Abstract
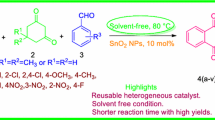
An efficient green approach for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amine derivatives, via a three-component one-pot condensation reaction of o-phenylenediamine, aromatic aldehydes and cyclohexyl isocyanide in the presence of perlite–SO3H nanoparticles (diameter/thickness of platelets < 100 nm) under ultrasound irradiation has been demonstrated. The present method offers advantages such as shorter reaction time, easy work-up, excellent yields, recovery and reusability of catalyst. In addition, the methodology has been prosperous in getting the green chemistry purposes such as natural catalyst, using ultrasound irradiation instead of conventional heating and stirring, and a non-hazardous products in the thus combining the features of both economic and environmental advantages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade, isocyanide-based multicomponent reactions (IMCRs) achieved important concern within the scientific association as an effective, appropriate, time-saving, and atom-economical procedure to quickly produce chemical variety. IMCRs are quickly done using readily accessible starting materials and sustain a diversity range of functional groups. Alteration and following transformations prepare achievement to a justly great number of unique structures that would otherwise need prolix preparations [1,2,3,4,5,6]. Therewith, IMCRs have been extensively used in the pharmaceutical industry [7,8,9].
Quinoxaline derivatives are a usual incidence in plenty pharmacological active materials of natural or synthetic sources [10,11,12]. Many recognized antibiotics containing echinomycin, actinomycin, triostin A and leromycin have the basic frame (Fig. 1) [13, 14]. The quinoxaline ring is also a constituent of many biologically and pharmacologically active compounds such as anthelmintics, fungicides, insecticides and herbicides [15, 16]. Quinoxaline derivatives have gained usage in dyes [17], electron luminescent substances [18], organic semiconductors [19], chemically controllable switches [20], as building blocks for the synthesis of anion receptors [21], cavitands [22], dehydroannulenes [23], DNA unzipping agents [24], and also minister as effective strict subunits in macrocyclic receiver or in molecular diagnosis [25, 26].
The usage of heterogeneous catalysts [27,28,29,30] has gained significant gravity in organic synthesis because of their facility of operation, elevated reaction rates, greater selectivity, easy work-up, and recoverability of catalysts. Between the different heterogeneous catalysts, exclusively, nano-perlite has advantages of eco friendly, low cost, ease of preparation and catalyst recycling [31]. Perlite is an amorphous volcanic glass that has a relatively high water value, typically formed by the hydration of obsidian. Because of its low density and relatively low price, many commercial applications for perlite have developed. In the construction and manufacturing fields, it is used in lightweight plasters and mortars, insulation and ceiling tiles. There are few reports about using perlite as suitable support for catalytic applications [31,32,33,34,35].
Ultrasound irradiation is well known to speed up chemical reactions [36, 37]. This is due to the event of acoustic cavitation, that is, the formation, development and crash of micrometrical bubbles, formed by the propagation of a pressure wave through a liquid. The crashes are quasiadiabatic processes, resulting in the production of high temperatures and pressures in the nanosecond time size, attended by sonoluminescence and mechanical effects. These effects can be applied to speed up chemical reactions, decreasing the reaction time and optimizing the profit–charge relevance. Moreover, this energetic flow can help to push forward both desirable and undesirable chemical reactions [36]. Therefore, the yields received with ultrasound-assisted reactions are various than those received under the similar conditions with classical synthetic methods [37].
As part of our continuing interest to develop the new ultrasound-promoted isocyanide-based multicomponent reactions [38,39,40], herein, we wish to report a green, effective and suitable method for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amine derivatives 4 using perlite–SO3H nanoparticles as catalyst through one-pot condensation reaction of o-phenylenediamine 1, aromatic aldehydes 2 and cyclohexyl isocyanide 3 (Scheme 1). We think that our work is in good agreement with the principles of green chemistry [41,42,43,44] such as inhibition of the formation of waste materials, having very high atom economy, eschewing of hazardous products, modeling safer products, using natural volcanic rock as catalyst, energy economy with operating reaction under ultrasonic irradiation and elimination of chemical stages with one-pot reaction.
Experimental
General
All reagents were purchased from Merck (Germany) and Fluka (Switzerland) and used without further purification. Infrared spectra were recorded on a Jasco 6300 FTIR spectrometer. Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected. Sonication was performed in a Bandelin SONOPULS ultrasonic homogenizers (made in Germany) with 20 kHz processing frequency, a nominal power 250 W and uniform sonic waves.
