Abstract
This work shows that ultrasound irradiations and mesoporous SBA-SO3H together can have a synergic effect on the catalysis of highly substituted imidazole, dihydropyrimidinone and dihydropyridine derivatives syntheses via multicomponent coupling method. In this paper, we have presented a system which is combination of mesoporous nanoreactors and ultrasonic irradiation for efficient synthesis of the biologically interesting heterocycles. Induction of ultrasonication on SBA-SO3H has led to a remarkable acceleration on the mass transfer through the mesochannels (or nanoreactors). This modification on the mesochannels caused to development of mild conditions, high selectivity and tolerance with various functional groups and high yields in short reaction time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ultrasound irradiation as an assisting agent has been utilized not only to accelerate the reaction rate, also to develop green approaches by increasing the reaction rate and yields and decreasing the unfavourable products [1, 2]. A large number of reactions can be carried out in higher yields, shorter reaction times or milder conditions if ultrasonic irradiation is exerted [3–6]. On the other hand, the catalysis of multicomponent reactions are widely developed by the catalytic applications of porous materials in the last decade [5–8]. Various mesoporous silica materials with different surface functionalities including acidic and basic groups or metal supported species are of promising candidates for development of green protocols in the field of catalysis [7–9]. Among them, utilization of SBA-SO3H as a heterogeneous Bronsted acid is been more preferable due to its intrinsic capabilities in the catalysis of the organic reactions [10, 11]. However, a major concern is in its catalytic mass transfer through the mesochannels which leads to decrease in catalytic efficiency. To solve this problem, functionalization of the surface with a hydrophobic alkyl chain or embedding it within the pore walls are some alternatives [12, 13]. However, their synthesis is somehow more difficult and costly than SBA-15 itself. Moreover, functionalization onto surface could play the role of a barrier inside the pores. Application of fluorous alcohols is another alternative to resolve the problem [14–16]. However, the use of fluorous alcohols as a solvent is costly and sometimes is not affordable.
Recently, we found that ultrasonic irradiation can have drastic effect on the resolving of this problem [17]. Therefore, in this work we applied this synergistic technique as a combination of the sonochemistry and nanocatalysis of SBA-SO3H in multicomponent reactions. Among multicomponent reactions, syntheses of the imidazoles, dihydropyrimidinone and dihydropyridine heterocycles were selected to develop this catalytic system (Scheme 1).
2 Experimental
2.1 Chemicals and apparatus
All reagents were obtained from Merck (Germany) and Fluka (Switzerland) and used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. A multi-wave ultrasonic generator (Sonicator_3000; Misonix Inc., Farmingdale, NY, USA), equipped with a converter/transducer and titanium oscillator (horn), 12.5 mm in diameter, operating as continues irradiation with a maximum power output of 600 W, was used for the ultrasonic irradiation. We selected 19.6 kHz for sonication. The lower frequencies was insufficient for reaction progress while the effect of higher frequencies than 19.6 kHz was insignificant. Progress of reactions was monitored by Thin Layer Chromatography (TLC).
2.2 General procedure for the synthesis of imidazoles under ultrasonic/nonoreactor method
A mixture of benzil (2 mmol), aromatic aldehyde (2 mmol), ammonium acetate (2.2 or 4.5 mmol) and amine (2 mmol) was irradiated under ultrasonic for appropriate time in the presence of the SBA-15/SO3H mesoporous (5 mol%) at 100 °C. The progress of reaction was monitored by TLC. When the reaction was complete as judged by TLC, ethanol (8 mL) was added and the reaction mixture was filtered and the remaining solid was washed with warm ethanol (3 × 5 mL) in order to separate the SBA/SO3H solid catalyst. The products were recrystallized from ethanol.
2.3 General procedure for the synthesis of DHPMs and DHPs under ultrasonic/nonoreactor method
A mixture of an aldehyde (2 mmol), β-dicarbonyl compound (2 or 4 mmol) and urea or NH4OAc (2.5 mmol) and catalytic amount of SBA-15/SO3H (5 mol%) was sonicated (or was stirred for HSS method) at room temperature. After complete disappearance of starting material as indicated by TLC, the resulting mixture was diluted with hot ethyl acetate (10 mL) and filtered. The catalyst was completely recovered from the residue.
