Abstract
230Th/234U radiochronometer was commonly used for uranium age determination which could help to reveal the origin of the nuclear material. 229Th was a relatively appropriate spike for measuring 230Th by isotope dilution mass spectrometry and its concentration needs to be calibrated before using as spike. A prepared 229Th solution which was used as Th isotope spike for age dating of CRM U850 sample were calibrated separately by 232Th and 230Th standard materials. The calibrated results were compared and evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
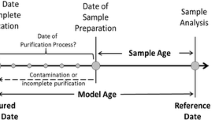
Uranium age could be defined as the time since the uranium material was last subjected to a separation process capable of separating uranium from its radioactive-decay daughters [1, 2]. It is a dynamic fingerprint of nuclear material and could help to reveal the origin of the nuclear material and trace back the possible route of the sample to its source, which could be verified against the declared origin in nuclear forensics [3, 4].
Several isotope ratios such as 230Th/234U, 231Pa/235U and 214Bi/234U have been successfully applied for the determination of the age of uranium [5,6,7]. However, in case of 231Pa/235U lack of suitable isotopic spike inhibits its application, while for 214Bi/234U ratio the method is restricted only to highly enriched uranium (HEU) samples or old nuclear materials due to the slow ingrowth of this daughter nuclide [8]. 230Th/234U isotope ratio was commonly used for uranium age dating. For most samples of uranium, ages determined with this 230Th-234U chronometer are considered as “model ages” by R. W. Williams [9], because they are based on the assumptions of (a) some initial amount of 230Th in the sample, and (b) closed-system behavior of the sample since production. Under the condition of meeting these assumptions the accuracy of dates determined with the 230Th-234U chronometer only relies upon accurate 230Th and 234U measurements.
In the developed isotopic dilution mass spectrometric methods for determining ratio of 230Th/234U, 229Th solution was used as spike for 230Th. It was usually prepared by separating the 229Th from old 233U solution [7,8,9]. The 229Th solution should be calibrated by reverse isotope dilution measurement with another isotope standard material before use. Usually, it is a long and complicated process to determine the age of uranium material.
Both the 230Th and 232Th isotope standard reference material could be used for 229Th calibration [10]. The aim of this study was to develop a more exact 229Th calibration method for the age determination of uranium materials using 229Th solution as spike by isotope dilution multi-collector inductively coupled plasma mass spectrometry.
Experimental
Instrumentation and materials
The mass spectrometric analysis was carried out using an inductively coupled plasma mass spectrometer equipped with 7 Ion counters, 1 Daly detector and 9 Faraday cups (Micromass Isoprobe-T, GV). The 1.5 mg uranium oxide reference material (CRM 850) was dissolved in 10 mL HNO3 for age analysis. The 233U isotopic standard (IRMM051) was used to spike the samples for 234U measurements. 229Th spike for 230Th analysis were calibrated by natural Th-solution (Relative standard uncertaintyof certified value is 0.3% (k = 1), SPEXertificate) and 230Th standard material provided by LLNL (prepared from NIST 4342A, Relative standard uncertainty of certified value is 0.19% (k = 1)). Standard solutions (prepared from ERM-199, CRM U030, CRM U200 and working standard UTB900) were used to correct for instrumental mass discrimination and relative detector gain factors. The TEVA (100–150 μm) extraction chromatographic resin was used for chemical separation. All sample preparation procedures were carried out under clean room conditions (Class 100,000).
229Th spike calibration by 232Th
The primary 229Th solution which is as 229Th spike used for the 230Th determination was prepared by separating the Th from an old 233U solution. The 229Th concentration in the primary solution obtained was determined by reverse isotope dilution ICP-MS measurement applying a 0.870(7) µg g−1 232Th standard solution which was obtained by successively diluting the certified natural Th-solution with 2% HNO3 solution. Three aliquots of 0.5 mL 229Th solution were added in 0, 5 and 2.5 mL 0.870(7) µg g−1 232Th standard solution respectively. After being isotopic equilibrium, 229Th/232Th atom ratios in three samples were measured by MC-ICP-MS. Both the 229 Th and 232Th ionic beams were collected by faraday cups (FC).
