Abstract
Extant hyraxes are small, rabbit-sized, herbivorous paenungulates, with a notable feeding mechanism: they crop with the molars instead of the incisors, unlike most modern hoofed mammals. We investigated the postnatal development of the skull, and their functional correlates, in two extant representative forms of hyraxes, the terrestrial grazer Procavia capensis and the arboreal browser Dendrohyrax arboreus. We measured 18 linear variables representing fundamental descriptive and functional aspects of the skull, and estimated allometric equations on the basis of bivariate and multivariate analyses of an ontogenetic series of 32 specimens of P. capensis and 27 specimens of D. arboreus. Results showed that the ontogenetic trajectories of both species are similar in overall shape and size, but differ in localized regions of the skull associated with the masticatory apparatus. Examples include both differences in degree of allometry (upper postcanine row more positively allometric in Procavia) as well as opposing trends (positive allometry of diastema length in Dendrohyrax versus negative allometry in Procavia). In the mandible, the height of mandible and the length of the lower postcanine row showed significant differences. These changes could be associated with the acquisition of contrasting herbivorous specialization along the browsing-grazing gradient as exemplified in hyraxes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hyracoidea are small-sized hervibores that originated in the Afro-Arabian landmass during the Eocene (Barrow et al. 2010) as part of the diversification of Paenungulata, a group of afrotherian hoofed mammals that also include proboscideans and sirenians (Tethytheria; Simpson 1945; Seiffert 2007; Asher and Helgen 2010). Extant hyraxes are restricted to tropical and subtropical habitats in continental Africa and the Middle East, across a wide range of elevations from 400 m below sea level near the shores of the Dead Sea, to 3500 m above sea level in East Africa (Shoshani 1992). However, this order was more diverse in the past, with 17 extinct genera (Rasmussen and Gutierrez 2010). Recent species are united in a single family, Procaviidae, with three extant genera, Procavia, Heterohyrax, and Dendrohyrax (Hoeck 2011). The three hyrax genera are quite similar and share many morphological character states, but nonetheless they can be differentiated by anatomical, physiological, and behavioral characters (Hoeck 1975; Corbert 1979; Shoshani 1992, 2001; Eley 1994).
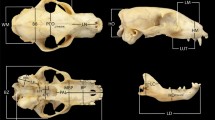
Hyraxes lack sexual size dimorphism (Hoeck 2011). The incisors are hypertrophied and resemble tusks (Fig. 1); unsuitable for food processing, incisors are not used in the ingestion of plants that compose their diets (Sale 1966). Thus, hyraxes exhibit a rather unconventional mode of food intake using the molar teeth; for browsing they turn the head sideways (at 90° to the body) and use the molar teeth to bite of the shoot or leaf and take it in through the side of the mouth (Sale 1966). Hyraxes lack permanent canine teeth and exhibit a large diastema between incisors and premolars (like many ungulates), more pronounced in the upper than in the lower jaw (Eley 1994); the upper diastema is longer in D. arboreus (~17 mm) than in the similar sized P. capensis (~9 mm) (Fig. 1b, d; Thomas 1892). Procavia and Heterohyrax have a short muzzle and a high and broad cranium with an incomplete postorbital bar, while Dendrohyrax have a large muzzle and a narrow and flattened cranium, with a depression above the orbit and a complete postorbital bar (Fig. 1; Shoshani 1992). Hyraxes are agile climbers: Procavia (rock hyrax) and Heterohyrax (bush hyrax) occupy rocky outcrops (kopjes) and are gregarious and diurnal, while Dendrohyrax species (tree hyraxes) are arboreal, usually solitary, and nocturnal (Eley 1994; Hoeck 2011). All species are herbivores with a diet consisting mostly of leaves, twigs, grasses, fruit, and some insects. Although they do not ruminate, the digestive system is complex, with three separate areas of microbial fermentation for food, similar to that of ruminant ungulates (see Hoeck 2011). Procavia capensis and D. arboreus exhibit both similarities and differences in their biology, which are summarized in Table 1.
Most authors refer to the dietary habits of extant hyraxes as either grazers or browsers, including the use of mixed-feeders categories, which is also true, for most herbivorous mammals (e.g., Janis 1990; Mendoza and Palmqvist 2008; Cassini 2013 and references therein). Bargo and Vizcaíno (2008) remarked upon the ambiguous nature of the terms browsing and grazing, because the terms have been used to refer both to the mode of food acquisition (i.e., selectivity) and to the type of food ingested (i.e., botanical connotation). Following Janis (2008), we adopt the latter concept in our work. As such, browsers and grazers differ in many traits including behavior, physiology, and morphology (Clauss et al. 2008). In particular, several authors agree that grazing ungulates tend to have higher hypsodonty index (i.e., relative crown height), wider muzzle and palate, and longer molar versus premolar tooth rows than browsers do (see also Table 1 in Mendoza 2005 and Tables 3.4 and 3.5 in Clauss et al. 2008). Hypsodonty has evolved numerous times in mammals and probably represents a fairly simple developmental change, involving delaying the closure of the tooth roots (Janis 1988) or any of a series of heterochronic changes from crown to root (Koenigswald 2011). Hypsodonty is present for instance in tusks, as in hyraxes (Koenigswald 2011), but it is more often referred to cheek teeth and it has been associated with grass consumption (Williams and Kay 2001) and foraging in open habitats (Mendoza and Palmqvist 2008). Compared with artiodactyls and perissodactyls, the rock hyrax P. capensis has a low hypsodonty index (HI ~ 1.69; Janis 1988) but higher than the value we calculated for D. arboreus (mean HI = 1.13; sd. = 0.0305; n = 3). Mendoza et al. (2002) related this lower HI to the lower metabolic rate and the consequently lower than expected daily intake for an ungulate of its size. Thus, hyraxes are shown to vary along the brachyodont-hypsodont gradient, with Dendrohyrax being typically brachyodont and Procavia exhibiting a tendency to hypsodonty.
