Abstract
In this research, a hydroxyl-terminated polydimethylsiloxane (HTPDMS) was modified by the incorporation of siliconized epoxy resin (SER) containing a segment of siloxane on the side chain of epoxy resin. The chemical structure of the SER was studied by FT-IR, GPC and NMR techniques. Adhesion test showed that the adhesion strength of silicone rubber systems improved markedly after modification. Contact angle (CA) measurements showed the presence of the epoxy block at the surface, which improved adhesion properties of the modified HTPDMS. Morphology studies (SEM) displayed that enhancement in the mechanical properties of the silicone rubber systems due to good compatibility between SER and silicone rubber matrix. Thermogravimetric analysis (TGA) indicated that the residual yield at 800 °C of modified HTPDMS increased compared with that of the neat HTPDMS, but the onset decomposition temperature was reduced with the increase in SER content. These observations highlight the significant role of SER in the modification of HTPDMS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polydimethylsiloxane (PDMS) has been one of the most explored materials for the fabrication of adhesive structures among all polymers [1, 2]. The interest in this polymer is owing to its superior thermal and thermo-oxidative stability, excellent moisture resistance, partial ionic nature, low surface energy, good flame retardancy and free rotation of chains about Si-O bonds, good hydrophobicity, compressivity and doping action [3–10]. These are all important requirements for its possible use as anti-corrosion coatings and structure bonding materials.
However, this potential application is limited by the risk of adhesion failure due to its low surface free energy (19.9 mJ/m) and extremely low chemical reactivity [11, 12]. In addition, the poor mechanical properties of silicone also make it easy to be destroyed in the process of application. In light of these problems, researchers have increased the PDMS surface energy and reactivity via the introduction of reactive functional groups onto the polymer surface [13–24]. The functionalization of polymer surfaces is usually achieved by plasma treatment. For instance, Karkhaneh et al. [23] successfully grafted Acrylic acid (AAc) and 2-hydroxyethyl methacrylate (HEMA) mixtures onto the surfaces of polydimethylsiloxane (PDMS) films using a two-step oxygen plasma treatment (TSPT). The effects of pretreatment and polymerization time length, monomer concentration, and ratio on peroxide formation and graft amount were studied. However, a major drawback of plasma is that the PDMS surface can be transformed into a brittle silica-like layer and the modified PDMS will lose its hydrophilicity in a very short time after such treatments [12, 18, 21]. Polymer brushes synthesized on the silicone rubber that is also an effective method to modify the polymer surface [25–27]. The stimuli responsive ultra-thin diblock copolymer brushes were developed on the surface of silicone rubber by Jalili et al. [25]. The authors used temperature dependent two-dimensional correlation spectroscopy to study the thermally-induced evolution mechanism of various interactions. But polymer brushes can only change surface properties of PDMS without improving the mechanical properties.
In contrast, the modification of PDMS with epoxy resins as a second component can improve the mechanical and adhesion properties without damaging its surface. Epoxy resin has excellent mechanical, electrical, and bonding properties. This resin is widely utilized as a high performance thermosetting resin in many industrial fields [28–30]. The good mechanical, chemical property and versatility in formulation made epoxy resins widely used in industry for surface coating and paint formulations [31]. The incorporation of epoxy in polydimethylsiloxane will result in the enhancement of mechanical and adhesion properties. However, the solubility parameter of polydimethylsiloxane: 7.4–7.8, is much lower than that of the epoxy resin, which is about 10.9 [32]. Polydimethylsiloxane is therefore not compatible with epoxy resin. More recently, the addition of copolymers to PDMS has been proved to be a valuable pathway to tune the properties of silicone coatings [33–37]. So far there have few reports focused on the mechanical and adhesion properties of epoxy modified silicone-based coatings.
In this work, we synthesized grafted copolymers containing a segment of siloxane on the side chain of epoxy resin. Then, the copolymers were dispersed into a HTPDMS, followed by matrix cross-linking at room temperature. The effects of the copolymer on the mechanical, thermal and morphology properties of the silicone rubber systems were analyzed in detail.
