Abstract
The patterns of development and skull ontogeny in caenolestids have been poorly studied, resulting in a limited knowledge. In this work, we report and compare the allometric growth trends of 15 variables in the three living groups of the Family Caenolestidae, represented by Caenolestes fuliginosus, Lestoros inca, and Rhyncholestes raphanurus. We analyzed the bivariate and multivariate allometry in comparison with morphologically convergent Australasian peramelids, as well as with other marsupials and placentals previously studied. We also report the phylogenetic signal and optimization of the confidence intervals of the variables analyzed in two alternative hypotheses, where Ameridelphia is considered as monophyletic and paraphyletic. Rhyncholestes raphanurus and C. fuliginosus shared more allometric trends than any other between-taxa comparisons. Notwithstanding, several statistics were higher in R. raphanurus, except for those variables related to temporal muscles and bite. The close relationship between R. raphanurus and L. inca is also supported by the longitudinal growth of the rostrum, although with a clear growth extension in R. raphanurus. The allometric trends reported for L. inca reflect a more predaceous condition compared to other caenolestids. Bandicoots and caenolestids did not show a particularly shared growth pattern, with the latter being morphologically more conservative. Ameridelphia was paraphyletic in the shortest tree regarding the optimization of the confidence intervals. However, the growth of several variables supported monophyletic groups in both hypotheses. Skull ontogeny in marsupials is informative in several aspects of the mandible and neurocranium reflecting the high phylogenetic signal displayed by variables related to these cranial regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extant marsupials exhibit high morphological variation in skull morphology (e.g., Wroe and Milne 2007; Goswami et al. 2011, 2012; Bennett and Goswami 2013). Recent comparative studies in marsupials and placentals (e.g., Wilson and Sánchez-Villagra 2010; Wilson 2013) suggest that the former show less disparity in the general morphology of the skull, but higher integration, mostly in the oral region (e.g., Shirai and Marroig 2010; Porto et al. 2013). In marsupials, the rostral region is initially constrained by its functional compromise from attachment for suckling during early stages of development (Bennett and Goswami 2013). Such differences are probably caused by intrinsic factors (especially the mode of marsupial reproduction and development, Goswami et al. 2012) and/or extrinsic ones (history of diversification and biogeography, Sánchez-Villagra 2013). However, in spite of the lower developmental disparity reported for marsupials in comparison with placentals, the former group exhibits high morphological diversity including both specialized (e.g., strictly herbivorous, nectarivorous, or carnivorous groups) as well as generalized forms showing morphological convergences on skull shape (see Wroe and Milne 2007 for carnivores).
The Australasian bandicoots (Peramelidae) and the South American shrew opossums (Caenolestidae), exhibit similar skull features such as a greatly elongated rostrum and large palatine vacuities, which distinguish both from other extant marsupials. This resemblance between bandicoots and shrew opossums, which is apparent in spite of well-marked differences in body size (e.g., Osgood 1921; Retief et al. 1995; Krajewski et al. 1997; Palma and Spotorno 1999), represented the basis for old hypotheses of a close relationships between both groups (see Osgood 1921; Gregory 1922). Several studies have placed caenolestids in different relationships with other marsupials (Beck 2008; Meredith et al. 2008). They have been recovered as the sister group to Didelphimorphia within Ameridelphia (Szalay 1982, 1994; Gemmell and Westerman 1994; Retief et al. 1995; Colgan 1999); sister group to the entire Australian marsupial radiation and separate from Ameridelphia (Springer et al. 1998; Amrine-Madsen et al. 2003; Horovitz and Sánchez-Villagra 2003; Asher et al. 2004; Cardillo et al. 2004; Nilsson et al. 2004; Munemasa et al. 2006; Beck 2008; Beck et al, 2008); sister to both Didelphimorphia and all Australian marsupials (Kirsch et al. 1991; Szalay and Sargis 2001); or as the sister group to Peramelemorphia and different from the rest of all marsupials (Palma and Spotorno 1999). Another recent phyogeny placed the caenolestids in a position closely related to bandicoots (Lavedèze and Muizon 2010). Despite this conflicting phylogenetic information, caenolestids exhibit a plesiomorphic tarsal morphology similar to that of other New World marsupials (Szalay 1982, 1994), paired spermatozoa as in most New World marsupials, albeit with unique characteristics (Biggers and DeLamater 1965; Temple-Smith 1987), enlarged and forwardly projected first lower incisors (Marshall 1980; Patterson 2008), large antorbital vacuities (Marshall 1980; Patterson and Gallardo 1987), and a shrew-like external appearance (Palma 2003). The pair of large, forwardly directed incisors in the lower jaw resemble those of Diprotodontia (Osgood 1924), but they are apparently homologous to i2 in other marsupials and not to i1 (Hershkovitz 1995). In turn, bandicoots possess a tarsal morphology typical of Australasian marsupials (Szalay 1982), unpaired spermatozoa (Tyndale-Biscoe 2005), and a chorioallantoic placenta (Hughes et al. 1990; Tyndale-Biscoe 2005), which is functionally related to a fast rate of development in this group (Gordon and Hulbert 1989).
In the balance of all evidence, the two long-nosed clades of living marsupials appear to be convergent and not closely related phylogenetically. Caenolestids are the only extant representatives of the once diverse order Paucituberculata (Abello 2007; Patterson 2008), which includes several specialized forms that had their richness peak during the middle Tertiary (Abello 2007, 2013), convergent with other “pseudodiprotodont” fossils such as Polydolopimorphia (Goin et al. 2003).
Regarding bandicoots, until recently the scarce ontogenetic data published were limited to aging of specimens on the basis of tooth eruption (e.g., Kingsmill 1962; Lyne 1964; Lyne and Mort 1981). Recently, Travouillon et al. (2014) described some cranial ontogenetic changes in the fossil Madju variae, and Flores et al. (2013) quantified the pattern of skull growth in Echymipera kalubu and Isoodon macrourus. This study showed that positive allometries apply to longitudinal dimensions in E. kalubu, whereas positive allometries are restricted to vertical dimensions of the skull in I. macrourus. By contrast, information on ontogeny is lacking in caenolestids, beyond a reported slow rate of development for the group (Tyndale-Biscoe 2005), and just a few studies dealing with dental homologies and tooth replacement / eruption (e.g., Luckett and Hong 2000; Martin 2007, 2013). Breeding data are restricted to anecdotal records (see Patterson and Gallardo 1987).