Preparation of perlite NPs
Perlite NPs were prepared from perlite mineral powder according to the preparation of silica nanoparticles from organic laboratory waste of silica gel HF254 method [45] The morphology and grainsize of the perlite NPs was investigated by SEM and XRD (Figs. 1, 2, 3).
Preparation of perlite–SO3H NPs
A 250-mL suction flask was equipped with a constant pressure dropping funnel containing chlorosulfonic acid (11.6 g, 0.1 mol) and a gas inlet tube for conducting HCl gas over an adsorbing solution, i.e., H2O. Then 30.0 g of nanoperlite was charged in to the flask. Chlorosulfonic acid was added drop wise over a period of 30 min at room temperature. HCl gas evolved from the reaction vessel immediately. After the addition was complete, the mixture was shaken for 30 min. Perlite–SO3H was obtained as a white solid. The SEM image of the synthesized perlite–SO3H nanoparticles has been showed in Fig. 4.
Acid strength measurement
Color test was done by moving 0.1 g of dried, powdered solid perlite–SO3H NPs to a test tube, adding a 0.1% solution of methyl red in toluene as an indicator (three drops). From the color changing to red, it was easy to decide that the solid perlite–SO3H NPs was acidic [46].
Acid amount determination
The quantity of acid sites on perlite–SO3H NPs can be evaluated by amine titration directly after determination of acid strength by mentioned method [45]. The method includes titration of the sample suspended in toluene with n-butylamine, using an indicator. The 0.1 N n-butylamine solution was provided by weighing 1.0 mL of n-butylamine in a 100-mL volumetric flask and making up the volume using dried toluene. Then, 0.2 g of dried perlite–SO3H sample was transferred to a 50-mL Erlenmeyer flask. 9 mL of dry toluene and 3 mL of indicator solution in toluene were added to the sample suspension. Then, enough 0.1 N n-butylamine in toluene was added from a 2-mL burette to the sample so as to bracket the expected titer by the appropriate number of millimoles of n-butylamine per gram of perlite–SO3H sample. The tightly capped sample was then equilibrated in a rotator at least for 4 h at room temperature. The titration was then continued using smaller stepwise increases in n-butylamine content until the end point.
General procedure for the one-pot synthesis of N-cyclohexyl-3-phenyl-quinoxaline-2-amines
To a mixture of o-phenylenediamine 1 (1 mmol), benzaldehyde 2a (1 mmol), and cyclohexyl isocyanide 3 (1 mmol) in EtOH (10 mL), a catalytic amount of perlite–SO3H (100 mg) was added and the mixture was irradiated under an ultrasonic processor at 150 W. The improvement of the reaction was monitored by TLC (ethyl acetate–hexane 1:3). After completion of the reaction, the resulting solid (catalyst) was separated by simple filtration and EtOH was removed and the reaction mixture was diluted with water (15 mL), and CH2Cl2 (15 mL) was added. The organic layer was separated and dried over MgSO4. The solvent was evaporated under reduced pressure, and the product was obtained without any further purification.
Results and discussion
The amount of acid in the perlite–SO3H NPs samples was investigated. It can be seen that the amount of acidity was observed in the case of perlite–SO3H NPs as 2.46 mmole/g.
To get appropriate conditions for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amine (4a–i), different reaction conditions have been studied in the reaction of o-phenylenediamine 1, benzaldehyde 2a and cyclohexyl isocyanide 3 as a model reaction.
Effects of the catalyst under ultrasound irradiation
In a primary study, for examination of the amount of catalyst in this reaction, o-phenylenediamine 1, benzaldehyde 2a and cyclohexyl isocyanide 3 were first reacted in EtOH (10 mL) for 40 min under ultrasound irradiation in the presence 0, 10, 20, 50, 100 and 200 mg of perlite–SO3H nanoparticles separately. The best results were taken using 100 mg of catalyst (yield = 94%). Using lower amounts of catalyst resulted in lower yields, while higher amounts of catalyst did not affect the reaction times and yields and in the absence of catalyst, the yield of the product was found to be very low [33] (Table 1).
Effects of the solvents under ultrasound irradiation
Here, we have varied the solvents condition such as water, methanol, acetonitrile, THF, dioxane and ethanol, the observation showed that the reaction using EtOH as solvent gave the best result, which gave the products not only in good yield but also with higher reaction rates (94% yield in 40 min) (Table 2, entry 6). Acetonitrile afforded moderate yields of desired products but took comparatively longer reaction time (Table 2, entry 3).