The Hyracoidea have received much attention in ecological, systematic, molecular, and behavioral research (e.g., Roche 1975; Prinsloo and Robinson 1992; Shoshani 1992, 2001; Eley 1994; Milner and Harris 1999; Visser 2013). Anatomy of fossil and extant forms has also been intensely investigated (e.g., Thomas 1892; Beddard 1909; Churcher 1956; Bothma 1967; Klein and Cruz-Uribe 1996; Pickford 1996, 2005; Barrow et al. 2010). However, the paucity in developmental data precludes in-depth evolutionary comparisons with paenungulates and other hoofed mammals. An account of the morphological changes with functional significance during any period of development is lacking for procaviids as a group.
Here, we contribute a quantitative study of postnatal skull ontogeny in two contrasting hyrax species in a comparative framework, the terrestrial, predominantly grazing rock hyrax P. capensis, and the arboreal, browsing tree hyrax D. arboreus. Cranial ontogeny is highly relevant to understanding the relationship between function and trophic ecology; here, we describe the ontogenetic basis of cranial differences between two species that constitute one important example of the key browsing-grazing functional gradient in the evolution of ungulates.
Material and Methods
Study Specimens
We analyzed postnatal ontogenetic series of 32 specimens (with 18 females and nine males) of Procavia capensis, and 27 specimens (with 14 females and seven males) of D. arboreus. Specimens (listed in Table 2) are housed in the Mammal collections of the Field Museum of Natural History (FMNH, Chicago, USA), National Museum of Natural History, Smithsonian Institution (USNM, Washington, D.C., USA), and the American Museum of Natural History (AMNH, New York, USA).
We assigned relative individual dental age stages or IDAS, following Anders et al. (2011). Our sample covered all stages in the ontogenetic series of both species, infant, juveniles, adults, late adults, and seniles (IDAS 1 to 5, respectively; Table 2). This methodology has the advantage to be generalized and useful for both fossil and extant placental mammals, providing a wide comparative basis for ontogenetic studies. Thus, we provide this classification as informative and for future comparative purposes. The Dendrohyrax sample was composed of nine infants (IDAS 1), of which the smallest specimen was 51.37 mm in condyle-basal length (FMNH 17505, Table 2); eight juveniles (IDAS 2), five adults (IDAS 3), three late adults (IDAS 4), and two senile specimens (IDAS 5), of which the largest specimen was 94.12 mm in condyle-basal length (FMNH 17508, Table 2). The Procavia sample consisted of six infants, of which the smallest specimen was 44.77 mm in condyle-basal length (USNM 468208, Table 2), 13 juveniles, five adults, four late adults, and four senile specimens, of which the largest specimen was 92.41 mm in condyle-basal length (FMNH 47371, Table 2).
Study of Growth and Measurements
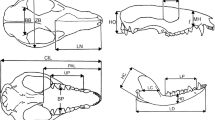
Allometry refers broadly to how specific traits grow at a given rate as compared with the body growing as a whole. In morphometric studies, the term refers to the association between size and shape variation (Huxley 1932; Mosimann 1970; Gould 1977; Mitteroecker et al. 2013). Ontogenetic allometry (or growth allometry) deals with covariation among characters (modifications in structures) as the animal grows (Klingenberg and Zimmermann 1992; Klingenberg 1996). In addition, allometry explicitly considers timing of changes as the rate of development (i.e., the rate of change in shape) and the rate of growth (i.e., the rate of change in size) throughout the lifetime (Piras et al. 2011 and references therein). Allometry of size compares shape changes against overall size along a growth series (i.e., rate of development versus rate of growth) wherein the time frame is implicit. Consequently, the association between size and shape across different age stages can be used as an estimate of a population’s ontogenetic trajectory, especially when the calendar ages are not known (Mitteroecker et al. 2013). In our study, we use a Mixed Cross-Sectional Data (sensu Cock 1966) collected in different specimens in several known relative age stages (i.e., IDAS, Table 2). For the allometric analysis, we used 18 skull linear measurements (Fig. 2; Table 3) including length, breadth, and height of neurocranial and splanchnocranial components describing overall skull structure and general functional attributes. The craniomandibular dimensions analyzed herein partially overlap with those considered in other morphometric studies of mammalian skulls (e.g., Abdala et al. 2001; Giannini et al. 2004,2010; Cassini et al. 2012, 2015; Flores et al. 2013; Tarnawski et al. 2014a, b, 2015). We pooled males and females of all ages in a single sample given the lack of sexual size dimorphism in hyraxes (Hoeck 2011), and then analyzed the data in both bivariate regression and multivariate allometry frameworks. Both methodologies fall in the concept of allometry of size in which developmental time is implicit in the growth series, and ontogenetic changes are compared against a change in size (see Abdala et al. 2001).The regression approach is suitable for statistical comparison of slopes, intercepts, and shifts of regressions between both species, whereas the multivariate approach focuses on allometric coefficients all simultaneously affected by size as a latent variable (see below).
Skull and mandible measurements used in this study. Variable abbreviations as in Table 3
Bivariate Analyses
The ontogenetic trajectories were obtained by bivariate linear regressions after log transformation (log10) of the measurements (Weston 2003). In the comparisons of ontogenetic trajectories, there are different growth patterns in a common size-shape space, where size is a proxy of time (see Reilly et al. 1997; Abdala et al. 2001; Piras et al. 2011; Sheets and Zelditch 2013). The trajectory itself may differ in direction (slope), position (y-intercept), and in length (shift). There are three basic types of evolutionary change (Weston 2003; Tarnawski et al. 2014a, b): 1. ontogenetic scaling: trajectories overlap so direction and position are the same and trajectories differ in their offset; 2. lateral shift: in which the trajectories share direction but the position is different (parallel trajectories with different intercept), indicative of change in the onset (i.e., juveniles of one group resembles an older or younger age-class of the other group); and 3. directional change, in which trajectories are different in the direction and position as reflected in the slope, indicative of novel modes of postnatal growth (e.g., convergent to the same offset shape or divergent from the same onset shape).