Extant caenolestids represent excellent candidates for comparative studies of skull growth with convergent Australasian forms, the bandicoots, and with other marsupials, given the wealth of recent studies on the allometry of skull growth in marsupials from several groups from both the Australasian and American radiations (e.g., Abdala et al. 2001; Flores et al. 2003, 2006, 2010, 2013; Giannini et al. 2004). Flores et al. (2013) compared the allometric growth pattern of two bandicoots with previously studied marsupials and placentals (three didelphids, two australidelphians, two primates, and two carnivorans). The two bandicoots showed ontogenetic trajectories that differed in several measurements, suggesting a clear divergence in the growth patterns between them. However, and in agreement with those studies that suggest a low morphological disparity in the neurocranium of marsupials (e.g., Bennett and Goswami 2013), the allometric trends of sensorial capsules scaled mostly with negative allometry in all groups (Flores et al. 2013). The higher degree of integration of the viscerocranium in marsupials (Bennett and Goswami 2013) is in general agreement with the stability of the negative allometry of the palate width and the positive allometry of the rostral length, across all previously studied species (see Flores et al. 2013).
In this work we report on the skull growth of one representative species per extant genus of caenolestids: Caenolestes fuliginosus, Lestoros inca, and Rhyncholestes raphanurus. We integrated our results with previously published data on cranial ontogeny of marsupials, particularly the convergent bandicoots, and established a phylogenetic framework of comparison to place the particulars of the caenolestid cranial ontogeny in the broader context of skull evolution in metatherians.
Materials and Methods
The Group
Extant caenolestids comprise only three recent genera, Caenolestes, Lestoros, and Rhyncholestes (the first one polytypic) known only from South America (Cabrera 1957; Patterson 2008). Their broadly relictual and disjunct distribution (Patterson 2008) suggests a wider distribution and higher diversity of extinct taxa, which is supported by its substantial fossil record (see Abello 2007; Goin et al. 2007, 2009). In this study we included representatives of the three genera. Caenolestes fuliginosus is distributed in the Páramo (i.e., the Neotropical high mountain biome with a vegetation composed mainly of giant rosette plants, shrubs, and grasses; Luteyn 1999) of Colombia and Ecuador and is the species of Caenolestes best represented in systematic collections (Brown 2004; Patterson 2008; Solari et al. 2013). Lestoros inca inhabits wet, mossy elfin forests, as well as highly disturbed scrub and second-growth forests of southern Perú (Myers and Patton 2008; Medina et al. 2012). Rhyncolestes raphanurus is an extremely rare species that has been collected only in the temperate Valdivian rain forests of southern Chile and adjacent Argentina (Pearson 1995; Birney et al. 1996; Martin 2011), separated from the northern caenolestids by the arid diagonal of South America (Bruniard 1982). Although caenolestids exhibit an old evolutionary history in South America, and high species richness in the past (Abello 2007), their extant representatives show a very low diversity when compared to other marsupial groups including the sympatric didelphids (Gardner 2005; Patterson 2008). The extant species exhibit a relatively conservative skull morphology as well as little variation in body size (Patterson 2008). Old and recent evidence of development in shrew opossums only covered patterns of tooth replacement, function, and wear, which revealed a consistent pattern of tooth use and so important inferences on food habits and behavior were made in this poorly known group (Luckett and Hong 2000; Martin 2007, 2011, 2013). The principal aspects of reproduction in shrew opossums remain poorly understood, and the scarcity of young specimens in collections represents a serious limitation for ontogenetic studies. Extant caenolestids are very difficult to capture in the field and almost impossible to study alive, given that no individual has survived for more than a few days in captivity (Patterson and Gallardo 1987; Tyndale-Biscoe 2005). In addition, capture of a female with attached young has never been reported. Knowledge about reproduction and breeding in the group is based upon scattered field data (e.g., Kirsch and Waller 1979; Patterson and Gallardo 1987; Tyndale-Biscoe and Renfree 1987), which suggested a pattern of seasonal breeding, a single reproductive event per year, small litter size, and a very slow rate of development (Tyndale-Biscoe 2005).
Sample
We studied aged skull series of species representative of the three extant genera in the Family Caenolestidae deposited in the mammal collections of the Universidad de Antioquia (CTUA), Field Museum of Natural History (FMNH), Instituto de Ciencias Naturales, Universidad Nacional de Colombia (ICN), and United States National Museum, Smithsonian Institution (NMNH): Caenolestes fuliginosus (n = 119; 49 females, 70 males), Lestoros inca (n = 62; 31 females, 31 males), and Rhyncholestes raphanurus (n = 26; 13 females, 13 males; Supplementary Information). The lack of specimens with deciduous dentitions in systematic collections worldwide limited our sample to subadult and adult specimens, defined as individuals with emerging P3/p3, and individuals with fully erupted dentition, respectively (Luckett and Hong 2000). As discussed by Luckett and Hong (2000) and Martin (2013), the retention of a late erupting P3, associated with the derived condition of a greatly reduced dP3 that probably does not erupt, is a combination unique to caenolestids. However, it is still possible to establish a relationship between size and age. The larger specimens in the three species exhibit advanced degrees of molar cusp wear. For instance, in L. inca a small cusp anterolingual to stylar cusp B (apparently the paracone, see Goin et al., 2007) forms a small socket increasing in size from M1 to M3, which is highly susceptible to wear and can only be observed in young specimens (Martin 2013). Other dental characters for subadult specimens of C. convelatus and C. fuliginosus were described by Luckett and Hong (2000). The youngest specimen of C. fuliginosus (FMNH 70858, condylo-insicive length [CIL] 25.24 mm) exhibited the lower third premolar in the process of eruption (see Luckett and Hong 2000: fig. 9) whereas our largest specimen (ICN-PSP1247, unknown sex, CIL 33.85) presented heavily worn molar cusps. Additionally, seven specimens (FMNH 70832, 70888, 70859, ICN 16534-6, 11654) showed incompletely erupted third lower premolars. Our youngest specimen of L. inca (FMNH 172048, male, CIL 25.25) showed its fourth upper molar barely emerging above the alveolar line, and its third upper premolar not fully erupted with its main cusp still not reaching the height of the stylar cusp B of the next molar (lateral view; see Martin, 2013). Two additional specimens (FMNH 75113, USNM 194407) were of small size, showing similar dental conditions. By contrast, our larger specimen (FMNH 75115, male, CIL 30.75) presented heavily worn molar cusps. Finally, the youngest specimen in our sample of R. raphanurus (FMNH 129828, female, CIL 28.8) exhibited an adult dentition with little cusp wear, whereas our largest specimen (FMNH 50071, male, CIL 34.78) showed heavily worn molar cusps.