Experimental section
Materials
Bisphenol-A type epoxy resins (DGEBA) with the epoxide equivalent weight of 220–230, was supplied by Jiangsu Wuxi Resin Plant, China. A methoxy group-containing methyl phenyl silicone intermediate (PMPS) was synthesized by our lab, with the methoxy content of 18 wt % and a 50/50 ratio of Si-phenyl/Si-CH3. The hydroxyl terminated polydimethylsiloxane (HTPDMS) (viscosity is about 10000cst) which was a kind of room temperature vulcanized silicone rubber (RTVSR) was purchased from China BlueStar Chengrand Research Institute of Chemical Industry. Tetraisopropyl titanate (TPT) obtained from J &K Scientific Ltd. was used as catalyst directly. The curing agent, 3-Triethoxysilylpropylamine (APTES) was supplied kindly by China BlueStar Chengrand Research Institute of Chemical Industry. Dibutyltindiaurate (DTBDL), which was used as catalyst during the vulcanization, was purchased from Chengdu Chemical Reagent Company, China. All of the materials utilized in this study were used directly without further purification.
Synthesis of siliconized Epoxy Resin (SER)
DGEBA (200 g) was firstly fed into a three-necked round-bottom flask equipped with reflux condenser, mechanical stirrer, and thermometer and heated until the DGEBA melted. Then, 33 g of PMPS with 0.5 wt % of TPT was added. The blends were heated to 130 °C and carried out for 7 h under N2 atmosphere. The crude product named was then washed twice with toluene to remove the unreacted PMPS. The reaction process was illustrated in Scheme 1.
Preparing procedure of SER filled silicone rubber systems
HTPDMS, SER were mixed together in a beaker at 100 °C under mechanical stirring for 15 min. After the mixture was cooled to room temperature, a mixture of curing agent (APTES) and catalyst (DTBDL) were added into it. The liquid compounds were completely mixed by a mechanical stirrer and degassed with a vacuum pump to eliminate air bubbles. The bubble-free mixtures were then poured into a polytetrafluoroethylene (PTFE) mold to fabricate samples. The curing reaction was conducted at room temperature for 7 days. The process of preparing the silicone rubber materials can be seen in Scheme 2. The cured samples were carefully removed from the mold and used for various tests. The additive amounts of SER, APTES, and DTBDL used to prepare the silicone rubber systems in this study are presented in Table 1. For simplicity, silicone rubber systems containing SER were designated as S0, S10, S20, S30, S40, and S50.
Characterization
FTIR spectroscopy
Infrared spectra of monomer and polymer (liquid sample as well as film) were recorded in the range of 4000–400 cm−1 with a resolution of 1 cm−1 with a Nicolet 570 FTIR spectrophotometer (Nicolet, USA). The liquid samples were directly dropped on KBr pellets, and the samples were scanned from 4000 to 500 cm−1.
NMR measurements
The NMR measurements were carried out on a DRX-400 (Bruker Company, Germany) 400 MHz NMR spectrometer to obtain 1H-NMR spectra at 25 °C. The samples were dissolved in CDCl3.
Gel permeation chromatography (GPC)
Polymer molecular weight (Mw) was measured using a gel permeation chromatography (GPC), which consists of a HLC-8320 chromatograph (Tosoh, Japan) equipped with two columns (TSK gel super HZM-H 6.0 × 150 mm) in serials. The samples were analyzed at 40 °C with THF as an eluent and the flow rate was set at 0.6 mL/min. Polystyrene (PS) was used as calibration standards.
Mechanical properties
The tensile properties of the cured specimens were measured with an Instron (Instron 5567, Instron, USA) universal testing instrument at a rate of 500 mm/min according to ISO 37:2005. The cured films were cut into strips of 100 mm × 5 mm in size. Test specimens were examined for each composition and the average result of five highest readings at peak load was reported as tensile strength. The strain values at the breaking point were used to obtain % elongation. All mechanical values were taken from an average of five samples.
