attaching C.I. Reactive Blue 21 to SiO2 particles, several parameters were varied relating to both the amount of the 3APTMS and the amount of C.I. Reactive Blue 21 which were added into the SiO2-water suspension.
3.1 Optimization of 3APTMS amount
Initial experiments indicated that reactive dyes were not able to be efficiently adsorbed on the surface of unmodified SiO2 SMPs [9]. Efficiency of adsorption of the dye on the silica surface was significantly improved by modification of the surface with various organosilane reagents. SEM and DLS were used to examine the effect of 3APTMS amount on the morphology and particle size distribution. Figure 2a–h is SEM photographs of blue SiO2 SMPs obtained by variation of amount of 3APTMS. As shown in the Fig. 2a–d, when 0.5–20 μL of 3APTMS are added into the SiO2-water dispersion, the blue SiO2 SMPs show good dispersity. But severe aggregation or flocculation occurrs when the amount of 3APTMS is added up to 40 μL as shown in Fig. 2e–h. The alkoxy group of 3APTMS hydrolyzes first, then forms Si–O–Si bonds with surface silanol group of SiO2 SMPs in the presence of water [16], excess aminosilane causes crosslinking between particles [17]. The size distribution also almost matched above mentioned conclusion. As shown in Fig. 3, hydration radius of blue SiO2 SMPs increases with the amount of 3APTMS in the range of 0–60 μL. When the amount of 3APTMS added is 40 μL, the particles show slight aggregation. The more 3APTMS added, the more severe aggregation occurred, this resulted the size distribution moved right and became wider.
The amount of 3APTMS also affected coupling efficiency of C.I. Reactive Blue 21 onto SiO2 SMPs within a certain range. The maximum absorption wavelength at 614 nm of C.I. Reactive Blue 21 was determined by UV–vis spectrophotometer. Then obtained supernatants were collected to calculate the coupling efficiencies of the C.I. Reactive Blue 21 by multiskan spectrum at 614 nm. Figure 4 demonstrates that the coupling efficiency increased from 11.3 to 89.2% with the increase of 3APTMS in the range of 0.5–10 μL and kept at 84.6–89.0% in the range from 10 to 80 μL. From a visual point of view, when the amount of 3APTMS reached 10 μL, the increase of 3APTMS amount has a very small impact on color of blue SiO2 SMPs. The SEM, DLS and coupling efficiency results prove that dispersity and C.I. Reactive Blue 21 density on blue SiO2 SMPs are influenced by the amount of 3APTMS. Inadequate 3APTMS causes poor color of blue SiO2 SMPs and excess 3APTMS leads to agglomeration between blue SiO2 SMPs. In order to reduce the probability of aggregation between particles, the amount of 3APTMS was selected to be 10 μL.
3.2 Synthesis of the core-shell blue SiO2 SMPs in a Stöber system
Silica protection layer was deposited on the blue SiO2 SMPs to prevent the shedding of C.I. Reactive Blue 21. The blue SiO2 SMPs was introduced into a pre-hydrolyzed Stöber system to carry out regrowth of the silica layer onto blue SiO2 SMPs. SEM observations indicate that there is almost no change in morphology characteristic of the SiO2 SMPs after coupling with the dye and coating with a protection layer, as shown in Fig. 5a–c. The core-shell blue SiO2 SMPs are uniform and well dispersed after silica shell is grown on the blue SiO2 SMPs in the pre-hydrolyzed Stöber system. In this system, water accelerated the hydrolysis of Si-O-Si bond [15], large amount of water caused the agglutination of SiO2 SMPs. When core-shell blue SiO2 SMPs were synthesized, small amount of water was added to avoid the agglutination.
SEM image of SiO2 SMPs (a), blue SiO2 SMPs (b), and core-shell blue SiO2 SMPs (c); Size distribution of SiO2 SMPs, blue SiO2 SMPs and core-shell blue SiO2 SMPs (d); Zeta potentials of SiO2 SMPs, blue SiO2 SMPs and core-shell blue SiO2 SMPs dispersed in water with different pH (e) (color figure online)
Figure 5d shows that particle size distribution curve of SiO2 SMPs, blue SiO2 SMPs, and core-shell blue SiO2 SMPs. The diameter of core-shell blue SiO2 SMPs moved slightly to the right compared with original unmodified SiO2 SMPs, which proved that there was a little increase in particle size. According with particle size distribution curve, the average diameter of unmodified SiO2 SMPs was 195 nm, and that of the core-shell blue SiO2 SMPs was determined to be 207 nm, corresponding to a shell thickness of 6 nm. As can be seen from the size distribution curve, the hydrated radius of blue SiO2 SMPs is the biggest of three. Here we didn’t come up for discussion on the diameter of blue SiO2 particles which have the unknown quantity exposed groups such as amino groups and sulfonic acid groups (These groups were from dye molecules) that would increase the hydrated radius of blue SiO2 SMPs in the procedure of measure before regrowth of silica layer. The decrease hydrated radius of core-shell blue SiO2 SMPs proved that the deposition of the silica shell covered a lot of exposed groups of C.I. Blue Reactive 21, and made the blue SiO2 SMPs be pure silica surface.