In bivariate analysis, the scaling of any measurement can be affected strongly by the choice and scaling of the independent variable (Smith 1981; Wayne 1986). Several researchers have taken the total skull length (TSL), or a correlated measurement such as the condyle-basal length (CBL), as a proxy of overall size and as an independent variable (e.g., Abdala et al. 2001; Flores et al. 2003; Weston 2003; Giannini et al. 2004; Cassini et al. 2012; Kruska and Steffen 2013), because they demonstrated that it was isometric. However, this was not the case in all mammalian groups (see Tarnawski et al. 2014a, b, 2015). In hyraxes, CBL exhibited negative allometric growth (see below). To avoid the bias of such trends, we used the geometric mean of all cranial and mandibular measurements as the proxy of size and so the independent variable (Mosimann 1970; Tarnawski et al. 2015 and references therein). To examine the allometric changes of each variable to overall size (geometric mean), we used the log transformation of the power growth function, y = b0.xb1.e, to obtain the equation of allometry: log(y) = log b0 + b1 log (x) + log(e), where y is the focus variable, log(b0) is the y-intercept, x is the geometric mean, b1 is the slope of the line or coefficient of allometry, and e is the error term (see Alexander 1985; Giannini et al. 2004). We use the standardized major axis (SMA) regression to determine an axis or line of best fit because is more appropriate than the ordinary least squared regression (OLS) for dealing with allometric approaches (for extensive overviews on the subject, see Warton et al. 2012). Following Mitteroecker et al. (2013), in the classic concept of allometry a trait is considered as “negatively” or “positively” allometric if it increases less or more in size than other traits, or overall size, do(es). The relationship between the two variables is isometric when the slope is equal to one (a value expected under geometric similarity between two variables with the same dimensionality; Peters 1993). As a first step, we performed F-tests to assess the significance of the deviations from isometry in the allometry coefficients under the null hypothesis b1 = 1.0 (Warton and Weber 2002). Consequently, if b1 < 1.0 the case was one of “negative” allometry, and of “positive” allometry if b1 > 1.0 (Emerson and Bramble 1993). Testing for a common interspecific coefficient of allometry between P. capensis and D. arboreus for a given variable was the second step in the analysis. We used the likelihood ratio test to find a common SMA slope following Warton et al. (2006). If both species shared a common coefficient of allometry, the significance of a common constant of normalization (y-intercepts) was compared using the Wald statistic (Warton et al. 2006). Finally, if both slopes and y-intercepts were shared, the data points were scattered around a common axis with no difference in elevation. The Wald statistic was to test the hypothesis that there might be an ontogenetic shift along the axis, as in Warton et al. (2006). All coefficients, statistical parameter estimations, and tests were performed using smatr 3.4–3 package in R (Warton et al. 2012).
Multivariate Analysis
We used a multivariate approach to allometry based on the generalized allometry equation proposed by Jolicoeur (1963). In contrast with bivariate analyses, multivariate allometry considers size as a latent variable affecting all original variables simultaneously (see also Giannini et al. 2004). The various allometric relationships of all variables with the latent size can be expressed in the first eigenvector of a principal components analysis (PCA). This eigenvector is extracted from a variance-covariance matrix of log-transformed variables and is scaled to unity. In this approach, allometry is a deviation with respect to a hypothetical isometric eigenvector, which represents pure size change. All eigenvector elements are the same and represent the expected isometric value; it is calculated as the squared root of 1/p with p equal to the number of variables. The hypothetical isometric vector equals 0.236 for 18 skull variables. To estimate the statistical deviation from isometry of our variable set, we used the application of jackknife developed by Giannini et al. (2004) and applied in several studies (see Flores et al. 2015 and references therein). This technique generates confidence intervals for each of the empirically derived first-eigenvector elements (Giannini et al. 2010). If the confidence interval for a given variable includes the isometric value (here 0.236), the variable is statistically indistinguishable from isometry. Alternatively, the interval may exclude the isometric value, being “positive” if the observed element is >0.236, or “negative” otherwise. Statistical departures from isometry were estimated using a jackknife procedure (Quenouille 1956; Manly 1997), removing one specimen at a time. Here, we report results from untrimmed (full set of jackknife pseudovalues used) and trimmed analyses (with the m smallest pseudovalues removed in the calculations, with m = 1), and report 95% confidence interval for each allometry coefficient (for details see Giannini et al. 2004). The multivariate statistical analysis (PCA + jackknife resampling; Giannini et al. 2010) was programmed in an R script (R Development Core Team 2004) and is available from the authors.
Several approaches were used to explore and compare ontogenetic and multivariate allometric trends in different groups (e.g., ordinations such as PCA or between-group PCA: Mitteroecker and Bookstein 2011; plot growth trajectories and allometric vectors on PCA: Coquerelle et al. 2011; compare multiple allometric vectors by PCA: Mitteroecker et al. 2013). In our study we used the angle between the first eigenvectors obtained for each species in this section to compare the ontogenetic trajectories, by means of this R command: angle = acos(sum(a*b)/(sqrt(sum(a*a))*sqrt(sum(b*b)))), where a is the first eigenvectors of P. capensis, and b is the first eigenvectors of D. arboreus. In addition, we performed a permutation test to assess the significance of directional difference between vectors under the null hypothesis that the angle value is smaller than obtained by chance. All data generated during our analyses in the current study are available from the corresponding author on reasonable request.
Data Availability
All data generated during our analyses in the current study are available from the corresponding author on reasonable request.
Results
Bivariate Analysis
Allometry in Dendrohyrax arboreus
All regression coefficients were significant (Table 4) except those of y-intercepts of length of palate (PAL), the breadth of palate (PAB), and the height of coronoid process (CPH) (Table 4). Regressions resulted in high determination coefficients values for almost all variables (i.e., 0.75 or higher) except for breadth of braincase (BCB) and (CPH). The observed allometric trends were isometric in five from 18 variables (i.e., 27.8%; PAB, BZB, CSL, CPH, and CPW). In the remaining measurements, positive allometry was detected in seven variables (i.e., 38.9%; PAL, MZL, LPD, UPL, LPL, MCDr, and MCDc), while allometric negative coefficients were found in six variables (i.e., 33.3%; CBL, OCH, MZHr, ORL, BCB, and BMB).