Morphology of cured blends
The morphology of the fracture surfaces was observed using a scanning electron microscope (SEM: JSM-5900, JEOL, Japan) at an accelerating voltage of 10 kV. Prior to the observation, the surfaces of all the samples were coated with gold to enhance conductivity and prevent charging.
Contact angle
Contact angle was measured by the sessile drop method using a DSA30 contact angle analyzer (KRUS3, Germany). In this method the contact angle is formed between the liquid/solid interface and liquid/air interface. A micrometric syringe was used to dispense a 10 μL droplet of deionized water on the surfaces. The contact angles of five different points on the samples were measured (25 °C) and their averages were reported. The surface energies of different samples were calculated using equation of state (EOS) method by the contact angle instrumental. The equations are as follows:
Where \( {\sigma}_s \) is the surface energy of the solid, \( {\sigma}_l \) is the surface energy of the liquid, \( {\gamma}_{sl} \) is the interfacial tension solid/liquid and θ is the contact angle.
Adhesion properties
The shearing strength the cured specimens were conducted with the help of Universal tensile instrument (Instron 5567) according to ISO 4587:2003. The test was performed under a rate of 5 mm/min at room temperature. All adhesion values were taken from an average of five samples. The substrate used in this method is aluminum sheet.
Thermogravimetric analysis (TGA)
TGA was conducted on a thermogravimetric analyzer (TG 209F1 Iris, Netzsch, Germany) to investigate the thermal stability of the samples under dry nitrogen gas with a flow rate of 60 mL/min. The samples were heated at a rate of 10 °C/min, and the relative mass loss of the samples was recorded from 50 to 800 °C.
Dynamic mechanical analysis (DMA)
The conversion and glass transition temperature of the cured samples were detected by DMA on TA Instruments (TA Instruments Q800 apparatus, USA). The experiment was performed in tensile mode at a heating rate of 3 °C/min from −150 to 150 °C and with a frequency of 1Hz and 0.2 % strain. The temperature corresponding to the peak in the loss tangent (tan δ) versus temperature plot was taken as the glass transition temperature (Tg).
Results and discussion
Characterization of siliconized Epoxy Resins (SER)
The FTIR spectra of DGEBA, PMPS, and SER are shown in Fig. 1. The disappearance of characteristic peak for –OCH3 of PMPS, which is located at 2845 cm−1, can be deduced from the spectra. In the meanwhile, the weakened signal for –OH groups at 3479 cm−1 was noted and the stretching vibration of Si-O-Si and Si-O-C bonds is overlapped at the range from 1184 to 1036 cm−1 in the FTIR spectrum of SER. These all results indicate that DGEBA was successfully reacted with PMPS. Moreover, The infrared spectrum of SER exhibits major absorptions at 3494 cm−1 (OH), 2967 cm−1, 1259 cm−1, 805 cm−1 (Si-CH3), 1607 cm−1, 1459 cm−1, 489 cm−1 (Si-phenyl), 1360 cm−1 [(CH3)2C], and 913 cm−1 (oxirane).
The 1H-NMR of SER is shown in Fig. 2. The 1H-NMR of pure epoxy resin (δ, ppm) is: 7.12–6.83 (aromatic ring protons), 4.17 (−CH2-O-Ar), 3.33 (−CH, oxirane), 2.9–2.75 (−CH2, oxirane), 1.63 (−CH3). The presence of characteristics peaks in 1H-NMR spectra (δ, ppm, 2.17 (CH-O-Si)) of SER attributed to the reaction between Si-OCH3 and CH-OH of PMPS and epoxy resin (Scheme 1). These results further support the formation of the SER copolymer.
GPC test shows that the weight average molecular weight (Mw) of epoxy resin is 393 g/mol and the Mw of SER is 816 g/mol, which verify that the reaction between PMPS and epoxy occurred.