Sexual dimorphism was detected in L. inca and C. fuliginosus involving size, whereas shape dimorphism was detected in C. fuliginosus and partially in L. inca (Astúa 2010; Martin 2013). The study of Astúa (2010) did not find evidence for sexual dimorphism in size or shape for R. raphanurus. Despite sexual variation detected in L. inca and C. fuliginosus, both males and females clearly shared the same ontogenetic trajectory in the three species (i.e., non-significant slope or intercept differences in bivariate analyses of selected variables; see below). Therefore, within each species we pooled male and female specimens of all ages in a single sample per species, as in our recent study on bandicoots (Flores et al. 2013).
Study of Growth
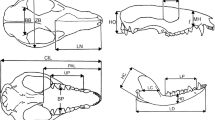
Following our previous studies on marsupials (Abdala et al. 2001; Flores et al. 2003, 2006, 2010; 2013; Giannini et al. 2004), we used 15 cranial measurements (Fig. 1) to estimate allometric growth in caenolestids. We used bivariate and multivariate analyses to estimate growth trends. In bivariate analyses, the scaling of any measurement can be affected by the choice of the independent variable (Smith 1981; Wayne 1986). Because our multivariate analyses indicated that the total length of the skull, or the condylo-incisive length (CIL), was isometric, this measurement was taken as a reliable indicator of size variation and therefore it was used as an independent variable in bivariate allometry. We used linear (log10) transformation of the power equation of growth for each variable (see Abdala et al. 2001 for details) and tested deviations from isometry by means of 2-tailed t-tests, after corroborating that the independent variable was normally distributed (Shapiro–Wilk test, C. fuliginosus: w = 0.981, P = 0.466; L. inca: w = 0.958, P = 0.44; R. raphanurus: w = 0.987, P = 0.987). Statistically significant deviations from isometry represent cases of “negative” allometry if the slope b < 1.0 and “positive” allometry if b > 1.0. We applied standarized major axis regression (SMA) and followed Warton et al. (2006) in using a likelihood ratio test for the common SMA slope using a chi-squared distribution (Warton and Weber 2002). If the species shared a common slope, we compared the significance of the common normalization constant (y-intercepts) using the Wald test (as described in Warton et al. 2006). All these regression coefficients, statistical parameters, and tests were performed using the SMATR package of R (Warton and Weber 2002). We statistically compared the parameters of the trajectories among the three species.
Skull and mandible measurements considered in this study. Abbreviations: CIL Condylo-insicive length, BB Breadth of the braincase, BP Breadth of the palate, HC Height of the coronoid process, HD Height of the dentary, HO Height of the occipital plate, LC Length of the coronoid process, LD Length of the dentary, LN Length of the nasals, LP Lower postcanine toothrow, MH Muzzle height, ORB Orbit length, PAL Palate length, UP Upper postcanine toothrow, ZB Zygomatic breadth
Multivariate coefficients of allometry were obtained from the first (unit-scaled) eigenvector of a principal component analysis (PCA) based on a variance–covariance matrix of log10 transformed data (Jolicoeur 1963). We tested the significance of multivariate coefficients of allometry with a resampling strategy based on the jackknife, implemented in such a way that each specimen was removed from the sample at a time, generating n pseudovalues to calculate confidence intervals (CIs) for the original coefficients (see Giannini et al. 2004 and Flores et al. 2006 for further details on this method). If the interval excluded an expected value of isometry, the variable was considered positively or negatively allometric. For all multivariate coefficients of allometry, the expected value of isometry, which depends only on the number of variables (p), is calculated as 1/p0.5 (0.258 for our set of 15 variables). Trimming the largest and smallest m pseudovalues (with m = 1) for each variable may significantly decrease the standard deviations calculated under jackknife and allow for more accurate allometric estimations (Manly 1997; Giannini et al. 2004). Here we report untrimmed and trimmed calculations, but opting for the results with either lower average standard deviation or lower bias (with the latter defined as the difference between the observed and jackknifed allometry coefficient; Giannini et al. 2004). For the multivariate statistical analyses (PCA + jackknife resampling), we used the R script of Giannini et al. (2010; available from the authors).
Comparative Analysis
Here we followed the quantitative approach of character optimization and phylogenetic signal that we used in our recent report on bandicoots (Flores et al. 2013), and integrated the results obtained herein for extant caenolestids with a taxonomic sample of seven additional marsupials (see below) and four placentals included as outgroup (Lycalopex culpaeus, Puma concolor, Cebus apella, and Alouatta caraya). We used the same dataset and included a re-analysis under multivariate allometry of the ontogenetic raw data of Didelphis albiventris, Lutreolina crassicaudata, and Dromiciops gliroides (previously reported using bivariate allometry), as well as placental outgroups (see Flores et al. 2013), considering the exact same 15 cranial variables as in caenolestids and bandicoots (Fig. 1).