Allometry in Procavia capensis
Bivariate regressions coefficients were significant except those of y-intercepts of length of palate (PAL) and meatal breadth (BMB) (Table 4). Regressions resulted in high determination coefficients values for almost all variables (i.e., 0.75 or higher) except for breadth of braincase (BCB), breadth of palate (PAB), length of diastemata (LPD), and meatal breadth (BMB). The observed allometric trends were isometry in six from 18 variables (i.e., 33.3%; e.g., CBL, PAB, BMB, BZB, CSL, and CPW). In the remaining measurements, positive allometry was detected in eight variables (i.e., 44.4%; PAL, MZL, LPD, UPL, LPL, MCDr, MCDc, and CPH), while negative coefficients were found in four variables (i.e., 22.2%; OCH, MZHr, ORL, and BCB).
Interspecific Comparisons
In both species, the condyle-basal length (CBL), length of palate (PAL), and length of rostrum (MZL) showed the higher determination coefficient (R2) among variables, and so the highest correlation with size (Table 4). Although we detected common allometric trends in the SMA analyses (i.e., the same allometric sign in 15 of 18 variables, 83.3%), the statistical analyses show that both species shared a common slope in only nine of the 18 variables (i.e., 50%; PAL, MZL, OCH, LPD, BZB, CSL, LPL, CPH, and CPW; Table 5).
Comparison of growth trajectories showed three patterns of developmental changes, or their lack thereof, illustrated with examples in Fig. 3. Two patterns corresponded with trajectories with the same slope (Fig. 3a, b), and one with trajectories with different slopes (Fig. 3c). The first pattern (Fig. 3a) occurred only in three of the total variables and shows differences neither in slope, intercept, or shift (BZB, CSL, and CPH). The second pattern has the same slope but different intercept (i.e., parallel trajectories; Fig. 3b), and occurred in six variables. In three (PAL, MZL, and LPD) of these six variables, Dendrohyrax showed greater intercept values than Procavia whereas the reverse was true in OCH, LPL, and CPW. The third pattern corresponded with significant differences in slopes (Fig. 3c), and occurred in seven variables, in which, we observed convergent trajectories (TBL, PAB, BCB, MCDr, and MCDc) and divergent trajectories (UPL and ORL).
Bivariate plots of three skull measurements against geometric mean. a, same slope and intercepts; (b), same slope and different intercepts; and (c), different slopes between the growth trajectories. Open symbols and continuous are Procavia capensis, solid symbols and dashed line are Dendrohyrax arboreus. Variable abbreviations as in Table 3
Multivariate Analyses
The results of multivariate analysis of allometry are shown in Table 6. Fig. 4 shows the distribution of confidence intervals of each species with respect to the expected isometric value (0.236). Procavia capensis and D. arboreus shared fourteen allometric trends (77.7%). Of the common set, seven variables (CBL, OCH, MZH, ORL, BCB, BMB, and BZB) were negatively allometric; six (PAL, MZL, UPL, LPL, MCDr, and MCDc) were positive and only one (HCP) was isometric (Table 6). The remaining four variables (LPD, PAB, CSL, and CPW) differed between species (Table 6). In D. arboreus, PAB, CSL, and CPW showed negative allometry (isometric in P. capensis) and LPD positive allometry (negative in P. capensis; Table 6). Still, in PAB, CSL, and CPW, the confidence intervals (ci) overlapped between the species (Fig. 4). The angular comparison yielded an 8.95° angle between the first eigenvectors obtained for each species. The permutation test showed that both ontogenetic trajectories were significantly different (P-value = 0.0011 with 10,000 random permutations).
Distribution of confidence intervals for each species with respect to the isometric value (0.236). Solid lines are Procavia capensis; open lines are Dendrohyrax arboreus. Variable abbreviations as in Table 3
It is noteworthy that ten of 18 variables showed the same trend in bivariate and multivariate analyses. The remaining variables showed partially similar trends between methods (i.e., in one of the two species; CBL, PAB, LPD, BMB, CSL, CPH, and CPW) or different trends (i.e., in both species; BZB).
Discussion
Analyses of allometry in the hyraxes P. capensis (terrestrial, grazer, tendency to hypsodonty) and D. arboreus (arboreal, browser, brachyodont) indicated common growth trends in three-quarters of the cranial variables investigated. Multivariate ontogenetic trajectories were statistically different between species, but the relatively small angle of 8.95° between them suggested that both allometric vectors were not quite divergent.
Hyraxes are morphologically similar to each other, but differ in ecologically important features (see Table 1). Conservation of a majority of allometric patterns in Procavia and Dendrohyrax suggests that they simply follow the same growth processes as their common ancestor, but with some significant particularities in each species. The bivariate analysis showed that adults of both species converged to approximately the same total skull length. However, functionally important trends have been recovered in both bivariate and multivariate analyses and relate to differences in life style, specifically the grazing versus browsing habit. We interpret these trends as follows.