Figure 3 shows the photographs of two cured silicone rubber systems added by 30 phr DGEBA and 30 phr SER, respectively. As seen in Fig. 3a, it is obvious that HTPDMS modified with epoxy resin directly could not be cured owing to the big difference in solubility parameter of them, and it is not suit for using as adhesive materials. However, HTPDMS modified by SER (Fig. 3b) not only can be completely cured but also possesses good performance. This means that SER has been successfully prepared and shows good compatibility with HTPDMS.
Mechanical properties
To investigate the mechanical properties of the SER modified silicone rubber samples, tensile measurements were carried out and the results were presented in Fig. 4a and b. Figure 4a shows that SER modification leads to the growth of tensile strength as well as modulus of the silicone rubber systems when the content of SER increased from 0 to 30 phr. This was mainly ascribed to the higher amount of stress withstood by the rigid epoxy chain. In this circumstance, SER modification essentially has an effect equivalent to that of internal reinforcing filler. However, when the content of SER was increased further, the tensile strength did not fulfill the expectation. This was attributed to that high content of SER is easy to cause stress concentration and advanced damage.
As shown in Fig. 4b, the elongation at break of silicone rubber systems reaches 337 % at the SER content of 40 phr, increasing almost 2-folds compared with the unmodified silicone rubber systems. By contrast, when SER is added from 0 to 40 phr, the entanglements of molecular chain in the silicone rubber systems increased rapidly owing to the rigid molecular chain and strong interaction between molecular chains of epoxy. Therefore, the elongation of silicone rubber systems improved markedly. With further increasing SER to 50 phr, the reduction of the elongation can be ascribed to that too much rigid epoxy molecular chain is easy to cause the stress concentration.
Surface properties
Figure 5 demonstrates how the water contact angles of silicone rubber systems change after the silicone rubber was modified by SER. The water contact angles of pure RTV silicone rubber is 115°. The value is consistent with the value reported by J. Li et al. [38]. They reported that the water contact angles of current RTV silicone rubber coatings are smaller than 120°. Then, we observe a steady decrease in the contact angles as the weight fraction of SER increased. This could be attributed to that the epoxy chains can improve the hydrophilic properties of the RTV silicone rubber systems via oxygen containing groups such as hydroxyl and oxirane [39–41]. However, the slope of the decrease was very small. On one hand, the contact angles will decrease after SER was incorporated. On the other hand, the rearrangement of silicone segments outward the surface that increases hydrophobicity [2] leading the increase of contact angle.
Adhesion properties
Adhesion to the protected material is an important factor because improper adhered coatings can peel off protected material surfaces under strong mechanical stress. As shown in Fig. 6, the unmodified silicone rubber has an adhesion strength of 0.41 MPa. This could be attributed to the non-polar nature and poor mechanical properties of the RTV silicone rubber. We observe that the adhesion strength of silicone rubber systems increases significantly after filling the SER from 10 to 30 phr, but decreases with the weight fraction of SER from 30 to 50 phr.
According to S.K. Rath [21], the relative adhesion of the fouling organisms with the coating varies linearly with the (δ · E)1/2, where δ is the surface energy and E is the modulus of the coatings. The surface energy (δ), modulus (E), the (δ · E)1/2 values and adhesion strength of the different silicone rubber samples are is presented in Table 2.
The results are consistent with the results of the Adhesion strength. Nicolas Amouroux et al. [42] reported that the peel energy is dominated either by frictional losses associated with slip on the interface or by viscous dissipation due to shear deformations distributed in the volume of the adhesive. So, the notable improvement of adhesion strength is mainly ascribed to two reasons. First, epoxy chains can interact with the matrix via polar group such as hydrogen bond and epoxy group [43] thus increasing frictional losses. Second, as explained in the mechanical analysis, the mechanical properties would be enhanced by incorporating SER. This effect could generate much more viscous dissipation than the pure silicone rubber systems. These results clearly highlight that the SER play a significant role in the attachment of silicone rubber systems to the aluminum sheet. The supreme value of adhesion was observed for the 30 phr SER modified silicone rubber systems.