229Th spike calibration by 230Th
The primary 229Th solution was successively diluted with 2% HNO3 solution to get around 100 pg g−1 229Th solution (named as solution2). Each dilution was approximately 100-fold and 30-fold respectively. Three aliquots of 0.8 mL solution2 were added in around 0, 80 and 80 pg 230Th standard reference solution which was from gravimetrically diluting 230Th standard reference material (NIST 4342A) respectively. After being isotopic equilibrium, 229Th/230Th ratios in three samples were measured by MC-ICP-MS. Both the 229Th and 230Th ionic beams were collected by ionic counters (IC).
Sample preparation for 234U determination
Approximately 15 μg aliquot of the dissolved uranium oxide sample (CRM 850) was used for the analysis. The sample was diluted twice successively with 2% ultrapure nitric acid solution. Each dilution was approximately tenfold and 100-fold respectively. The 234U concentration following the second dilution was determined by isotope dilution ICP-MS measurement using 233U standard as spike.
Sample preparation for 230Th determination
The separation method of Th from U in Ref. [2] was adopted. Approximately 300 μg aliquot of the dissolved uranium oxide sample (CRM 850) was placed in a Teflon cup and spiked with approximately 2 pg of 229Th spike gravimetrically, respectively. The Th-content of the sample was separated from uranium by 1.6 mL TEVA resin. In order to further purify the sample, the extraction chromatographic separation was repeated.
Data evaluation
All raw data were corrected taking into account instrumental mass bias using linear correction. The overall uncertainty was calculated taking into account the uncertainty of the weight measurements, spike concentrations, isotope ratios measurement and half-lives according to “A Guide to Expression of Uncertainty in Measurement” (ISO/1995).
Results and discussion
The 229Th concentration in the working solution obtained was determined twice by reverse isotope dilution ICP-MS measurement applying certified natural Th-solution with different additive quantum. CRM U200 was used to correct instrumental mass bias. The results were showed in Table 1. The twice determined isotope concentrations were identical within the range of number of significant digit.
The 229Th concentration in the working solution were obtained according to the gravimetrical dilution and the calibrated Th isotope concentration in dilution2 which was repetitively determined twice by reverse isotope dilution ICP-MS measurement applying 230Th standard reference solution. CRM U005 and CRM U200 were used to correct instrumental mass bias and relative detector gain factors. The results were showed in Table 2. The relative deviation between two determined isotope concentrations was in 2%.
Two calibration methods could be expressed in the Eqs. (1–2). Assumed \( 9_{\text{p}} \) was the 229Th concentration of primary 229Th solution and \( x_{\text{p}} \) was the 232Th or 230Th concentration of primary 229Th solution. \( \left( {{\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 x}}\right.\kern-0pt} \!\lower0.7ex\hbox{$x$}}} \right)_{\text{pm}} \) was the determined Th isotope ratios of primary 229Th solution. \( \left( {{\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 x}}\right.\kern-0pt} \!\lower0.7ex\hbox{$x$}}} \right)_{\text{mm}} \) was the determined Th isotope ratios of spiked aliquot primary 229Th solution by 232Th or 230Th standard reference material. \( m \) and \( n \) were the weight of aliquot primary 229Th solution and weight of 232Th or 230Th standard reference material respectively. \( x_{\text{s}} \) was the concentration of 232Th or 230Th standard reference material.
\( 9_{\text{p}} \) could be expressed to Eq. (3) by the solution of Eq. (1) and Eq. (2).
Linear propagation of uncertainty on the weight of aliquot primary 229Th solution \( m \), weight of 232Th or 230Th standard reference material \( n \), the concentration of 232Th or 230Th standard reference material \( x_{\text{s}} \), the determined Th isotope ratios of primary 229Th solution \( \left( {{\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 x}}\right.\kern-0pt} \!\lower0.7ex\hbox{$x$}}} \right)_{\text{pm}} \), and the determined Th isotope ratios of spiked aliquot primary 229Th solution \( \left( {{\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 x}}\right.\kern-0pt} \!\lower0.7ex\hbox{$x$}}} \right)_{\text{mm}} \) results to:
For calibration by 230Th, the uncertainty on the weight of aliquot primary 229Th solution \( m \) was including of uncertainty of dilution process. Two representative uncertainty budgets for the 229Th spike calibration by 232Th and 230Th were given in Table 3.