Optimization is a tool used to determine the cost of a phylogenetic topology and ancestral character states; this way of mapping evolutionary information onto phylogenetic trees represents one of the essentials of comparative biology (see discussion in Giannini and Goloboff 2010). Here we followed Giannini (2014) who demonstrated that allometry should be mapped using the estimated confidence interval as a continuous character (see Giannini 2014 and Segura 2014 for applications in rodents and carnivorans, respectively). Optimization of continuous characters using either point estimates or intervals for terminal values is implemented in the computer program TNT (Goloboff et al. 2008) as an extension of Farris’ (1970) multistate character optimization (Goloboff et al. 2006). Thus, allometric similarities can be explained by common ancestry and ancestral allometry vectors can be optimally estimated (see Giannini 2014 and citations therein). We reconstructed the allometric vector at all internal nodes, calculated the optimal cost of reconstruction (steps on the tree), and examined the evolutionary changes (increases and decreases in individual allometric coefficients) as implied by the tree topology, to uncover the general phylogenetic pattern of cranial ontogeny evolution and its functional implications.
We included the didelphids Didelphis albiventris, Lutreolina crassicaudata, and Caluromys philander, the microbiotheriid Dromiciops gliroides, the dasyurid Dasyurus albopunctatus, the peramelid Echimypera kalubu and Isoodon macrourus, and the caenolestids studied herein, and four placental outgroups, arranged in two possible topologies. The first one (called H1 hereafter) considered Ameridelphia as monophyletic (i.e., caenolestids sister to Didelphidae; Szalay 1982; Beck 2008). The second topology (called H2 hereafter) considered Ameridelphia as paraphyletic (i.e., caenolestids were sister to Australidelphia; Horovitz and Sánchez-Villagra 2003; Asher et al. 2004; Nilsson et al. 2004; Beck et al. 2008; Meredith et al. 2008). In both topologies, Dromiciops was placed as sister to the remaining australidelphian marsupials (Meredith et al. 2008).
Phylogenetic signal was obtained using random swapping of characters (allometric coefficients) in terminal branches (Laurin 2004). The distribution of character states (i.e., resampled allometric coefficients) on the observed phylogenies was compared with the distribution of states on 20,000 randomly generated trees. The number of evolutionary steps of each character on the randomly generated trees was also compared with the number of steps of that character in the observed phylogenies. We considered a character to have significant phylogenetic signal if the observed number of steps was larger than the steps calculated for at least 95 % of the randomly generated trees. For phylogenetic signal calculation, we used the R script of Prevosti et al. (2010), computed with the program TNT v. 1.1 (Goloboff et al. 2008).
Results
Cranial Allometry in Caenolestes fuliginosus
Bivariate Analysis
All variables were correlated with the total length of the skull; variables with r 2 < 0.7 included UP (upper postcanine length; r 2 = 0.63); ORB (orbital length; r 2 = 0.41); BB (breadth of the braincase; r 2 = 0.31); HO (height of the occipital plate; r 2 = 0.38); LC (length of the coronoid process; r 2 = 0.52); HD (height of the dentary; r 2 = 0.54); and LP (lower postcanine length; r 2 = 0.64) (Table 1). UP, LD (length of the dentary), and LP were the only isometric variables (Table 1). Positive allometry was observed in ZB (zygomatic breadth); HM (height of the muzzle), PAL (length of the palate), ORB, LN (length of the nasals), HC (height of the coronoid process), LC, and HD. Three variables scaled negatively with condylo-incisive length (CIL), from both the neurocranium and the splanchnocranium: BB, HO, and BP (breadth of the palate). The latter exhibited the slowest growth rate (brma = 0.64).
Multivariate Analysis
The first principal component explained 64.46 % of the total variation. The multivariate approach showed a pattern similar to that of the bivariate analysis, although with weaker trends in five variables (Table 2). Indeed, the only differences among multivariate (untrimmed, see below) and bivariate approaches were limited to the negative allometry of LP and UP, and the isometry of ZB, PAL, and HC. Remarkably, CIL and LD (the two variables representing overall length of the skull and mandible, respectively) departed less from the point estimate of isometry (between −0.005 and −0.004), while BP, BB, HO, and LP departed the most (between −0.12 and −0.18).
Cranial Allometry in Lestoros inca
Bivariate Analysis
Variables were not strongly correlated with the total length of the skull, with r 2 > 0.7 in just two variables (LN, and LD). The lowest correlations were detected in ORB (r 2 = 0.14), LP (r 2 = 0.22), HD (r 2 = 0.32), LC (r 2 = 0.06), HO (r 2 = 0.05) and BB (r 2 = 0.31; Table 1). Variables showing isometry with respect to the condylo-incisive length included measurements from both neurocranium and splanchnocranium (i.e., ZB, BP, BB, HO, LD, HC, and LC). Positive allometry was observed in seven variables: HM, PAL, UP, ORB, LN, HD, and LP. Finally, no variables scaled negatively in L. inca, although BP exhibited the slowest growth rate (brma = 0.89).
Multivariate Analysis
The first principal component explained 49.81 % of the total variation. The smallest departure from isometry was PAL (−0.028) and the largest departure was ORB (0.44). Multivariate analysis showed several different trends as compared with its bivariate counterpart, as only three variables (ORB, LD, and HC) shared allometric trends (Table 2). In a general sense, the allometric coefficients observed under multivariate analysis were lower than observed under bivariate analysis, that is, a scale shift was apparent: those variables with positive trends in bivariate analysis showed isometry in multivariate analysis (i.e., HM, PAL, UP, LN, HD, and LP), whereas those variables that were isometric under bivariate allometry, became negatively allometric under multivariate analysis (i.e. ZB, BP, BB, OH, and LC). Finally, only ORB conserved its positive allometry also in this analysis.
Cranial Allometry in Rhyncholestes raphanurus
Bivariate Analysis
All variables were well correlated with CIL, with r 2 > 0.5, with the lowest correlation obtained in HD (r 2 = 0.51) and LP (r 2 = 0.57), and the highest in LD (r 2 = 0.92). Eight variables were isometric with respect to CIL, most of which were related with trophic function (ZB, PAL, BP, UP, LN, LD, and HC) plus one neurocranial variable (BB). Positive allometry was detected in three splanchnocranial (HM, LC, and HD) and one neurocranial (ORB) variables. Only two variables were negatively allometric, also belonging to both cranial regions (LP and HO).