Morphology of the silicone rubber systems
The morphology of the fracture surface of the silicone rubber systems with the contents of SER for 0 and 30 phr are shown in Fig. 7. The pictures indicated that the pure silicone rubber has a smooth surface, while the surface of SER filled silicone rubber systems become rough and has some wrinkles. As it can be seen in Fig. 7, it is obvious that SER filled silicone rubber systems exhibit a typical sea-island structure. The average diameter of the dispersed phases of SER was 0.7 μm at the loading of 30 phr and the size distribution was narrow. The reasons for good compatibility between SER and HTPDMS may be that 1) The siloxane part of siliconized epoxy (SER) acts as an effective compatibilizer. 2) the vulcanizing agent (APTES) can not only react with silicone rubber via alkoxy group but also react with epoxy via amino group, thus further improving the compatibility of the SER and HTPDMS (Scheme 2). This morphological feature is conjectured to be responsible for the observed increase in the mechanical properties.
Figure 8a and b show the morphology of the silicone rubber systems with the contents of SER for 0 and 30 phr at low magnification, respectively. The pure silicone rubber systems (blank sample) exhibited a smooth microstructure without plastic deformation (Fig. 8a). However, some cracks could be found in the blank sample, this is because that HTPDMS was easy to be destroyed under the stress due to its poor mechanical properties. By contrast, lots of wrinkles were obviously observed in Fig. 8b. This means that SER enhanced the mechanical properties of HTPDMS and the silicone rubber systems have absorbed more energy during the process of destruction.
TGA analysis of the cured silicone rubber systems
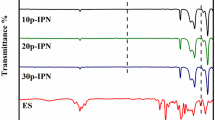
The TGA and derivative thermograms (DTG) as a function of temperature for different silicone rubber systems are presented in Fig. 9a and b, respectively. It is shown in Fig. 8 that the thermal degradation of silicone rubber systems takes a two mass loss steps. The first step during the temperature range from 300 to 440 °C was attributed to depolymerization of HTPDMS caused by hydroxyl group, producing cyclic trimers and higher cyclomers [44, 45]. The other might be ascribed to the random degradation along the HTPDMS chain during 440–640 °C by producing cyclic trimers and higher cyclomers [44–47]. Unlike the neat HTPDMS, the main decomposition reaction and weight loss of the silicone rubber systems mainly occurred in the second process. In addition, it can be seen from Fig. 9a that most prat of TGA curve of SER modified silicone rubber are located above the pure sample, which revealed that thermal stability of the silicone rubber systems were improved compared with that of the neat HTPDMS.
The characteristic data of TGA and DTG curves are listed in Table 3. The onset decomposition temperature (Tonset) is the temperature at 5 % weight loss, and the residual yield at 800 °C is defined as R800. As shown in Table 3, the degradation of epoxy observed from 210 to 340 °C in the silicone rubber systems was responsible for the decrease of Tonset. However, R800 increased gradually with the increasing content of SER which was mainly attributed to the dense residual layer formed in the surface during the heat treatment process, hindering the heat flux from penetrating into the internal matrix.
DMA analysis of the cured silicone rubber systems
Effective information of the polymer blend can be observed through the intermolecular interaction in the DMA [48, 49]. It is well known that the peaks in the loss tangent (tan δ) versus temperature curves are closely related to the glass transition. Figure 10 shows the effect of SER content on the tan δ for the silicone rubber systems. The variation in Tg for the silicone rubber systems with respect to the SER content is given in Table 4. It can be seen in S0 that Tg of pure HTPDMS is −120.6 °C. As the content of SER increases, the Tg of the silicone rubber systems increase from −120.6 to −117.4 °C, the reason may be that the hard molecular chain of epoxy resin can restrict molecular movement of silicone rubber. The new Tgs which located at −77.5 and 98.1 °C (see S30) were assigned to the glass transition temperature of PMPS and epoxy resin, respectively. Especially, it should be noted that the Tgs located at 17.4 (S10) and 31.8 °C (S30) were attributed to the structure formed by the reaction in Scheme 2 [49]. What’s more, since the molecular motion of the molecular chains became more restricted by epoxy resin and the amino group of APTES would be consumed completely as the proportion of SER increases, the Tg in S30 (31.8 °C) was higher than in S10 (17.4 °C).