The calibrated Th isotope concentrations by two methods were close, but in calibration by 232Th, the twice determined isotope concentrations were identical within the range of number of significant digit and in calibration by 230Th, there was a deviation between twice measurements. The difference between two calibration methods could also be concluded by the math theory. For convenience, the computation (3) of 229Th concentration in primary solution could be simplified into Eq. (5), as assuming the amount of 232Th or 230Th in original primary solution could be ignored. \( x \) is the added true amount of 232Th or 230Th standard material. \( 229 \) is the amount of 229Th in primary 229Th solution.
When the uncertainty of measurement could be ignored, we could assume that \( \left( {{\raise0.7ex\hbox{${229}$} \!\mathord{\left/ {\vphantom {{229} x}}\right.\kern-0pt} \!\lower0.7ex\hbox{$x$}}} \right)_{\text{measure}} \) is the true isotope ratio value and \( \Delta x \) is the distance between reference value used for calculation and true value \( x \).Then 229Th concentration in primary solution could be expressed by Eq. (6).
By math operation, the Eq. (7) could be gotten from Eq. (6).
Similarly, when added different amount of th isotope standard expressed by \( kx \). The apparent amount used for calculation is equal to the value of \( k \) multiplied by sum of \( x \) and \( \Delta x \). The calculated expression of 229Th concentration in primary solution from Eq. (8) is the same to the Eq. (7).
That means when adding different amount of Th standard material, if the uncertainty of measurement could be very little to be ignored the calibrated 229Th concentration in every determination are same. Because the \( \Delta x \) is a fixed value which is independent on the measurement process. From the uncertainty budget in Table 3, we can see the contribution from the measurement to total uncertainty could be ignored relative to the standard reference material. So it could be explained the twice calibrated 229Th concentration were identical.
From the above deducing we know the deviation of reference value of standard material didn’t affect the point-to-point reproducibility of calibrated results. So it could be assumed the apparent added amount of Th isotope standard material is true value \( x \) and \( \Delta^{1} \) is the distance between measured isotope ratio value and true isotope ratio value. In calibrating by 230Th, when the deviation of measurement could not be ignored, the calibrated 229Th concentration in primary solution could be expressed by Eq. (9).
By math operation, the Eq. (10) could be gotten from Eq. (9).
Similarly, when adding different amount of Th isotope standard material, the calculated Eq. (11) of 229Th concentration in primary solution is different with the Eq. (10). Deltas were different in every measurement due to fluctuant parameter. So it could be explained two calibrated results by 230Th have deviation.
As the uncertainty budget in Table 3 shown, the largest contribution to combined uncertainty of 229Th concentration calibrated by 230Th was from sample ratio measurement. Standard reference material for Mass bias correction and concentration of 230Th standard reference material were two significant contributors to combined uncertainty of 229Th concentration. But for calibration by 232Th the only one significant contributor was from the reference concentration value of 232Th standard reference material. The difference may be due to that the precision of measurement by IC for relative low concentration sample is lower than the precision of measurement by FC for relative high concentration sample. The calibration process contribution to combined uncertainty of 229Th concentration calibrated by 232Th could be less with one order of magnitude than the reference concentration value of 232Th standard reference material. It was reflected by the twice identical determined isotope concentrations within the range of number of significant digit in calibration by 232Th. It seems that the calibration method by 232Th is superior to calibration method by 230Th. The combined uncertainty of 229Th concentration in single observation calibrated by 232Th is a little higher than by 230Th just because the relative uncertainty of used 232Th standard reference material is 8.5‰ which is much larger than 2.5‰ of 230Th standard reference material. Considering about the reproducibility of two independent measurements, the combined uncertainty of average 229Th concentration in spike calibrated by 230Th was larger than by 232Th.
According to the weight of spikes and determined isotope ratios, the 234U/230Th ratio could be calculated. The model age was calculated according to simplified age-dating Eq. (12) where R is the 230Th/234U atomic ratio, and λ 1 and λ 2 are the decay constants of 234U and 230Th, respectively. Half-lives for 230Th and 234U of 75690 ± 230 years and 245250 ± 490 years were used for these decay constants, respectively [11].
The results of age determination were shown in Table 4. The purification date was calculated: 1957-4-15 ± 209 days for CRM U850 with 229Th spike calibrated by 232Th and 1956-2-18 ± 301 days for CRM U850 with 229Th spike calibrated by 230Th. The U850 analyzed give the determined age older than the purification dates of record (1957-12-31). This indicates that excess 230Th in U850 standard results from incomplete purification during production.