3 Selected spectroscopic data
1,2,4,5-tetraphenyl-1H-imidazole (1a)
IR (KBr v max/cm−1): 1443, 1479, 1496, 1601. 1H NMR: δ = 7.05 (2 H, d, J 7.8 Hz), 7.15 (2 H, d, J 6.9 Hz), 7.18–7.27 (12 CH, m), 7.44 (2 H, t, J 7.5 Hz), 7.62 (2 H, t, J 7.5 Hz). 13C NMR: δ = 126.60, 127.41, 127.95, 128.09, 128.16, 128.24, 128.34, 128.43, 128.96, 129.06, 130.52, 130.65, 130.85, 131.12, 134.43, 137.11, 138.27, 146.93.
2-(4-nitrophenyl)-1,4,5-triphenyl-1H-imidazole (1c)
IR (KBr v max/cm−1): 1460, 1492, 1520, 1608. 1H NMR: δ = 7.07–7.15 (4 CH, m), 7.26–7.35 (8 CH m), 7.60 (3 H, t, J 7.2 Hz), 8.09 (2 H, d, J 8.1 Hz). 13C NMR: δ = 123.43, 127.03, 127.30, 128.25, 128.29, 128.38, 128.48, 129.05, 129.55, 129.91, 131.00, 132.46, 133.84, 136.50, 136.61, 144.30, 147.03.
1-Benzil-2-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole (1f)
IR (KBr v max/cm−1): 1416, 1448, 1499, 1600. 1H NMR: δ = 6.81 (2 H, d, J 3.6 Hz), 7.14–7.27 (9 CH, m), 7.36 (4 H, t, J 8.1 Hz), 7.59–7.62 (4CH, m). 13C NMR: δ = 48.29, 125.87, 126.54, 126.80, 127.02, 128.16, 128.73, 128.79, 128.84, 128.89, 129.07, 130.24, 130.45, 130.75, 130.02, 134.28, 134.99, 137.31, 138.28, 146.86.
2-(4-chlorophenyl)-1,4,5-triphenyl-1H-imidazole (1h)
IR (KBr v max/cm−1): 1446, 1479, 1496, 1597. 1H NMR: δ = 7.04–7.15(4 CH, m), 7.21–7.30 (11 CH, m), 7.38 (2 H, d, J 7.8 Hz), 7.41 (2 H, d, J 7.5 Hz). 13C NMR: δ = 126.73, 127.36, 128.09, 128.21, 128.38, 128.51, 129.00, 129.25, 130.09, 130.42, 131.09, 131.17, 134.25, 134.32, 136.90, 138.44, 145.75.
2-(4-chlorophenyl)-1-cyclohexyl-4,5-diphenyl-1H-imidazol (1l)
IR (KBr v max/cm−1): 1408, 12484, 1508, 1599, 2933. 1H NMR: δ = 1.03–1.66 (10 H, m), 3.91 (1 H, m), 7.12–7.15 (4CH, m), 7.40–7.47 (8 CH, m), 7.56(2 H, t, J 7.2 Hz). 13C NMR: δ = 24.99, 26.17, 33.63, 58.44, 126.116, 126.64, 127.94, 128.62, 128.70, 128.89, 129.40, 130.98, 131.31, 132.12, 132.32, 134.48, 134.98, 146.44.
2,4,5-triphenyl-1H-imidazole (2a)
IR (KBr v max/cm−1): 1441, 1461, 1488, 1587, 1601, 3037 1H NMR: δ = 7.19–7.46 (9 CH, m), 7.55(4 H, t, J 6.6 Hz), 7.90 (2 H, d, J 8.1 Hz). 13C NMR: δ = 125.31, 127.50, 127.83, 128.60, 128.90, 129.02, 129.91.
Dimethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate (3a)
1H NMR: 2.28 (s, 6 H), 3.54 (s, 6 H), 4.89 (s, 1 H), 7.09–7.45 (m, 5 H), 8.87 (s, 1 H). 13C NMR: 18.47, 37.27, 51.10, 101.23, 127.27, 127.31, 128.37, 129.69, 146.13, 148.29, 168.64.
Diethyl 2,6-dimethyl-4-Phenyl-1,4-dihydropyridine-3,5-dicarboxylate (3b)
IR (KBr): 3342, 1700, 1657, 1473, 1198, 1129. 1H NMR: 1.23 (t, 3 J HH = 7.0 Hz, 6 H), 2.34 (s, 6 H), 4.12 (q, 3 J HH = 7.0 Hz, 4 H), 4.91 (s, 1 H), 5.68 (s, 1 H), 7.07–7.43 (m, 5 H). 13C NMR: 14.91, 19.56, 39.43, 59.82, 103.14, 127.25, 127.32, 128.34, 129.61, 146.19, 148.25, 167.34.