Conclusion
To improve the mechanical and adhesion properties of the HTPDMS, a siloxane/epoxy graft copolymer (SER) was synthesized and added as a modifier. A superior adhesion strength was obtained at 1.1 MPa in the 30 phr SER modified silicone rubber systems, and this value was much higher than the 0.41 MPa of pure silicone rubber. The tensile strength of the silicone rubber systems increased 3 times higher than that of the neat HTPDMS, and the elongation at break was nearly double of the unmodified silicone rubber systems. These results showed that SER produced better enhancement of mechanical properties of HTPDMS owing to its good compatibility with the silicone rubber matrix. Besides, silicone rubber systems showed good heat resistance. Therefore, it is believed that the modified HTPDMS will have potential applications in anti-corrosion coatings and structure bonding materials.
References
Huang JC (2012) Fabrication of a gecko seta-like structure using polydimethylsiloxane. Int J Adhes Adhes 36:25–31
Tsai MF, Lee YD, Long YC (2000) Synthesis of a polydimethylsiloxane-block-hydroxyl grafted acrylate prepolymer copolymer to improve the adhesion between silicone rubber and polyurethane by induced surface reconstruction. J Polym Res 7(2):73–79
Xiang K, Huang G, Zheng J, Wang X, Li GX, Huang J (2012) Accelerated thermal ageing studies of polydimethylsiloxane (PDMS) rubber. J Polym Res 19(5):1–7
Zhang M, Wu Y, Wu H, Zhang Q (2011) Study on reactive polydimethylsiloxane-modified waterborne polyurethanes. J Polym Res 19(1):1–10
Liao SK, Jang SC, Lin MF (2005) Phase behavior and mechanical properties of siloxane-urethane copolymer. J Polym Res 12(2):103–112
Shi Y, Huang G, Liu Y, Qu Y, Zhang D, Dang Y (2013) Synthesis and thermal properties of novel room temperature vulcanized (rtv) silicone rubber containing poss units in polysioxane main chains. J Polym Res 20(9):1–11
Lv P, Wang Z, Hu Y, Yu M (2009) Study on effect of polydimethylsiloxane in intumescent flame retardant polypropylene. J Polym Res 16(2):81–89
Chen WH, Chen PC, Wang SC, Yeh JT, Huang CY, Chen KN (2009) Uv-curable pdms-containing pu system for hydrophobic textile surface treatment. J Polym Res 16(5):601–610
Lauter U, Kantor SW, Schmidt-Rohr K, MacKnight WJ (1999) Vinyl-substituted silphenylene siloxane copolymers: novel high-temperature elastomers. Macromolecules 32(10):3426–3431
Chojnowski J, Cypryk M, Fortuniak W, Scibiorek M, Rozga-Wijas K (2003) Synthesis of branched polysiloxanes with controlled branching and functionalization by anionic ring-opening polymerization. Macromolecules 36(11):3890–3897
Roth J, Albrecht V, Nitschke M, Bellmann C, Simon F, Zschoche S, Michel S, Luhmann C, Grundke K, Voit B (2008) Surface functionalization of silicone rubber for permanent adhesion improvement. Langmuir 24(21):12603–12611
Eddington DT, Puccinelli JP, Beebe DJ (2006) Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sensors Actuators B Chem 114(1):170–172
Pinto S, Alves P, Matos CM, Santos AC, Rodrigues LR, Teixeira JA, Gil MH (2010) Poly (dimethyl siloxane) surface modification by low pressure plasma to improve its characteristics towards biomedical applications. Colloids Surf B: Biointerfaces 81(1):20–26
Abbasi F, Mirzadeh H, Katbab AA (2001) Modification of polysiloxane polymers for biomedical applications: a review. Polym Int 50(12):1279–1287
Sia SK, Whitesides GM (2003) Microfluidic devices fabricated in poly (dimethylsiloxane) for biological studies. Electrophoresis 24(21):3563–3576