Zeta potential of SiO2 SMPs, blue SiO2 SMPs, and core-shell blue SiO2 SMPs were also measured (Fig. 5e). The unmodified SiO2 SMPs were negatively charged in the range of pH 3–12. But after amino-modification and coloration, blue SiO2 SMPs presented an isoelectric point between 9 and 10. The isoelectric point shifted to much higher pH values suggesting that the density of 3APTMS (amino groups) on the blue SiO2 SMPs surface was high. Isoelectric point moved around 6.5 which demonstrated that there was a lot of negative charge on the surface of the core-shell blue SiO2 SMPs due to growth of silica protection layer onto blue SiO2 SMPs. The resulting core-shell blue SiO2 SMPs are stabilized electrostatically by virtue of the negatively charged hydroxyl due to regrowth of silica protection layer. The surface of core-shell blue SiO2 SMPs rich in hydroxyl groups, and thus can be grafted different groups through different kinds of silane coupling agent.
3.3 Dye leakage test
After silica protection layer was deposited on the blue SiO2 SMPs, a dye leakage test was done by measuring the absorbance of supernatant of blue SiO2 SMPs and core-shell blue SiO2 SMPs. These two submicron particles were washed with water for 18 times once an hour, and then were washed again after six hours, after centrifugation and supernatants were collected. Figure 6 demonstrates that C.I. Reactive Blue 21 is easy to shed from blue SiO2 SMPs. Severe dye shedding occurred at first, with the increase of water washing, the amount of dye leak decreased, but a small amount of dye still be washed off after 20 times of wash. There was almost no dye shedding from core-shell blue SiO2 SMPs during the dye leakage test, which was similarly reported by Xu et al. [18]. This proved that a growth of the silica protection layer was coated onto the blue SiO2 SMPs, and the silica protection layer effectively prevented the shedding of C.I. Reactive Blue 21.
3.4 Preparation of six other colored SiO2 SMPs
In order to verify the universal applicability of this method, six other colored SiO2 SMPs were prepared from five kinds of reactive dyes. As shown in Fig. 7, the bright colored SiO2 SMPs indicate that those reactive dyes coupling occur efficiently in aqueous dispersions with SiO2 SMPs in the presence of 3APTMS. The colors of colored SiO2 SMPs closely match with the color of the reactive dyes solution, reflecting the fact that the chromophore is not affected by these reactions and the color of colored SiO2 SMPs is easy to control. The color of the colored SiO2 SMPs can be altered widely with this method, depending on the kind and amount of organic dye. The resulting core-shell colored SiO2 SMPs prepared by this method were bright and stable. This confirms the fact that other reactive dyes may also be used to synthesize colored SiO2 SMPs by our method.
Colored SiO2 SMPs produced by coupling different organic reactive dyes from the left to right: C.I. Reactive red 136, C.I. Reactive Orange 1, C.I. Reactive Yellow 160, mixture of C.I. Reactive Blue 21 and C.I. Reactive Yellow 160 with the ratio of 1: 1(w/w), C.I. Reactive Blue 21, C.I. Reactive Blue 49, C.I. Reactive violet 5, respectively (color figure online)
4 Conclusion
In this work, we developed a two-step approach for the high efficiently coupling of reactive dyes onto SiO2 SMPs to synthesize monodispersed core-shell colored SiO2 SMPs for the first time. The C.I. Reactive Blue 21 was highly efficient covalently coupled on SiO2 SMPs by 3APTMS in aqueous solution. The prepared core shell blue SiO2 SMPs not only avoided from dye leakage, but also showed good dispersion, particle size uniformity, and homogeneous morphology, which make them a kind of good visible submicron label used in immunoassay. This approach is simple, economical saving, time saving, and environment friendly. And this two-step procedure takes advantage of the large inventory of commercial reactive dyes employed in the textile industry. Colored SiO2 SMPs not obtained any attention from immunoassay applications as colloidal gold, colored latex submicron particles, and quantum dots. No applications with colored SiO2 SMPs have been reported at the time of writing. In the following research, we would develop more immunoassay based on the core-shell colored SiO2 SMPs synthesized in this work.