Multivariate Analysis
The first component explained 75.29 % of total variation. The lowest departure from isometry was in MH (0.029). These results differed from the bivariate analysis in five variables (HM, HD, UP, ORB, and BB; Table 2). As in L. inca, the allometric coefficients observed under multivariate analysis were lower than those obtained with bivariate analysis. Most variables with positive trends in bivariate analysis were isometric in multivariate analysis (i.e., HM, ORB, and HD), whereas some isometric variables under bivariate allometry, became negatively allometric in multivariate analysis (i.e., UP and BB). Finally, several variables conserved their allometric trend in both analyses (i.e., positively allometric in LC; negatively allometric in HO and LP, and isometric in ZB, PAL, BP, LN, LD, and HC).
Comparative Allometry of Bivariate Results in Extant Caenolestids
In all multivariate analyses the condylo-insicive length (CIL) was recovered as isometric, for which our choice of it as independent variable in bivariate analyses was supported. Caenolestes fuliginosus and L. inca shared eight out of 14 allometric trends (i.e., ZB, HM, PAL, BB, LN, HO, LD, and HC; Table 3; Fig. 2a). Statistical differences in slopes between both species were found in the remaining six variables (i.e., BP, UP, ORB, LC, HD, and LP; Fig. 2b); in all but one case (LC), slopes were lower in C. fuliginosus. Y-intercepts were statistically different in those variables sharing the same slope (i.e., ZB, HM, PAL, BB, LN, HO, and HC) except in LD, and those of L. inca were lower except in ZB and HC.
Bivariate SMA regressions for Caenolestes fuliginosus (crosses), Lestoros inca (squares), and Rhyncholestes raphanurus (filled squares). a Same slope and intercepts for L. inca and R. raphanurus, but lower intercept for C. fuliginosus (PAL); b different slope in the growth trajectory of C. fuliginosus, and different intercepts between R. raphanurus and L. inca (BP). Lines SMA regression: dashed line C. fuliginosus; black line R. raphanurus; grey line L. inca. Abbreviations as in Fig. 1
Comparisons of bivariate analyses between L. inca and R. raphanurus (Table 3) indicate that both species shared slopes in eight out of 14 variables (i.e., ZB, PAL, BP, UP, BB, LN, LD, and HC; Fig. 2b). Among these eight variables, the y-intercept was higher in L. inca just in ZB and BP. In LN, BB, and LD, the intercept was higher in R. raphanurus. Both slope and intercept were shared in PAL (Fig. 2a) and UP, but with an extended trajectory in R. raphanurus. The remaining six variables (i.e., HM, ORB, HO, LC, HD, and LP) showed statistical differences in slopes, these being higher in most variables for L. inca with the exception of LC.
Finally, our comparison of bivariate analysis between C. fuliginosus and R. raphanurus (Table 3) showed that both species shared slopes in ten out of 14 variables (ZB, HM, PAL, UP, ORB, BB, LN, HO, LD, and HD). Among these variables, six showed higher values of y-intercepts favoring R. raphanurus (HM, PAL, ORB, BB, LN, and HO), whereas the remaining four variables (ZB, UP, LD, and HD) favored C. fuliginosus. The remaining three variables showed differences in slopes favoring R. raphanurus in two variables (BP and LC) and one (LP) favoring C. fuliginosus.
Comparison of Ontogenetic Patterns with Extant Bandicoots
No variables showed a particular or unique trend in shrew opossums, which was true also for bandicoots (Flores et al. 2013), given that each trend was also observed in some other marsupial group (Table 4). However, in the comparative context of shrew opossums and bandicoots, two variables showed a somewhat noteworthy distribution of ontogenetic trends. First, the condylo-insicive length (CIL), an important measurement because it is frequently used as proxy of overall size and therefore is a usual independent term in bivariate analyses, was recovered as isometric in both bandicoots and shrew opossums, and also in the carnivorous didelphid Lutreolina crassicaudata. Second, the dentary length was recovered as isometric in the three shrew opossum species, but positively allometric in bandicoots (Table 4). The remaining variables showed no common or contrasting pattern between both long-nosed groups, and the allometric trends shared between both groups were actually widely distributed among other marsupial and placental groups, such as trends in neurochranial variables (e.g., HO, BB) or palate breadth (BP; Table 4).
Ontogenetic Character Mapping and Phylogenetic Signal
Tree length for the topology considering Ameridelphia as paraphyletic (i.e., H2) was slightly shorter than the topology where Ameridelphia was monophyletic (5.51 vs. 5.57 steps, Table 5). Both cases exhibited phylogenetic signal, although statistically significant in just four variables. For both hypotheses, significant phylogenetic signal was present in two variables related with the neurocranium (BB and HO), and two related with mandibular growth (LD and HC; Table 5). A marginal significance was observed for ZB in H2 (i.e., where Ameridelphia is paraphyletic; Table 5).
Our character mapping of ontogenetic trends as continuous characters showed that most changes concentrated in terminals. However, we also recovered phylogenetic adjustment in the trend of several variables on internal nodes in both systematic hypotheses (H1 and H2; see Material and Methods section). For instance, caenolestids as a group were supported by two ontogenetic changes in both hypotheses (Figs. 3 and 4): a decrease in the ontogenetic interval of the breadth of the palate (BP) and length of the dentary (LD). Two additional characters supported caenolestids depending on their position in the marsupial tree: when Ameridelphia was considered paraphyletic (Fig. 4) we observed an increase of the orbit interval (ORB), but when caenolestids were placed as sister group to Australidelphia (Fig. 3) we also detected a decrease in growth rate of the length of the palate (PAL). The proposed sister relationship between R. raphanurus and L. inca (Ojala-Barbour et al. 2013) was supported herein by a decrease in the intervals of height of the coronoid process (HC) and in length of the nasal (LN) for both topologies (Figs. 3 and 4). Didelphids were supported by an increase in growth rate of the height of the dentary (HD), height of the occipital plate (HO), and upper postcanine toothrow (UP) for both hypotheses, whereas an increase in growth rates of the condylo-incisive length (CIL) and zygomatic breadth (ZB) were recovered as a synapomorphy for the group in the topology where Ameridelphia is monophyletic (Fig. 3). Bandicoots (Peramelidae) were supported by an increase in HO and PAL growth rate in both hypotheses, and a rate increase in CIL in H2 (Fig. 4). The clade Ameridelphia as recognized in H1 (Fig. 3) was supported by rate changes in two variables: increase of growth rate in breadth of the braincase (BB) and decrease in length of the coronoid process (LC). The clade Australidelphia was supported by only one change in growth rate affecting the mandible in each hypothesis: an increasing rate in LC in H2, and a decreasing rate in HC in H1 (Figs. 2 and 3). Finally, the clade including Caenolestidae + Australidelphia recognized in H1 was supported by three changes, all related to the trophic apparatus, with a decreasing rate of growth in BP, ZB, and HC (Fig. 4).