Mikhail AS, Jones KS, Sheardown H (2008) Dendrimer-grafted cell adhesion peptide–modified PDMS. Biotechnol Prog 24(4):938–944
Boxshall K, Wu MH, Cui Z, Cui Z, Watts JF, Baker MA (2006) Simple surface treatments to modify protein adsorption and cell attachment properties within a poly (dimethylsiloxane) micro-bioreactor. Surf Interface Anal 38(4):198–201
Sugiura S, Edahiro JI, Sumaru K, Kanamori T (2008) Surface modification of polydimethylsiloxane with photo-grafted poly (ethylene glycol) for micropatterned protein adsorption and cell adhesion. Colloids Surf B: Biointerfaces 63(2):301–305
Iwasaki Y, Takamiya M, Iwata R, Yusa SI, Akiyoshi K (2007) Surface modification with well-defined biocompatible triblock copolymers: improvement of biointerfacial phenomena on a poly (dimethylsiloxane) surface. Colloids Surf B: Biointerfaces 57(2):226–236
Lee S, Vörös J (2005) An aqueous-based surface modification of poly (dimethylsiloxane) with poly (ethylene glycol) to prevent biofouling. Langmuir 21(25):11957–11962
Bodas D, Khan-Malek C (2006) Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron Eng 83(4):1277–1279
Cordeiro AL, Nitschke M, Janke A, Helbig R, D’Souza F, Donnelly GT, Willemsen PR, Werner C (2009) Fluorination of poly (dimethylsiloxane) surfaces by low pressure CF4 plasma–physicochemical and antifouling properties. eXPRESS Polym Lett 3:70–83
Karkhaneh A, Mirzadeh H, Ghaffariyeh AR (2007) Simultaneous graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto polydimethylsiloxane surfaces using a two-step plasma treatment. J Appl Polym Sci 105(4):2208–2217
Lippens E, Smet ND, Schauvliege S, Martens A, Gasthuys F, Schacht E et al (2013) Biocompatibility properties of surface-modified poly(dimethylsiloxane) for urinary applications. J Biomater Appl 27(6):651–660
Jalili K, Abbasi F, Milchev A (2013) Surface microdynamics phase transition and internal structure of high-density, ultrathin PHEMA-b-PNIPAM diblock copolymer brushes on silicone rubber. Macromolecules 46(13):5260–5278
Ercole F, Davis TP, Evans RA (2010) Photo-responsive systems and biomaterials: photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym Chem 1(1):37–54
Böhmer MR, Klibanov AL, Tiemann K, Hall CS, Gruell H, Steinbach OC (2009) Ultrasound triggered image-guided drug delivery. Eur J Radiol 70(2):242–253
Ochi M, Shimaoka S (1999) Phase structure and toughness of silicone-modified epoxy resin with added silicone graft copolymer. Polymer 40(5):1305–1312
Lee SS, Kim SC (1997) Morphology and properties of polydimethylsiloxane-modified epoxy resin. J Appl Polym Sci 64(5):941–955
Ahmad S, Gupta AP, Sharmin E, Alam M, Pandey SK (2005) Synthesis, characterization and development of high performance siloxane-modified epoxy paints. Prog Org Coat 54(3):248–255
Rath SK, Chavan JG, Sasane S, Patri M, Samui AB, Chakraborty BC (2010) Two component silicone modified epoxy foul release coatings: effect of modulus, surface energy and surface restructuring on pseudobarnacle and macrofouling behavior. Appl Surf Sci 256(8):2440–2446
Takahashi T, Nakajima N, Saito N (1989) In: Riew CK (ed) Rubber-toughened plastics, Adv. Chem. Ser. 222. American Chemical Society, Washington, D. C., p 243
Marabotti I, Morelli A, Orsini ML, Martinelli E, Galli G, Chiellini E, Lien ME, Pettitt EM, Callow EM, Callow AJ, Conlan LS, Mutton JR, Clare SA, Kocijan A, Donik C, Jenko M (2009) Fluorinated/siloxane copolymer blends for fouling release: chemical characterisation and biological evaluation with algae and barnacles. Biofouling 25(6):481–493