References
Winnik FM, Keoshkerian B, Fuller JR, Hofstra PG (1990) New water-dispersible silica-based pigments: synthesis and characterization. Dyes Pigments 14(2):101–112
Yu H, Zhao G, Dou W (2015) Simultaneous detection of pathogenic bacteria using agglutination test based on colored silica nanoparticles. Curr Pharm Biotechnol 16(8):716–723
Sun Q, Zhao G, Dou W (2016) An optical and rapid sandwich immunoassay method for detection of Salmonella pullorum and Salmonella gallinarum based on immune blue silica nanoparticles and magnetic nanoparticles. Sens Actuators B: Chem 226:69–75
Sun Q, Zhao G, Dou W (2015) A nonenzymatic optical immunoassay strategy for detection of Salmonella infection based on blue silica nanoparticles. Anal Chim Acta 898:109–115
Sun Q, Zhao G, Dou W (2015) Blue silica nanoparticle-based colorimetric immunoassay for detection of Salmonella pullorum. Anal Methods 7(20):8647–8654
Giesche H, Matijevi E (1991) Well-defined pigments: I. Monodispersed silica-acid dyes systems. Dyes Pigments 17(4):323–340
Binkowski S, Jesionowski T, Krysztafkiewicz A (2000) Preparation of pigments on modified precipitated silicas. Dyes Pigments 47(3):247–257
Krysztafkiewicz A, Binkowski Sa, Wysocka I (2003) Pigments on amorphous silica carriers. Powder Technol 132(2-3):190–195
Krysztafkiewicz A (2004) Application of silica-based pigments in water-borne acrylic paints and in solvent-borne acrylic paints. Dyes Pigments 60(3):233–242. https://doi.org/10.1016/s0143-7208(03)00149-9
Wang W, Vaughn MW (2008) Morphology and amine accessibility of (3-Aminopropyl) triethoxysilane films on glass surfaces. Scanning 30(2):65–77
Vandenberg ET, Bertilsson L, Bo L, Uvdal K, Erlandsson R, Elwing H, Lundström I (1991) Structure of 3-aminopropyl triethoxy silane on silicon oxide. J Colloid Interface Sci 147(1):103–118
Cuoq F, Masion A, Labille J, Rose J, Ziarelli F, Prelot B, Bottero JY (2013) Preparation of amino-functionalized silica in aqueous conditions. Appl Surf Sci 266(2):155–160
Wang W, Baohua G, Liyuan L, Hamilton W (2003) Fabrication of two- and three-dimensional silica nanocolloidal particle arrays. J Phys Chem B 107(15):3400–3404
Zhang T, Zhang Q, Ge J, Goebl J, Sun M, Yan Y, Liu Y, Chang C, Guo J, Yin Y (2009) A self-templated route to hollow silica microspheres. J Phys Chem C 113(8):3168–3175
Liang J, Xue Z, Xu J, Li J, Zhang H, Yang W (2013) Highly efficient incorporation of amino-reactive dyes into silica particles by a multi-step approach. Colloids Surfaces A Physicochem Eng Aspects 426(21):33–38
Choi H, Chen IW (2003) Surface-modified silica colloid for diagnostic imaging. J Colloid Interface Sci 258(2):435–437
Westcott SL, Oldenburg SJ, Lee T, Halas NJ (1998) Formation and adsorption of clusters of gold nanoparticles onto functionalized silica nanoparticle surfaces. Langmuir 14(19):5396–5401
Liu H, Wang Y, Li H, Wang Z, Xu D (2013) Luminescent rhodamine B doped core–shell silica nanoparticle labels for protein microarray detection. Dyes Pigments 98(1):119–124
Acknowledgements
This work was financially supported by a grant from National Natural Science Foundation of Zhejiang Province (LY17C200003), the Food and Engineering most important discipline of Zhejiang province (2017SIAR210, JYTSP20141062), Zhejiang public Innovation Platform Analysis and testing project (2015C37023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhu, C., Zhao, G. & Dou, W. A new synthesis method for bright monodispersed core-shell colored silica submicron particles. J Sol-Gel Sci Technol 85, 76–83 (2018). https://doi.org/10.1007/s10971-017-4515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4515-8