Optimization of confidence intervals obtained from multivatiate analysis of allometry for H1 hypothesis, where Ameridelphia is monophyletic (see Materials and Methods). Abbreviations on internal nodes and terminals indicate variables as in Fig. 1. Numbers indicate the difference between confidence interval of allometry between ancestor and descendent node (or terminal). Sign + or – indicate increase or decrease of the confidence interval
Optimization of confidence intervals obtained from multivatiate analysis of allometry for H2 hypothesis, where Ameridelphia is paraphyletic (see Materials and Methods). Abbreviations on internal nodes and terminals indicate variables as in Fig. 1. Numbers indicate the difference between the confidence interval of allometry between ancestor and descendent node (or terminal). Sign + or – indicate increase or decrease of the confidence interval
Discussion
Ontogenetic Patterns of Cranial Growth in Shrew Opossums
Our comparison of ontogenetic trends among the three extant caenolestid groups shed light onto the evolutionary and functional aspects of skull development in the group. Our ontogenetic evidence supports the clear distinction between Lestoros and Caenolestes, formerly suggested as synonyms (Bublitz 1987) but now considered different genera as firmly demonstrated in several studies (Myers and Patton 2008; Timm and Patterson 2008; Martin 2013; Ojala-Barbour et al. 2013). In fact, we observed more allometric trends shared between R. raphanurus and C. fuliginosus (ten out of 14 variables; Table 3) than between R. raphanurus and L. inca, which were recovered as sister taxa (Ojala-Barbour et al. 2013). However, R. raphanurus and L. inca shared the same slope and intercept for length of the palate (PAL) and the upper postcanine toothrow (UP), although with a clear growth extension in the extremely long-nosed R. raphanurus (Table 3). Y-intercepts were larger in R. raphanurus than in C. fuliginosus, except for those variables related to temporal muscles and bite (Osgood 1921), as zygomatic breadth (ZB), upper postcanine toothrow (UP), and length (LD) and height (HD) of the dentary, which favored C. fuliginosus (Table 3). Such differences in growth rate suggest possible differences in food habits of Rhyncolestes with respect to the more predaceous Caenolestes, as indicated in behavioral reports (Patterson and Gallardo 1987; Kirsch and Waller 1979; Patterson 2008). Despite the typical slender skull morphology exhibited by R. raphanurus (Osgood 1924, 1943), the allometric trends of variables related with the length of the skull (e.g., LCI, PAL, LN, UP, LD, and LP) were mostly isometric or negatively allometric (Tables 1 and 2), suggesting that its elongated appearance is already present from early post-weaned age stages and changes very little during post-weaning growth.
Lestoros inca develops a more robust skull with deeper masseteric fossae as compared with the other living shrew opossums (Martin 2013); the allometric trends reported here for L. inca are consistent with this observation and suggest distinct feeding habits. Although the zygomatic breadth was isometric (and negatively allometric in multivariate analysis; Table 2), other variables related with trophic function, such as height of the muzzle and dentary, length of the palate, and upper and lower toothrow, all were recovered as positively allometric in bivariate results (Table 1). Such findings and tooth morphology in L. inca (i.e., long, bladelike shearing structures, distinct morphology of I4 and its separation from other incisors, a double-rooted canine, and smaller dP1) are in agreement with the reported diet of soft-bodied invertebrates (Strait 1993; Myers and Patton 2008; Martin 2013). The intercepts of the bivariate analysis favored L. inca in most trajectories where the slope was shared with other caenolestids (Table 3). However, multivariate coefficients of C. fuliginosus showed more positive allometries than L. inca (Table 2), and the space for temporal muscles benefited more by the interplay between zygomatic and negative braincase growth. As in other mammals, the temporal muscle in caenolestids fits between the zygomatic arch and the lateral wall of the braincase so that the negative allometry of ZB and BB in L. inca (multivariate analysis; Table 2) suggests that this space for the temporal is expanded at a faster pace in C. fuliginosus. In fact, although our comparison of bivariate analysis showed slopes shared for both variables, the lower y-intercept for ZB and higher for BB observed in C. fuliginosus were both consistent with this trend (Table 3).
Although bandicoots and caenolestids did not share a particular growth pattern, the study of Flores et al. (2013) observed larger skull disparity in bandicoots as compared with that of living caenolestids presented here, which results in the latter being a morphologically conservative group, despite the longer rostrum of R. raphanurus. Extant bandicoots varied not only in size, but also in complex structures such as the tympanic region as a whole (e.g., Freedman and Joffe 1967; Freedman and Rightmire 1971; Archer 1976; Groves and Flannery 1990). However, this fact could be a consequence of the taxonomic level of our comparison, as bandicoots includes two extant families (Groves 2005, considering Chaeropodidae as extinct, Burbidge et al. 2008), whereas living caenolestids include only three closely related genera (Patterson 2008). The absence of a particular mode of growth in both long nosed groups (Table 4) can also be related to the basal position of both clades in the marsupial tree, either accepting or rejecting the monophyletic condition of Ameridephia (e.g., Horovitz and Sánchez-Villagra 2003; Asher et al. 2004; Cardillo et al. 2004; Beck 2008; Beck et al. 2008; Meredith et al. 2008). The long nosed pattern of skull morphology exhibited in both basal groups was already a common aspect in ancient mammal taxa, such as the Cretaceous Cronopio (Rougier et al. 2011) or the Miocene dryolestid Necrolestes (Rougier et al. 2012; Chimento et al. 2012), for which such an ancient condition could easily result in convergent morphology.