Dimethyl 4-(4-chlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (3c)
IR (KBr): 3329, 1697, 1650, 1470, 1219, 1127. 1H NMR: 2.35 (s, 6 H), 3.67 (s, 6 H), 4.99 (s, 1 H), 5.73 (s, 1 H), 7.19–7.28 (m, 4 H). 13C NMR: 19.56, 39.01, 50.99, 103.73, 128.14, 129.07, 131.84, 144.22, 145.98, 167.81.
Diethyl 4-(3-Chlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (3d)
IR (KBr): 3320, 1690, 1642, 1475, 1200, 1136. 1H NMR: 1.25 (t, 3 J HH = 7.1 Hz, 6 H), 2.37 (s, 6 H), 4.13 (q, 3 J HH = 7.1 Hz, 4 H), 4.99 (s, 1 H), 5.62 (s, 1 H), 7.11–7.29 (m, 4 H). 13C NMR: 15.18, 19.61, 39.74, 59.81, 103.82, 126.25, 126.31, 128.29, 129.04, 1433.60, 144.02, 149.73, 167.30.
Methyl 6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4a)
IR (KBr): 3320, 3315, 1700, 1661, 1585, 1420. 1H NMR: 2.36 (s, CH3), 3.63 (s, OCH3), 5.41 (1d, 3 J HH = 2.1 Hz, CHNH), 5.53 (broad, NH), 7.25–7.34 (m, C6H5), 7.56 (1broad, NH). 13C NMR: 14.08, 55.78, 60.36, 101.43, 126.49, 128.03, 128.81, 143.55, 146.21, 152.79, 166.04.
Methyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4d)
IR (KBr): 3345, 3090, 1706, 1631, 1507, 1425. 1H NMR: 2.45 (s, CH3), 3.73 (s, OCH3), 5.60 (s, CHNH), 5.75 (broad, NH), 7.58 (d, 3 J HH = 7.6 Hz, 2 CH of C6H4), 7.26 (d, 3 J HH = 7.6 Hz, 2 CH of C6H4), 8.37 (broad, NHCH).
Ethyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4f)
IR (KBr): 3290, 3185, 1692, 1641, 1533, 1433. 1H NMR: 1.17 (d, 3 J HH = 7.1 Hz, OCH2CH3), 2.33 (s, CH3), 4.08 (d, 3 J HH = 7.1 Hz, OCH2CH3), 5.37 (d, 3 J HH = 2.0 Hz, CHNH), 6.08 (broad, NH), 7.24 (d, 3 J HH = 8.1 Hz, 2 CH of C6H4), 7.28 (d, 3 J HH = 8.1 Hz, 2 CH of C6H4), 8.26 (broad, NHCH). 13C NMR: 14.17, 18.64, 55.12, 60.14, 101.17, 128.03, 128.88, 132.16, 142.24, 146.47, 153.39, 165.44.
4 Results and discussions
4.1 Synthesis and characterization of SBA-15/SO3H
The synthesis of SBA-15/SO3H has been achieved using three main steps: first step for preparation of the SBA-15 which known procedure described by Zhao et al. [18]. Second, which is thiol functionalization of the SBA-15 and third is oxidation of the SBA-15-Pr–SH to SBA-15-Pr–SO3H by hydrogen peroxide (Fig. 1). Finally, pH analysis for loading of SO3H showed 1.7 mmol g−1. CHNS analysis of SBA-15/SO3H indicated the successful presence of thiol grafting and the amount of sulfur atom in SBA-15/SO3H was 5.3 wt% according to this analysis.
In the FT-IR spectrum, the band from 806 to 1100 cm−1 is belonged to the vibrations of (Si–O–Si) bond, and the band at about 960 cm−1 is assigned of (Si–OH) bond and the SiO–H groups are appeared by the very broad IR absorption band in the 3100–3700 cm−1 region (Fig. 2). Also this broad band is more intense than that of SBA-15/SH which reveals the generation of new –O–H groups on the surface after oxidation of –SH group. Moreover, some peaks between 2900 and 3000 cm−1 can be assigned to aliphatic chain of the grafted thiol and sulfonic acid groups on the surface.
Diffraction peaks at the below 2° corresponding to the (1 0 0), (1 1 0), and (2 0 0) are readily recognized from the XRD pattern of SBA-15 (Fig. 3). The observed diffraction peaks agree with the 2D-hexagonal SBA-15 [18].
The SEM and TEM images as morphological and channels study of SBA-15 mesoporous material are presented in the Fig. 4.