Both species of hyrax shared negative growth trends in zygomatic breadth (BZB) and braincase variables (BCB, BMB, and OCH), as observed in other ungulates (e.g., cervids, Cassini et al. 2015; tapirs, Moyano and Giannini 2017). Churcher (1956) described the adult cranium of Dendrohyrax as narrow and less globular, and Thomas (1892) described the adult skull of Procavia as broad. Our study showed that the breadth of braincase is the most negative allometric of all variables in both species, but more so in Dendrohyrax (Fig. 4), which explains its adult shape. The meatal breadth suggests a relatively narrower skull in adults than in juveniles of both species. The height of occipital plate showed a higher intercept in Procavia; i.e., this character is juvenilized with respect to Dendrohyrax (Procavia with a taller occipital plate than Dendrohyrax). In the orbital region, the results of bivariate and multivariate analyses are the same (Tables 4 and 6). Zygomatic breadth of both species had the same slope and intercept, in both cases presenting slightly negative allometry. The same trend was reported in the extinct notoungulate Nesodon, the pampas deer Ozotoceros bezoarticus, and one species of Tapirus (Cassini et al. 2012, 2015; Moyano and Giannini 2017). Zygomatic breadth showed positive allometry in mammals with large temporalis muscles; space for the temporalis is created by the combination of the negative allometry of braincase breadth and the positive allometry of zygomatic breadth (e.g., as in Didelphis albiventris, an omnivorous didelphid: Abdala et al. 2001; Adinotherium ovinum, a herbivore notoungulate: Cassini et al. 2012; Lycalopex culpaeus, a carnivoran: Segura and Prevosti 2012). In hyraxes, as in most modern ungulates (Greaves 2012 and references therein), the temporalis is relatively less important than the masseter, and this is reflected in the modest expansion of zygomatic breadth but great development of the angular process of the mandible (insertion of the masseter; see below).
The allometric growth trends of muzzle variables (UPL, LPD, MZL, PAL, PAB, and MZHr) showed that hyraxes developed a relatively long rostrum (with upper postcanine row and palate length showing positive allometry), which is also relatively low and narrow (with breadth of palate and height of muzzle showing negative allometry). Nonetheless, differences exist between Dendrohyrax and Procavia, and these are very important functionally. In Procavia, the upper postcanine row (UPL) grows much faster than in Dendrohyrax, which is reflected in the strongly negative growth of the upper diastema. That is, in Procavia, space for a considerably larger postcanine upper toothrow is gained during development at the expense of the diastema. This can be directly associated with the greater degree of hypsodonty and also to the grazing habit in Procavia; cheek teeth take an increased space in both the maxillary tuber and the mandible (see below) to house larger molars (Clauss et al. 2008). In addition, the cheek-teeth occlusal surface area (OSA), here only tangentially considered in the length of the postcanine toothrow (see also Appendix I in Vizcaíno et al. 2006), is larger in grazers than in browsers among ungulates, suggesting more extensive oral food processing (Vizcaíno et al. 2006 and references therein), which is also evident in hyraxes (Fig. 1). In ungulates, chewing area is also inversely related to the nutritional value of food (less quality in grazers; Jarman 1974; Shipley 1999; Vizcaíno et al. 2006). Dendrohyrax had a greater intercept of palate and rostrum length than Procavia, suggesting that the former began postnatal growth with a longer rostrum. Also, the muzzle in Dendrohyrax is relatively lower than that in Procavia, all of which is indicative of browsing habits (longer and finer rostrum used to more effectively select foliage, see above). Length of the orbit showed different modes of growth in the two hyraxes. The juveniles of Dendrohyrax begin the ontogeny with an orbit slightly larger than Procavia (intercept = 0.434 in Dendrohyrax, 0.210 in Procavia; Table 4), but greater growth rate in Procavia led to adults with greater value than in Dendrohyrax. Orbital growth differences in Dendrohyrax and Procavia could be related to the slight differences in the change of the orientation during the ontogeny; we observed that the orbits in Dendrohyrax are more vertically and laterally positioned in the newborn, and become more dorsally (horizontally) and anteriorly oriented in the adult (Fig. 1). These differences could be due to their differential use of substrate (Table 1). Several studies have related the anterior convergence of left and right orbits with the formation of a bony postorbital bar (present in Dendrohyrax, Fig. 1) for increased stereoscopic visual acuity and depth perception, which has been linked to arboreality and nocturnal vision (Watson et al. 2000; Finarelli and Goswami 2009 and references therein). Pilatti and Astúa (2016) described, for Didelphidae, that the elongation of rostrum implies orbits more dorsally positioned and a more convergent orientation in the cranium, whereas the widening implies more vertical position and a little divergent orbit orientation. We have not found significant differences in the length of the muzzle to corroborate this hypothesis, but our data suggest an increase in the growth rate of palate breadth in Procavia, which could be limiting a more vertical position (see Pilatti and Astúa 2016).
Regarding the mandible, three variables showed positive allometry. These were height of mandibular body (at first and last functional lower postcanine) and the length of postcanine row (MCDr, MCDc, LPL; Fig. 4). In all three cases, Dendrohyrax presented greater coefficients, and the mandible height at last molar in Dendrohyrax showed a higher rate of growth than in Procavia (unbiased coefficient = 0.433 in Dendrohyrax and 0.313 in Procavia, respectively; MCDc, Fig. 4 and Table 6). Janis (1983, 2008) observed that the jaws of hyraxes can be distinguished by the extreme deepening and posterior extension of the angle of the mandible, which corresponds with the enlarged masseteric-pterygoid muscle complex commonly seen in herbivorous mammals (Greaves 2012 and references therein). In early age, the mandible of Procavia is more robust than that of Dendrohyrax, which can be related again with a tendency to hypsodonty in the young of Procavia, but the mandibular body in Dendrohyrax grew at a faster rate and so the trajectories converged in the adult of both species. Pickford (2005) observed that the mandibles of Procavia species are shallower relative to the level of the molar row, and the converse was true in Dendrohyrax. This can be corroborated in the relationship between MCDc and LPL in the bivariate results (Fig. 3). Our study showed that the mandible in Dendrohyrax grew rapidly and was posteriorly taller than that of Procavia. This could not be related to the dentition type, but to the anatomy of the mandibular angle in Dendrohyrax, which seems to extend more anteroventrally than in Procavia and receive a greater share of insertion of superficial and deep masseter and medial pterygoid (Fig. 1; Janis 1983).