Ontogenetic Evolution and Phylogenetic Significance
Our approach to the evolution of cranial ontogeny in marsupials took advantage of continuous character optimization with ontogenetic trends coded as confidence intervals extracted from intraspecific multivariate analyses (see Giannini 2014; Segura 2014). This avoids mistakes associated with discretization of ontogenetic trends, which is useful for some comparative purposes but not as much for reconstructing evolution of ontogeny in these characters. In addition, it makes use of the intraspecific variation around point estimates of allometry, as discovered by data simulation (e.g., our application of jackknife resampling; see Giannini 2014). We detected evolutionary changes both in the “sign” and “amount” of coefficients (i.e., changes along the continuous scale of allometry) at internal nodes. The shortest length recovered was that of the tree in which Ameridelphia was paraphyletic (i.e., H2; Table 5), thus providing additional support to the latest phylogenetic evidence from varied sources (e.g., Horovitz and Sánchez-Villagra 2003; Asher et al. 2004; Nilsson et al. 2004; Meredith et al. 2008). Our analysis contributed three unreversed synapomorphies from ontogenetic characters to the caenolestid + australidelphian clade (decreases in growth rate in HC, ZB, and BP; Fig. 4). H2 would indicate that the increase in braincase breadth (BB) growth rate and the decrease in length of the coronoid process (LC) growth rate in H1 (Fig. 3) are not actual synapomorphies of New World marsupials, but most likely common symplesiomorphies, as in other characters such as paired sperm morphology (Biggers and DeLamater 1965). Indeed, BB was one of the variables with statistically significant phylogenetic signal (Table 5). As suggested by Flores et al. (2013), skull ontogeny in marsupials may be phylogenetically informative in several aspects, which is confirmed here given the significant signal observed both in splanchnocranial (LD, HC) and neurocranial (BB, HO) variables (Table 5). Indeed, adding living caenolestids to the previously available sample of marsupials generated phylogenetic support from almost all ontogenetic cranial variables to some clade. The marsupial root did not show changes in H1 or H2 with respect to the placental outgroups (as reported by Flores et al. 2013 using discretized ontogenetic characters of the skull), meaning that the divergent life-history strategies between these groups (in organogenesis, integration, development and reproduction; e.g., Clark and Smith 1993; Smith 1997; Shirai and Marroig 2010; Goswami et al. 2012) were not directly reflected in radical ontogenetic changes in skull development at the marsupial ancestor.
Neurocranium and splanchnocranium maintain a close developmental interaction both in growth pattern and function. Pattern of growth in neurocranial variables were negatively allometric in most mammals (Table 4). As evidenced in some works (e.g., Emerson and Bramble 1993; Maunz and German 1996; Wilson 2011; Segura et al. 2013; Koyabu et al. 2014; Tarnawski et al 2014a, b), mammals exhibit an accelerated differentiation of the central nervous system and sensory capsules, producing newborns with large braincases, eyes, and auditory regions relative to the trophic components of the skull. Specifically in marsupials, much of neurogenesis occurs after birth and during lactation (Smith 1997), so during the post-weaning period most neurocranial variables scale negatively in all marsupials studied to date, except the orbit. The isometry of the orbit (which means that it grows faster than expected for a neurocranial component) in D. gliroides, C. philander, R. raphanurus, and E. kalubu, and the marginally positive allometry in L. inca and C. fuliginosus, suggests that this condition may be relatively common among marsupials. However, the marginally positive allometry of the orbit detected in L. inca and C. fuliginosus is likely an artifact of the low correlation index of this variable (Table 1), as the particular anatomy of the weak and thin zygomatic arch in shrew opossums makes it difficult to determine a clear landmark at the posterior margin of the orbit. Also, and in agreement with the consistent negative allometry in almost all neurocranial variables found in marsupials and placentals, the neurocranial region did not show any significant differences in distribution between groups in the morphospace reported by Bennett and Goswami (2013).
In spite of its alleged growth conservatism, character optimization of neurocranial variables was informative in several aspects (see Figs. 3 and 4), also demonstrated by significant phylogenetic signal (Table 5). The increase in braincase growth rate in New World marsupials and the R. raphanurus + L. inca clade (in H1; Fig. 3), suggests some adjustment of neurocranial growth along the evolution of the group. However, the phylogenetic adjustment shown by the height of the occipital plate (i.e., growth rate increasing in Didelphidae and Peramelidae, Figs. 3 and 4) is likely associated with the development of the nuchal and sagittal crests on the supraoccipital bone in such groups (i.e., D. albiventris, L. crassicaudata, C. philander, E. kalubu, and I. macrourus). A recent report dealing with heterochronic patterns of prenatal skull ossification in mammals (Koyabu et al. 2014) suggested an accelerated timing in the ossification of the supraoccipital bone especially in those highly encephalized groups. However, the increase of the growth rate observed in our postnatal period is a consequence of the sagittal and nuchal crests in adults of larger species. Probably, the inclusion of mouse opossums and small sized dasyurids in our analysis, whose adults lack sagittal and nuchal crests, would reduce the information content of this variable in a larger and more varied sample.
Growth of the trophic apparatus, including the rostrum and mandible, exhibited phylogenetic adjustment in several clades, some related to changes in food habits. For instance, changes in the rostral growth rate detected in both long-nosed groups (shrew opossums and bandicoots) demonstrated that both groups reach their typical rostral morphology in completely different ways. Bandicoots show an increased rate of palate growth, whereas shrew opossums exhibit a decreasing rate for dentary length and nasals, suggesting that, as already mentioned for R. raphanurus, its elongated morphology is reached early in its postnatal ontogeny. Indeed, the decreasing rate of palate breadth in shrew opossums (both topologies; Figs. 3 and 4) pointed in the same direction. In the larger carnivorous forms included in our sample (i.e., Didelphidae and the dasyurid D. albopunctatus), the height of the dentary (HD) increased its growth rate (Figs. 3 and 4), thereby strengthening the jaw against mechanical stress imposed by chewing or biting in adults, as they shift to larger prey items as they grow. A similar condition was observed for the zygomatic breadth (ZB) and its increased rate of growth for didelphids and D. albopunctatus (on H1; Fig. 3). As already mentioned, the combination of the negative allometry in braincase growth and the positive allometry of ZB increases the space for the temporal musculature (Abdala et al. 2001).