Effects of the ultrasound power
By enhancing the irradiation power from 50 to 150 W, the reaction time of 4a reduced from 60 to 40 min and the yield increased from 52 to 94%. The reaction time and yield of 4a did not shift from 150 to 200 W, thus, 150W of ultrasonic irradiation was enough to push the reaction forward. The best yield for 4a was gained by ultrasonic irradiation for 40 min 150 W (Tables 3, 4) [39, 40].
Reusability of catalyst
Next, we investigated the reusability and recycling of perlite–SO3H nanoparticles. At first, we put o-phenylenediamine 1 (1 mmol), benzaldehyde 2a (1 mmol), cyclohexyl isocyanide 3 (1 mmol) and perlite–SO3H NPs in EtOH (10 mL) as solvent at 150 W under sonication. When the reaction was completed (monitored by TLC), the resulting solid (catalyst) was separated by simple filtration and recovered perlite–SO3H was reused in subsequent reactions without significant decrease in activity even after five runs (Table 5).
The feasible mechanism for the formation of product 4 is shown in Scheme 2. Product 4 is formed by the primary formation of minimum intermediate A from diamine 1 and aldehyde 2. Intermediate B was made by the nucleophilic attack of isocyanide 3 to the iminium intermediate A and next by an intramolecular nucleophilic attack of NH2 group to the nitrile moiety to yield intermediate C. Imine–enamine tautomerization of intermediate C and then catalytic dehydrogenation leads to the formation*** of product 4.
Conclusion
In summary, we have extended a new, efficient and environment friendly one-pot three-component method for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amine derivatives under ultrasound irradiation in excellent isolated yields using EtOH as an almost non-hazardous, inexpensive and readily available solvent. The various substituents (electron-donating and electron-withdrawing) on each component of the coupling partner were well tolerated. The combination of the relatively fast reaction times, simple work-up methods and the aspects stated above mean that the methodology can be operated in a combi-chem mode to produce libraries organizing a various array of N-cyclohexyl-3-aryl-quinoxaline-2-amine derivatives.
References
I. Akritopoulou-Zanze, Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 12, 324–331 (2008)
S. Sadjadi, M. Heravi, N. Nazari, Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv 6, 53203–53272 (2016)
A. Domling, Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106, 17–89 (2006)
M. Jida, M. Soueidan, N. Willand, F. Agbossou-Niedercorn, L. Pelinski, G. Laconde, R. Deprez-Poulain, B. Deprez, A facile and rapid synthesis of N-benzyl-2-substituted piperazines. Tetrahedron Lett. 52, 1705–1708 (2011)
M. Jida, S. Malaquin, R. Deprez-Poulain, G. Laconde, B. Deprez, Synthesis of five- and six-membered lactams via solvent-free microwave Ugi reaction. Tetrahedron Lett. 51, 5109–5111 (2010)
S. Malaquin, M. Jida, G.D. Gesquiere, R. Deprez-Poulain, B. Deprez, G. Laconde, Ugi reaction for the synthesis of 4-aminopiperidine-4-carboxylic acid derivatives. Application to the synthesis of carfentanil and remifentanil. Tetrahedron Lett. 51, 2983–2985 (2010)
A.E.A. Porter, in Comprehensive Heterocyclic Chemistry, 3 ed. by A.R. Katritzky, C.W. Rees eds. (Pergamon Press, New York, 1984), pp. 191–196
A. Varadi, T.C. Palmer, R. Notis Dardashti, S. Majumdar, Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 21, 19–41 (2015)
G.W.H. Cheeseman, R.F. Cookson, in The Chemistry of Heterocyclic Compounds, 1 ed. by A. Weissberger, E.C. Taylor eds. 2nd edn. : (Wiley, New York, 1970)
S. Tariq, K. Somakala, M. Amir, Quinoxaline: an insight into the recent pharmacological advances. Eur. J. Med. Chem. 143, 542–557 (2018)
S. Ucar, S. Essiz, A. Dastan, Bromination of quinoxaline and derivatives: effective synthesis of some new brominated quinoxalines. Tetrahedron 73, 1618–1632 (2017)