Cassini (2013) observed that the presence of high-crowned molars was related to mandibular shape. A similar relationship was observed in the anterior dentition. Hypsodont incisors present in Procavia resemble the condition seen in notoungulates (see tomography of P. capensis in Seiffert 2011), which requires a wide space in the anterior portion of the mandible to accommodate the hypsodont root (Cassini et al. 2017). In the lower dentition, the typotheres (Notoungulata: Typotheria) have procumbent incisors, inserted following the direction of the anterior face of the symphysis, and the root heights are about the same as those of the crown. The allometric growth of the anterior mandibular height in Procavia is positive but less so than in Dendrohyrax (Fig. 4; Table 6). This could be a consequence of hypsodonty of milk incisors in Procavia; i.e., the space is already enlarged at the beginning of postnatal growth and so the rate of change is slower as compared with Dendrohyrax.
Hyraxes have a small coronoid process, which is considerably smaller than the coronoid process of most ungulates (Janis 1983), reflecting a lesser importance of the temporalis muscle in mastication as compared with the masseteric complex (see above). The temporalis muscle inserts onto the coronoid process; a relatively high and caudally curved coronoid process provides a large area for the insertion of the temporalis (Cassini and Vizcaíno 2012). A small process would result in longer fibers in the temporalis attachment, and hence it would permit a greater degree of stretch in this muscle (Janis 1983). The coronoid process is broader in Procavia than in Dendrohyrax, which functionally matches the presence of the sagittal crest in the former (absent in the latter).
Conclusion
Our study on skull growth in hyraxes provides the developmental basis for the understanding of structural dissimilarities between closely related species, as well as the developmental basis of their divergent ecological role as grazing versus browsing mammalian herbivores. These small species in a rather isolated, low-diversity paenungulate clade evolved opposing trends along the grazing-browsing gradient, and we show how subtle developmental processes of the skull explain the acquisition of the adult functionality in the diverging ecologies of the adults. The trends we observed in hyraxes could reflect a more general, convergent pattern in the many ungulates lineages that evolved along the browsing-grazing continuum, and here we provided a comparative basis to that end with the lineage of hyraxes. Further studies in different lineages will certainly contribute to understanding the evolution of development in ungulates.
References
Abdala F, Flores DA, Giannini NP (2001) Postweaning ontogeny of the skull of Didelphis albiventris. J Mammal 82(1):190–200
Alexander RM (1985) Body support, scaling and allometry. In: Hildebrand M, Wake DB (eds) Functional Vertebrate Morphology. Belknap Press of Harvard University Press, Cambridge, pp 27–37
Anders U, Koenigswald W von, Ruf I, Smith BH (2011) Generalized individual dental age stages for fossil and extant placental mammals. Paläontol Z 85:321–339
Asher RJ, Helgen KM (2010) Nomenclature and placental mammal phylogeny. BMC Evol Biol 10(1):1
Bargo MS, Vizcaíno SF (2008) Paleobiology of Pleistocene ground sloths (Xenarthra, Tardigrada): biomechanics, morphogeometry and ecomorphology applied to the masticatory apparatus. Ameghiniana 45(1):175–196
Barrow E, Seiffert ER, Simons EL (2010) A primitive hyracoid (Mammalia, Paenungulata) from the early Priabonian (late Eocene) of Egypt. J Syst Palaeontol 8(2):213–244
Beddard FE (1909) Contributions to the anatomy of certain Ungulata, including Tapirus, Hyrax, and Antilocapra. Proc Zool Soc Lond 1901:160–197
Bothma JdP (1967) Recent Hyracoidea (Mammalia) of southern Africa. Ann Transvaal Mus 25:117–152
Cassini GH (2013) Skull geometric morphometrics and paleoecology of Santacrucian (late early Miocene; Patagonia) native ungulates (Astrapotheria, Litopterna, and Notoungulata). Ameghiniana 50(2):193–216
Cassini GH, Flores DA, Vizcaíno SF (2012) Postnatal ontogenetic scaling of nesodontine (Notoungulata, Toxodontidae) cranial morphology. Acta Zool 93:249–259
Cassini GH, Flores DA, Vizcaíno SF (2015) Postnatal ontogenetic scaling of pampas deer (Ozotoceros bezoarticus celer: Cervidae) cranial morphology. Mammalia 79(1):69–79
Cassini GH, Hernández Del Pino S, Muñoz NA, Acosta WG, Fernández M, Bargo MS, Vizcaíno SF (2017) Teeth complexity, hypsodonty and body mass in Santacrucian (early Miocene) notoungulates (Mammalia). Earth Env Sci Trans R Soc Edinb 106(4):303–313
Cassini GH, Vizcaíno SF (2012) An approach to the biomechanics of the masticatory apparatus of early Miocene (Santacrucian age) South American ungulates (Astrapotheria, Litopterna, and Notoungulata): moment arm estimation based on 3D landmarks. J Mammal Evol 19(1):9–25
Churcher CS (1956) The fossil Hyracoidea of the Transvaal and Taung deposits. Ann Transvaal Mus 22:447–501
Clauss M, Kaiser T, Hummel J (2008) The morphophysiological adaptations of browsing and grazing mammals. In: Gordon IJ, Prins HHT (eds) The Ecology of Browsing and Grazing. Springer, Berlin, pp 47–88
Cock AG (1966) Genetical aspects of metrical growth and form in animals. Q Rev Biol 131–190
Coquerelle M, Bookstein FL, Braga J, Halazonetis DJ, Weber GW, Mitteroecker P (2011) Sexual dimorphism of the human mandible and its association with dental development. Am J Phys Anthropol 145(2):192–202