Martinelli E, Savothaman KM, Galli G, Pettitt EM, Callow EM, Callow AJ, Conlan LS, Clare SA, Sugiharto BA, Davies C, Williams D (2012) Poly(dimethyl siloxane) (PDMS) network blends of amphiphilic acrylic copolymers with poly(ethylene glycol)-fluoroalkyl side chains for fouling-release coatings. II. Laboratory assays and field immersion trials. Biofouling 28(6):571–582
Ngo TC, Kalinova R, Cossement D, Hennebert E, Mincheva R, Snyders R, Flammang P, Dubois P, Lazzaroni R, Leclère P (2013) Modification of the adhesive properties of silicone-based coatings by block copolymers. Langmuir 30(1):358–368
Lin ST, Huang SK (1994) Synthesis and characterization of siloxane-modified epoxy resin. J Polym Res 1(2):151–162
Lin ST, Huang SK (1997) Thermal degradation study of siloxane-DGEBA epoxy copolymers. Eur Polym J 33(3):365–373
Li J, Fan L, Wong C (2010) Insulator coating and method for forming same. U.S. Patent 7,722,951 B2
Zhang Y, Jing X, Jing K, Chang L, Bao W (2015) Study on the pore structure and oxygen-containing functional groups devoting to the hydrophilic force of dewatered lignite. Appl Surf Sci 324:90–98
Abbasi N (2014) The effects of plasma treated electrospun nanofibrous poly (ε-caprolactone) scaffolds with different orientations on mouse embryonic stem cell proliferation. Cell J 16(3)
Chen J, Chen Q, Ma Q (2012) Influence of surface functionalization via chemical oxidation on the properties of carbon nanotubes. J Colloid Interface Sci 370(11):32–38
Amouroux N, Petit J, Léger L (2001) Role of interfacial resistance to shear stress on adhesive peel strength. Langmuir 17(21):6510–6517
Abbasian A, Ghaffarian SR, Mohammadi N, Fallahi D (2004) The contact angle of thin-uncured epoxy films: thickness effect. Colloids Surf A Physicochem Eng Asp 236(1):133–140
Grassie N, Macfarlane IG (1978) The thermal degradation of polysiloxanes—I. Poly (dimethylsiloxane). Eur Polym J 14(11):875–884
Rode VV, Verkhoti MA, Rafikov SR (1969) The thermal degradation and stabilization of polydimethysiloxane. Vysoke Soedin Sect A 11(7):1529
Thomas TH, Kendrick TC (1969) Thermal analysis of polydimethylsiloxanes. I. Thermal degradation in controlled atmospheres. J Polym Sci Part A‐2: Polym Phys 7(3):537–549
Radhakrishnan TS (1999) New method for evaluation of kinetic parameters and mechanism of degradation from pyrolysis–GC studies: thermal degradation of polydimethylsiloxanes. J Appl Polym Sci 73(3):441–450
Woo EM, Barlow JW, Paul DR (1985) Thermodynamics of the phase behaviour of poly (vinyl chloride)/aliphatic polyester blends. Polymer 26(5):763–773
Chiu HT, Chiu SH, Wu JH (2003) Study on mechanical properties and intermolecular interaction of silicone rubber/polyurethane/epoxy blends. J Appl Polym Sci 89(4):959–970
Acknowledgments
The authors would thank the National Natural Science Foundation of China (51273118) and the Science and Technology Pillar Program of Sichuan (2013FZ0006) for financial support, and the Analytical and Testing Center of Sichuan University for providing SEM observation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, C., Li, R., Luo, W. et al. The preparation and properties study of polydimethylsiloxane-based coatings modified by epoxy resin. J Polym Res 23, 14 (2016). https://doi.org/10.1007/s10965-015-0903-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0903-3