R.N. Lima, A.L.M. Porto, Facile synthesis of new quinoxaline from ethyl gallate by green chemistry protocol. Tetrahedron Lett. 58, 825–828 (2017)
M. Sato, T. Nakazawa, Y. Tsunematsu, K. Hotta, K. Watanabe, Echinomycin biosynthesis. Curr. Opin. Chem. Biol. 17, 537–545 (2013)
H. Oveisi, M. Adharvana Chari, Ch.V. Nguyen, J.E. Chen, S.M. Alshehri, E. Ynmaz, Md.Sh. Hossein, Y Yamauchi, K.C.W. Wu, ZnO-loaded mesoporous silica (KIT-6) as an efficient solid catalyst for production of various substituted quinoxalines. Catal. Commun. 90, 111–115 (2017)
J.A. Pereira, A.M. Pessoa, M.N.D.S. Cordeiro, R. Fernandes, C. Prudencio, J.P. Noronha, M. Vieira, Quinoxaline, its derivatives and applications: a state of the review. Eur. J. Med. Chem. 97, 664–672 (2015)
J. Azuaje, A. El Maatouguri, X. Garcia-Mera, E. Sotelo, Ugi-based approaches to quinoxaline libraries. ACS Comb. Sci. 16, 403–411 (2014)
S. Achelle, Ch Baudequin, N. Ple, Luminescent materials incorporating pyrazine or quinoxaline moieties. Dyes Pigm. 98, 575–600 (2013)
H. Chavan, L.M. Adsul, B.P. Bandgar, Polyethylene glycol in water: a simple, efficient and green protocol for the synthesis of quinoxalines. J. Chem. Sci. 123, 477–483 (2011)
P.O. Patil, S.B. Bari, Nitrogen heterocycles as potential monoamine oxidase inhibitors: Synthetic aspects. Arab. J. Chem. 7, 857–884 (2014)
M.R. Islami, Z. Hassani, One-pot and efficient protocol for synthesis of quinoxaline derivatives. ARKIVOC 2008, 280–287 (2008)
H.R. Darabi, F. Tahoori, K. Aghapour, F. Taala, F. Mohsenzadeh, NH4Cl–CH3OH: an efficient, acid- and metal-free catalyst system for the synthesis of quinoxalines. J. Braz. Chem. Soc. 19, 1646–1652 (2008)
Ch Li, F. Zhang, Zh Yang, Ch Qi, Chemoselective synthesis of quinoxalines and benzimidazoles by silica gel catalysis. Tetrahedron Lett. 55, 5430–5433 (2014)
J.-F. Chen, Z.-Q. Liu, Synthesis of imidazo[1,2-a]quinoxalines by double Groebke reactions and inhibitory effects on radicals and DNA oxidation. Tetrahedron Lett. 72, 1850–1859 (2016)
I.H. Eissa, A.M. El-Naggar, N.E.A.A. El-Sattar, A.S.A. Youssef, Design and discovery of novel quinoxaline derivatives as dual DNA intercalators and topoisomerase II inhibitors. Anticancer Agents Med. Chem. 18, 195–209 (2018)
A. Dandia, V. Parewa, Sh Maheshwari, K.S. Rathore, Cu doped CdS nanoparticles: a versatile and recoverable catalyst for chemoselective synthesis of indolo[2,3-b]quinoxaline derivatives under microwave irradiation. J. Mol. Catal. A Chem. 394, 244–252 (2014)
M.M. Heravi, B. Baghernejad, H.A. Oskooie, A novel three-component reaction for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amines. Tetrahedron Lett. 50, 767–769 (2009)
J. Safaei-Ghomi, S. Rohani, A. Ziarati, CuI nanoparticles as a reusable heterogeneous catalyst for the one-pot synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amines under mild conditions. J. Nanostruc. 2, 79–83 (2012)
L.-Y. Fan, L. Wei, H. Wen-Jun, X.-X. Li, Yb modified NaY zeolite: a recyclable and efficient catalyst for quinoxaline synthesis. Chin. Chem. Lett. 25, 1203–1206 (2014)
H. Slimi, Y. Moussaoui, R.B. Salem, Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction promoted by bismuth(III)nitrate or PPh3 without solvent. Aran. J. Chem. 9, 510–514 (2016)
M. Jeganthan, A. Dhakshinamoorthy, K. Pitchumani, One-pot synthesis of 2-substituted quinoxalines using K10-montmorillonite as heterogeneous catalyst. Tetrahedron Lett. 55, 1616–1620 (2014)
S.N. Hosseini, S.M. Borghei, M. Vossoughi, N. Taghavinia, Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Appl. Catalysis B Environ. 18, 53–62 (2007)
Jahanshahi,R.,Akhlaghinia,B. Expanded perlite: an inexpensive natural efficient heterogeneous catalyst for the green and highly accelerated solvent-free synthesis of 5-substituted-1H-tetrazoles using [bmim]N3 and nitriles. RSC Adv. 5: 104087–104096 (2015).