Corbert GB (1979) The taxonomy of Procavia capensis in Ethiopia, with special reference to the aberrant tusks of P. c. capillosa Brauer (Mammalia, Hyracoidea). Bull Brit Mus Nat Hist (Zool) 36:251–259
Eley RM (1994) The hyrax, a most mysterious mammal. Biologist 41:141–144
Emerson SB, Bramble DM (1993) Scaling, allometry, and skull design. The skull 3:384–421
Finarelli JA, Goswami A (2009) The evolution of orbit orientation and encephalization in the Carnivora (Mammalia). J Anat 214:671–678
Flores DA, Abdala F, Giannini NP (2013) Post-weaning cranial ontogeny in two bandicoots (Mammalia, Peramelomorphia, Peramelidae) and comparison with carnivorous marsupials. Zoology 116:374–384
Flores DA, Abdala F, Martin GM, Giannini NP, Martinez JM (2015) Post-weaning cranial growth in shrew opossums (Caenolestidae): a comparison with bandicoots (Peramelidae) and carnivorous marsupials. J Mammal Evol 22(3):285–303
Flores DA, Giannini NP, Abdala F (2003) Cranial ontogeny of Lutreolina crassicaudata (Didelphidae): a comparison with Didelphis albiventris. Acta Theriol 48(1):1–9
Gaylard A, Kerley G (1997) Diet of tree hyraxes Dendrohyrax arboreus (Hyracoidea: Procaviidae) in the Eastern Cape, South Africa. J Mammal 78(1):213–221
Giannini N, Abdala F, Flores DA (2004) Comparative postnatal ontogeny of the skull in Dromiciops gliroides (Marsupialia: Microbiotheriidae). Am Mus Novitates 3460:1–17
Giannini NP, Segura V, Giannini MI, Flores DA (2010) A quantitative approach to the cranial ontogeny of the puma. Mammal Biol 75(6):547–554. doi:https://doi.org/10.1016/j.mambio.2009.08.001
Gould SJ (1977) Ontogeny and phylogeny. Harvard University Press, Cambridge
Greaves WS (2012) The Mammalian Jaw: A Mechanical Analysis. Cambridge University Press, Cambridge
Hoeck H (1975) Differential feeding behaviour of the sympatric hyrax Procavia johnstoni and Heterohyrax brucei. Oecologia 22:15–47
Hoeck HN (2011) Family Procaviidae (hyraxes). In: Wilson DE, Mittermeier RA (eds) Handbook of the Mammals of the World. Vol. 2. Hoofed Mammals. Lynx Edicions, Barcelona, pp 28–47
Huxley JS (1932) Problems of Relative Growth, 2nd ed. Dover Publications, New York
Janis CM (1983) Muscles of the masticatory apparatus in two genera of hyraces (Procavia and Heterohyrax). J Morphol 176(1):61–87
Janis CM (1988) An estimation of tooth volume and hypsodonty indices in Ungulate Mammals, and the correlation of these factors with dietary preference. In: Russell DE, Santoro JP, Sigogneau-Russell D (eds) Teeth Revisited: Proceedings of the VII International Symposium on Dental Morphology, Paris 1986. Mémoires du Muséum national d’Histoire naturelle (se´rie C), Sciences de la Terre 53:367–87
Janis CM (1990) Correlation of cranial and dental variables with dietary preferences in mammals: a comparison of macropodoids and ungulates. Mem Qd Mus 28(1):349–366
Janis CM (2008) An evolutionary history of browsing and grazing ungulates. In: Gordon IJ, Prins HHT (eds) The Ecology of Browsing and Grazing. Springer, Berlin Heidelberg, pp 21–45
Jarman PJ (1974) The social organization of antelope in relation to their ecology. Behaviour 48(1):215–267
Jolicoeur P (1963) The multivariate generalization of the allometry equation. Biometrics 9:497–499
Klein RG, Cruz-Uribe K (1996) Size variation in the rock hyrax, Procavia capensis, and late Quaternary climatic change in South Africa. Quaternary Res 46:193–207
Klingenberg CP (1996) Multivariate allometry. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE (eds) Advances in Morphometrics. Plenum Press, New York, pp 23–49
Klingenberg CP, Zimmermann M. (1992). Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders. Am Nat 601–620
Koenigswald W von (2011) Diversity of hypsodont teeth in mammalian dentitions—construction and classification. Palaeontographica Abt A 294: 63–94
Kruska DCT, Steffen K (2013) Comparative allometric investigations on the skulls of wild cavies (Cavia aperea) versus domesticated guinea pigs (Cavia aperea f. porcellus) with comments on the domestication of this species. Mammal Biol 78:178–186
Manly BFJ (1997) Randomization, Bootstrap, and Monte Carlo Methods in Biology. 2nd ed. Chapman and Hall, London
Mendoza M (2005) Hacia una caracterización ecomorfológica compleja: una revisión de la paleoautoecología de los ungulados. Ameghiniana 42(1):233–248
Mendoza M, Janis CM, Palmqvist P (2002) Characterizing complex craniodental patterns related to feeding behaviour in ungulates: a multivariate approach. J Zool 258(02):223–246
Mendoza M, Palmqvist P (2008) Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? J Zool 274(2):134–142
Millar RP (2015) Reproduction in the rock hyrax (Procavia capensis). Afr Zool 6:243–261
Milner JM, Gaylar A (2013) Family Procaviidae: Dendrohyrax arboreus southern tree hyrax (southern tree dassie). In: Kingdon J, Happold D, Butynski T, Hoffmann M, Happold M, Kalina J (eds) Mammals of Africa. Vol. 1. Bloomsbury Publishing, London, pp 152–155
Milner JM, Harris S (1999) Activity patterns and feeding behaviour of the tree hyrax, Dendrohyrax arboreus, in the Parc National des Volcans, Rwanda. Afr J Ecol 37:267–280
Mitteroecker P, Bookstein F (2011) Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. J Evol Biol 38(1):100–114
Mitteroecker P, Gunz P, Windhager S, Schaefer K (2013) A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix, Ital J Mammal 24(1):59–66
Mosimann JE (1970) Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. J Am Stat Assoc 65(330):930–945
Moyano SR, Giannini NP (2017) Comparative cranial ontogeny of Tapirus (Mammalia: Perissodactyla: Tapiridae). J Anat. doi: https://doi.org/10.1111/joa.12666
Olds N, Shoshani J (1982) Procavia capensis. Mammal Species 171:1–7