The determined model age of CRM U850 and some results in the literature were shown in the Fig. 1. The data showed good agreement with the ages determined in the earlier works by Wallenius et al. [2] and Williams and Gaffney [8] using the ratio 234U/230Th. The 230Th was determined with 232Th spike in the work by Wallenius et al. and the 230Th was determined with 229Th spike which was calibrated by the NIST SRM 4342A 230Th radioactivity solution in the work by Williams & Gaffney. The determined purification date of CRM U850 using the 229Th spike calibrated by 232Th which in this work was lying between the results in the literature was closer to the average model age of current published results than the determined purification date of CRM U850 using the 229Th spike calibrated by 230Th which was earlier than the results in the literature.
Two representative uncertainty budgets for the age-dating analyses were given for sample CRM U850 in Table 5, 6. The largest part of the uncertainty in these analyses comes from the 229Th spike, of which the uncertainty on the Mass bias correction and ratio measurement for 230Th/229Th analysis were two significant contributors to combined uncertainty of determined U850 age. The accuracy concentration of 229Th spike has a great effect on the result of determined age of Uranium material.
Conclusions
Precisely and accurately calibrating Th isotope spike was very important to determine uranium model age by 230Th/234U radiochronometer. Using higher concentration primary Th spike solution to be directly calibrated and be used as spike for uranium age dating could get more accuracy results than diluted to be calibrated especially in the case the uncertainty of standard materials for calibration were similar. Easily available 232Th standard material could be used to calibrate 229Th spike to get accuracy results instead of uncommon 230Th standard material.
References
Morgenstern A, Apostolidis C, Mayer K (2002) Age determination of highly enriched uranium: separation and analysis of 231Pa. Anal Chem 74:5513–5516
Wallenius M, Morgenstern A, Apostolidis C, Mayer K (2002) Determination of the age of highly enriched uranium. Anal Bioananl Chem 374:379–387
Varga Zsolt, Wallenius Maria, Mayer Klaus (2010) Age determination of uranium samples by inductively coupled plasma mass spectrometry using direct measurement and spectral deconvolution. J Anal At Spectrom 25:1958–1962
Varga Zsolt, Wallenius Maria, Mayer Klaus, Hrnecek Erich (2011) Alternative method for the production date determination of impure uranium ore concentrate samples. J Radioanal Nucl Chem 290:485–492
Eppich Gary R, Williams Ross W, Gaffney Amy M, Schorzman Kerri C (2013) 235U–231Pa age dating of uranium materials for nuclear forensic investigations. J Anal At Spectrom 28:666–674
Nguyen Cong Tam, Zsigrai József (2006) Gamma-spectrometric uranium age-dating using intrinsic efficiency calibration. Nuclear Instrum Methods Phys Res B 243:187–192
LaMont SP, Hall G (2005) Uranium age determination by measuring the 230Th/234U ratio. J Radioanal Nucl Chem 264:423–427
Varga Z, Surányi G (2007) Production date determination of uranium-oxide materials by inductively coupled plasma mass spectrometry. Anal Chim Acta 599:16–23
Williams RW, Gaffney AM (2011) 230Th-234U model ages of some uranium standard reference materials. Proc Radiochim Acta 1:31–35
Varga Z, Mayer K, Bonamici CE, Hubert A, Hutcheon I, Kinman W, Kristo M, Pointurier F, Spencer K, Stanley F, Steiner R, Tandon L, Williams R (2015) Validation of reference materials for uranium radiochronometry in the frame of nuclear forensic investigations. Appl Radiat Isot 102:81–86
Cheng H, Edwards RL, Hoff J, Gallup CD, Richards DA, Asmerom Y (2000) The half-lives of uranium-234 and thorium-230. Chem Geol 169:17–33
Acknowledgements
The authors wish to thank professor Ross W. Williams in Lawrence Livermore National Laboratory (LLNL) for providing the 230Th standard material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Zhao, YG., Li, LL. et al. A comparison of two calibration methods of the 229Th spike for uranium age dating by 230Th/234U radiochronometer. J Radioanal Nucl Chem 314, 499–505 (2017). https://doi.org/10.1007/s10967-017-5396-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5396-6