Ramazani,A.,Rouhani,M.,Mirhadi,E.,Sheikhi,M.,Ślepokura,K.,Lis,T, Perlite-SO3H nanoparticles as an efficient and reusable catalyst for one-pot three-component synthesis of 1,2-dihydro-1-aryl-naphtho[1,2-e][1,3]oxazine-3-one derivatives under both microwave-assisted and thermal solvent-free conditions: single crystal x-ray structure analysis and theoretical study, Nano. Chem. Res. 1:87–107 (2016).
Skubiszewska-Zięba,J.,Charmas,B.,Leboda,R.,Gun’ko,V.M., Carbon-mineral adsorbents with a diatomaceous earth/perlite matrix modified by carbon deposits, Micropor. Mesopor. Mat. 156:209–2016 (2012).
E. Kolvari, N. Koukabi, M.M. Hosseini, Perlite, A cheap natural support for immobilization of sulfonic acid as a heterogeneous solid acid catalyst for the heterocyclic multicomponent reaction. J. Mol. Catal. A Chem 397, 68–75 (2015)
G. Cravotto, P. Cintas, Forcing and controlling chemical reactions with ultrasound. Angew. Chem. Int. Ed. 46, 5476–5478 (2007)
A.C.M.P. Da Silva, C.G. Pancote, C.L. Brito, N.B.A.B. Da Silveira, Synthesis 2004, 1557–1558 (2004)
M. Rouhani, A. Ramazani, S.W. Joo, Y.Very Hanifehpour, Efficient and rapid catalyst-free one-pot three component synthesis of 2,5-dihydro-5-imino-2-methylfuran-3,4-dicarboxylate derivatives under ultrasound irradiation. Bull. Korean Chem. Soc. 33, 4127–4131 (2012)
A. Ramazani, M. Rouhani, S.W. Joo, Novel, fast and efficient one-pot sonochemical synthesis of 2-aryl-1,3,4-oxadiazoles. Ultrasoun. Sonochem. 20, 262–267 (2014)
A. Ramazani, M. Rouhani, S.W. Joo, Ultrasonics in isocyanide-based multicomponent reactions: a new, efficient and fast method for the synthesis of fully substituted 1,3,4-oxadiazole derivatives under ultrasound irradiation. Ultrasoun. Sonochem. 21, 391–396 (2015)
A. Ramazani, M. Rouhani, S.W. Joo, Catalyst-free sonosynthesis of highly substituted propanamide derivatives in water. Ultrason. Sonochem. 28, 393–399 (2016)
H. Ahankar, A. Ramazani, K. Slepokura, T. Lis, S.W. Joo, Synthesis of pyrrolidinone derivatives from aniline, an aldehyde and diethyl acetylenedicarboxylate in an ethanolic citric acid solution under ultrasound irradiation. Green Chem. 18, 3582–3593 (2016)
L. Wen, Zh..R. Li, M. Li, H. Cao, Solvent-free and efficient synthesis of imidazo[1,2-a]pyridine derivatives via a one-pot three-component reaction. Green Chem. 14, 707–716 (2012)
A. Palmieri, S. Gabrielli, C. Cimarelli, R. Ballini, Fast, mild, eco-friendly synthesis of polyfunctionalized pyrroles from β-nitroacrylates and β-enaminones. Green Chem. 13, 3333–3336 (2011)
A. Ramazani, A. Mahyari, A. Farshadi, M. Rouhani, Preparation of silica nanoparticles from organic laboratory waste of silica gel HF254 and their use as a highly efficient catalyst for the one-pot synthesis of 2,3-dihydro-1H-isoindolone derivatives. Helv. Chim. Acta. 94, 1831–1838 (2011)
M. Yurdakoc, M. Akcay, Y. Tonbul, K. Yurdakoc, Acidity of silica-alumina catalysts by amine titration using hammett indicators and FT-IR study of pyridine adsorption., Turk. J. Chem., 23: 319–327 (1999)
Acknowledgements
The authors thank Science and Research Branch, Islamic Azad University for the support and guidance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouhani, M., Ramazani, A. Perlite–SO3H nanoparticles: very efficient and reusable catalyst for three-component synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amine derivatives under ultrasound irradiation. J IRAN CHEM SOC 15, 2375–2382 (2018). https://doi.org/10.1007/s13738-018-1426-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1426-8