Peters TA (1993) The history and development of transaction log analysis. Library Hi Tech 11:41–66
Pickford M (1996) Pliohyracids (Mammalia, Hyracoidea) from the upper middle Miocene at Berg Aukas, Namibia. C R Acad Sci Paris 322:501–505
Pickford M (2005) Fossil hyraxes (Hyracoidea: Mammalia) from the late Miocene and Plio-Pleistocene of Africa, and the phylogeny of the Procaviidae. Palaeontol Afr 41:141–161
Pilatti P, Astúa D (2016) Orbit orientation in didelphid marsupials (Didelphimorphia: Didelphidae). Current Zool 63(4):403–415
Piras P, Salvi D, Ferrara G, Maiorino L, Delfino M, Pedde L, Kotsakis T (2011) The role of post-natal ontogeny in the evolution of phenotypic diversity in Podarcis lizards. J Evol Biol 24(12):2705–2720
Prinsloo P, Robinson TJ (1992) Geographic mitochondrial DNA variation in the rock hyrax, Procavia capensis. Mol Biol Evol 9:447–456
Quenouille MH (1956) Notes on bias in estimation. Biometrika 43:353–360
R Development Core Team (2004) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from http://www.Rproject.org. Accessed Jan 2016
Rasmussen DT, Gutierrez M (2010) Hyracoidea. In: Sanders WJ, Werdelin L (eds) Cenozoic Mammals of Africa. University of California Press, Berkeley, pp 123–146
Reilly SM, Wiley EO, Meinhardt DJ (1997) An integrative approach to heterochrony: the distinction between interspecific and intraspecific phenomena. Biol J Linn Soc 60(1):119–143
Rudnai J (1984) Suckling behaviour in captive Dendrohyrax arboreus (Mammalia: Hyracoidea). S Afr J Zool 19(2):121–123
Roche J (1975) Systematique du genre Procavia et des damans en general. Mammalia 36:22–49
Sale JB (1960) The Hyracoidea. J East Afr Nat Hist Soc 23(5):185–188
Sale JB (1966) Daily food consumption and mode of ingestion in the hyrax. J East Afr Nat Hist Soc 25:215–224
Segura V, Prevosti F (2012) A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131(1):79–92
Seiffert ER (2007) A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence. BMC Evol Biol 7(1):1
Seiffert E (2011) "Procavia capensis" (On-line), Digital Morphology. Accessed December 7, 2016 at http://digimorph.org/specimens/Procavia_capensis/
Sheets HD, Zelditch ML (2013) Studying ontogenetic trajectories using resampling methods and landmark data. Hystrix, Ital J Mammal 24(1):67–73
Shipley LA (1999) Grazers and browsers: how digestive morphology affects diet selection. In: Launchbaugh KL, Sanders KD, Moseley JC (Eds) Grazing Behavior of Livestock and Wildlife. Idaho Forest, Wildlife and Range Expeditions Station Bulletin, Moscow, pp 20–27
Shoshani J (1992) Ecology and taxonomy: a new approach, using Heterohyrax vs. Procavia as a case study. Isr J Zool 38:399–416
Shoshani J (2001) Hyracoidea. Encyclopedia Of Life Sciences. John Wiley and Sons, Ltd. Available from http://www.els.net
Simpson GG (1945) The principles of classification and a classification of mammals. Bull Am Mus Nat Hist 85:1–350
Smith RJ (1981) On the definition of variables in studies of primate dental allometry. Am J Phys Anthropol 55:323–329
Tarnawski BA, Cassini GH, Flores DA (2014a) Allometry of the postnatal cranial ontogeny and sexual dimorphism in Otaria byronia (Otariidae). Acta Theriol 59:81–97
Tarnawski B, Cassini G, Flores D (2014b) Skull allometry and sexual dimorphism in the ontogeny of the southern elephant seal (Mirounga leonina). Can J Zool 92:19–31
Tarnawski BA, Flores D, Cassini G, Cappozzo LH (2015) A comparative analysis on cranial ontogeny of South American fur seals (Otariidae: Arctocephalus). Zool J Linn Soc 173(1):249–269
Thomas O (1892) On the species of the Hyracoidea. Proc Zool Soc Lond 1892:50–76
Visser JH (2013) Gene-flow in the rock hyrax (Procavia capensis) at different spatial scales. MSc Dissertation. Stellenbosch University, Stellenbosch
Vizcaíno SF, Bargo MS, Cassini GH (2006) Dental occlusal surface area in relation to body mass, food habits and other biological features in fossil xenarthrans. Ameghiniana 43(1):11–26
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smatr 3 - an R package for estimation and inference about allometric lines, Meth Ecol Evol 3(2):257–259
Warton DI, Weber NC (2002) Common slope tests for bivariate errors-in-variables models. Biometrical J 44(2):161–174
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81(2):259–291
Watson RT, Noble IR, Bolin B, Ravindranath NH, Verardo DJ, Dokken DJ (2000) Land Use, Land Use Change, and Forestry. Cambridge University Press, Cambridge
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40:243–261. doi: https://doi.org/10.2307/2408805
Weston EM (2003) Evolution of ontogeny in the hippopotamus skull: using allometry to dissect developmental change. Biol J Linn Soc 80(4):625–638
Williams SH, Kay RF (2001) A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J Mammal Evol 8(3):207–229
Acknowledgements
We would like to thank Bruce Patterson and Bill Stanley (FMNH; Field Museum of Natural History, Chicago, USA), Eileen Westwig (AMNH; American Museum of Natural History, New York, USA), and Darrin P. Lunde (USNM; National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA). Visits to mammal collections were partially support by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) to SRM. This is a Contribution to the projects PICT-2015-2389, and CDD-CB 650/14 to GHC from the Universidad Nacional de Luján (UNLu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moyano, S.R., Cassini, G.H. & Giannini, N.P. Skull Ontogeny of the Hyraxes Procavia capensis and Dendrohyrax arboreus (Procaviidae: Hyracoidea). J Mammal Evol 26, 317–331 (2019). https://doi.org/10.1007/s10914-017-9424-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9424-7