Highly substituted imidazole derivatives are of important class of compounds in the field of organic chemistry and pharmaceuticals [19]. Herein, we report one-step synthesis of imidazoles via a multicomponent condensation of Benzil, aldehyde, NH4OAc and amines based on our discovered bifunctional sonochemistry/SBA-SO3H. For our study, Benzil, benzaldehyde NH4OAc and aniline were chosen as the benchmark substrates in the model reaction (Table 1).
In model reaction, to obtain the desired product (1,2,4,5-tetraphenyl imidazole), we tested the reaction using different conditions, both high speed stirrer (HSS) and ultrasonic (US), in presence of organic–inorganic hybrid –SO3H functionalized SBA-15 family nanoreactor. As shown in Table 1, the use of SBA-15/SO3H catalysts in high speed stirrer (HSS) method under solvent-free condition led to desired product after 4 h in 90 % yield (entry 5). Performing the reaction in the presence of sulfonic acid porous nanoreactor SBA-15 resulted at same condition, the yield of product was 94 % in sonicated condition after 8 min in compared of alternative HSS method (entry 5). For next steps, sonicated SBA-15/SO3H nanoreactor in reaction condition as an efficient and ultra-fast combined bifunctional method of ultrasonic/nonoreactor was selected.
To demonstrate the diversity of this combined ultrasonic and SBA-15/SO3H catalytic system and to expand the scope of the process, the optimized conditions were applied to a series of 1,2,4,5-tetrasubstituted imidazoles via a four-component condensation. Also, this new method was further explored for the synthesis of 2,4,5-trisubstituted imidazoles by the condensation of aldehyde, benzil with two equivalent of ammonium acetate under similar conditions. The obtained results are summarized in Table 2. As indicated in Table 2, when using benzyl amine instead of aniline, a raise on the yields can be observed.

With these results in hand we decided to explore the scope of this method (ultrasonic/nanoreactor combined system). In the other hand, dihydropyrimidinones (DHPMs) and dihydropyridines (DHPs) have attracted considerable interest in recent years because of their medicinally important. However, the developments in this area demand further searches for new methods and diversity in reagents that could be superior to the existing ones with regard to generality, simplicity, high yields and handling [20]. The catalytic activity of combined ultrasonic/nanoreactor system in the synthesis of DHPMs and DHPs by the condensation of aldehyde, β-dicarbonyl compound and ammonium acetate or urea was studied. The use of sonochemistry/nonoporous bifunctional received considerable attention as a heterogeneous and recyclable catalyst to afford the corresponding excellent yields of products in our study based on multicomponent synthesis of dihydropyrimidinones and dihydropyridines (Table 3). As can be seen, the presence of electron withdrawing group on benzaldehyde has positive effect on the product yields of dihydropyrimidinones. Nevertheless, there was no significant change on the product yields of dihydropyridines by inserting an electron-withdrawing group on benzaldehyde.

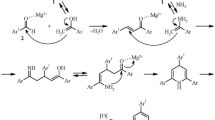
Although we don’t have a proven mechanism for ultrasonic/SBA-SO3H in an experimental manner, a possible schematic of the catalytic pathway is proposed in Fig. 5. Ultrasonication can have assisting effect on the reactions and a positive effect on the mass transfer through the pores. Beyond this, –SO3H can protonate the carbonyls and activate them in the reactions.
The possibility of recycling the SBA-SO3H was also studied under sonicated conditions. Therefore, We devised a set of experiments to recover and reuse the SBA-SO3H catalyst in the synthesis of methyl 6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate as a model reaction for synthesis of DHPMs class. It can be seen that the catalyst was highly reusable under the investigated reaction conditions while catalytic activity after six runs has been kept almost unaltered (Fig. 6).
5 Conclusion
In summary, covalently functionalized sulfonic acid on mesoporous SBA-15 in the presence of ultrasound medium acts synergistically in viewpoint of catalyst activity. As a result, an inexpensive heterogeneous, green and reusable catalyst for synthesis of the some heterocyclic compounds including imidazoles, DHPMs and DHPs based on multicomponent coupling method was developed. This new method, ultrasonication induction to SBA-SO3H raises the mass transfer with no need to anchor, confine or embed an additional hydrophobic group or ionic liquid and hence, increases the catalytic efficiency.
References
H. Mootabadi, B. Salamatinia, S. Bhatia, A.Z. Abdullah, Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel 89, 1818–1825 (2010)
Y.C. Fiamegos, C.G. Nanos, C.D. Stalikas, Ultrasonic-assisted derivatization reaction of amino acids prior to their determination in urine by using single-drop microextraction in conjunction with gas chromatography. J. Chromatogr. B 813, 89–94 (2004)
D. Kumar, G. Kumar, C. Singh, Ultrasonic-assisted transesterification of Jatropha curcus oil using solid catalyst, Na/SiO2. Ultrason. Sonochem. 17, 839–844 (2010)
R.S. Varma, H.M. Meshram, Solid state cleavage of semicarbazones and phenylhydrazones with ammonium persulfate-clay using microwave or ultrasonic irradiation. Tetrahedron Lett. 38, 7973–7976 (1997)
L.-P. Jiang, S. Xu, J.-M. Zhu, J.-R. Zhang, J.-J. Zhu, H.-Y. Chen, Ultrasonic-assisted synthesis of monodisperse single-crystalline silver nanoplates and gold nanorings. Inorg. Chem. 43, 5877–5883 (2004)
B. Salamatinia, H. Mootabadi, S. Bhatia, A.Z. Abdullah, Optimization of ultrasonic-assisted heterogeneous biodiesel production from palm oil: a response surface methodology approach. Fuel Process. Technol. 91, 441–448 (2010)
A. Taguchi, F. Schüth, Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 77, 1–45 (2005)
S. Rostamnia, E. Doustkhah, Nanoporous silica-supported organocatalyst: a heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 4, 28238–28248 (2014)
M. Nandi, J. Mondal, K. Sarkar, Y. Yamauchi, A. Bhaumik, Highly ordered acid functionalized SBA-15: a novel organocatalyst for the preparation of xanthenes. Chem. Commun. 47, 6677–6679 (2011)
J.A. Melero, R. van Grieken, G. Morales, Advances in the synthesis and catalytic applications of organosulfonic-functionalized mesostructured materials. Chem. Rev. 106, 3790–3812 (2006)
S. Rostamnia, F. Pourhassan, The SBA-15/SO3H nanoreactor as a highly efficient and reusable catalyst for diketene-based, four-component synthesis of polyhydroquinolines and dihydropyridines under neat conditions. Chin. Chem. Lett. 24, 401–403 (2013)
J.A. Melero, L.F. Bautista, J. Iglesias, G. Morales, R. Sánchez-Vázquez, K. Wilson, A.F. Lee, New insights in the deactivation of sulfonic modified SBA-15 catalysts for biodiesel production from low-grade oleaginous feedstock. Appl. Catal. A Gen. 488, 111–118 (2014)
C. Pirez, A. Lee, J. Manayil, C. Parlett, K. Wilson, Hydrothermal saline promoted grafting: a route to sulfonic acid SBA-15 silica with ultra-high acid site loading for biodiesel synthesis. Green Chem. 16, 4506–4509 (2014)
S. Rostamnia, E. Doustkhah, A. Nuri, Hexafluoroisopropanol dispersed into the nanoporous SBA-15 (HFIP/SBA-15) as a rapid, metal-free, highly reusable and suitable combined catalyst for domino cyclization process in chemoselective preparation of alkyl rhodanines. J. Fluor. Chem. 153, 1–6 (2013)
S. Rostamnia, E. Doustkhah, A mesoporous silica/fluorinated alcohol adduct: an efficient metal-free, three-component synthesis of indazolophthalazinetrione heterocycles using a reusable nanoporous/trifluoroethanol adduct (SBA-15/TFE). Tetrahedron Lett. 55, 2508–2512 (2014)
S. Rostamnia, E. Doustkhaha, Increased SBA-15–SO3H catalytic activity through hydrophilic/hydrophobic fluoroalkyl-chained alcohols (RFOH/SBA-15–Pr-SO3H). Synlett 26, 1345-1347 (2015)
S. Rostamnia, H. Xin, X. Liu, K. Lamei, Simultaneously application of SBA-15 sulfonic acid nanoreactor and ultrasonic irradiation as a very useful novel combined catalytic system: an ultra-fast, selective, reusable and waste-free green approach. J. Mol. Catal. A: Chem. 374, 85–93 (2013)
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998)
A. Da Settimo, G. Primofiore, F. Da Settimo, L. Calzolari, P. Cazzulani, A. Passoni, O. Tofanetti, 1-Substituted 2-benzylaminobenzimidazole derivatives: compounds with H 1-antihistaminic activity. Eur. J. Med. Chem. 27, 395–400 (1992)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavasi, Hantzsch reaction on free nano-Fe2O3 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 47, 9230–9232 (2011)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Doustkhah, E., Rostamnia, S. & Hassankhani, A. The raise of SBA-SO3H catalytic activity by inducing ultrasound irradiation in the multicomponent syntheses. J Porous Mater 23, 549–556 (2016). https://doi.org/10.1007/s10934-015-0108-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0108-5