Although extant shrew opossums and bandicoots exhibit a superficial convergence in general skull morphology, their postnatal allometries were different. The inclusion of Caenolestidae in the comparative analyses of cranial ontogeny in marsupials has demonstrated the importance of postnatal growth development as a source of information within a phylogenetic context. Even considering alternative topological relationships in the marsupial tree, ontogenetic characters (expressed as changes in growth rates) supported several monophyletic clades, such as Peramelidae, Didelphidae, Caenolestidae, Australidelphia, and Ameridelphia. By contrast, marsupials and placentals are not strictly supported by any specific change in growth rate (see also Flores et al. 2013). The integration of a broader taxonomic sample of small didelphids and dasyurids is crucial in order to detect if some functionally related characters (such as zygomatic breadth or height of dentary growth) are phylogenetically informative or depend on their association with alternative topologies. Finally, the further consideration of larger and smaller diprotodontians in a broad scale analysis would probably add new phylogenetic signals to this morphologically diverse group with strong anatomical specializations.
References
Abdala F, Flores DA, Giannini N (2001) Postweaning ontogeny of the skull of Didelphis albiventris. J Mammal 82:190–200. doi:10.1644/1545-1542(2001)082<0190:POOTSO>2.0.CO;2
Abello MA (2007). Sistemática y bioestratigrafía de los Paucituberculata (Mammalia, Marsupialia) del Cenozoico de América del Sur. Dissertation, Universidad Nacional de La Plata, Argentina
Abello MA (2013) Analysis of dental homologies and phylogeny of Paucituberculata (Mammalia: Marsupialia). Biol J Linn Soc 109:441–465. doi:10.1111/bij.12048
Amrine-Madsen H, Scally M, Westerman M, Stanhope MJ, Krajewski C, Springer MS (2003) Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol Phylogen Evol 28:186–196. doi:10.1016/S1055-7903(03)00122-2
Archer M (1976) The basicranial region of marsupicarnivores (Marsupialia), inter-relationships of carnivorous marsupials, and affinities of the insectivorous marsupial peramelids. Zool J Linn Soc 59:217–322
Asher R, Horovitz I, Sánchez-Villagra M (2004) First combined cladistic analysis of marsupial mammal interrelationships. Mol Phylogen Evol 33:240–250. doi:10.1016/j.ympev.2004.05.004
Astúa D (2010) Cranial sexual dimorphism in New World marsupials and a test of Rensch’s rule in Didelphidae. J Mammal 91:1011–1024. doi:10.1644/09-MAMM-A-018.1
Beck RMD (2008) A dated phylogeny of marsupials using a molecular supermatrix and multiple fossil constraints. J Mammal 89:175–189. doi:10.1644/06-MAMM-A-437.1
Beck RMD, Godthelp H, Weisbecker V, Archer M, Hand SJ (2008) Australia’s oldest marsupial fossils and their biogeographical implications. PLoS ONE. doi:e1858. doi:10.1371/journal.pone.0001858
Bennett CV, Goswami A (2013) Statistical support for the hypothesis of developmental constraint in marsupial skull evolution. BMC Biology 11. doi:10.1186/1741-7007-11-52
Biggers JD, Delamater ED (1965) Marsupial spermatozoa pairing in the epididymis of American forms. Nature 208:402–404
Birney EC, Sikes RS, Monjeau JA, Guthmann N, Phillips CJ (1996) Comments on Patagonian marsupials from Argentina. In: Genoways HH, Baker RJ (eds) Contributions in Mammalogy: A Memorial Volume Honoring Dr. J. Knox Jones, Jr. Museum of Texas Tech University, pp 149–154
Brown BE (2004) Atlas of New World marsupials. Fieldiana Zool 102:1–308
Bruniard ED (1982) La diagonal árida argentina: un límite climático real. Rev Geog 95:5–20
Bublitz J (1987) Untersuchungen zur Systematik der Rezenten Caenolestidae Trouessart, 1898: Unter Verwendung craniometrischer Methoden. Bonn Zool Monog 23:1–96
Burbidge A, Dickman C, Johnson K (2008) Chaeropus ecaudatus. IUCN Red List of Threatened Species. Version 2013.2. http://www.iucnredlist.org. Accessed 25 April 2014
Cabrera A (1957) Catálogo de los Mamíferos de América del Sur. Rev Mus Arg Cs Nat “Bernardino Rivadavia” 4:1–308
Cardillo M, Bininda-Emonds O, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264:11–31. doi:10.1017/S0952836904005539
Chimento N, Agnolin F, Novas F (2012) The Patagonian fossil mammal Necrolestes: a Neogene survivor of Dryolestoidea. Rev Mus Arg Cs Nat “Bernardino Rivadavia” 14:261–306
Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215:119–149. doi:10.1002/jmor.1052150203
Colgan DJ (1999) Phylogenetic studies of marsupials based on phosphoglycerate kinase DNA sequences. Mol Phylogen Evol 11:13–26. doi:10.1006/mpev.1998.0553
Emerson SB, Bramble DM (1993) Scaling, allometry and skull design. In: Hanken J, Hall BK (eds) The Skull, Volume 3. Functional and Evolutionary Mechanisms. The University of Chicago Press, Chicago, pp 384–416
Farris J (1970) Methods for computing Wagner trees. Syst Zool 19:83–92. doi:10.1093/sysbio/19.1.83
Flores DA, Casinos A (2006) Cranial ontogeny and sexual dimorphism in two New World monkeys: Alouatta caraya (Atelidae) and Cebus apella (Cebidae). J Morphol 272:744–757. doi:10.1002/jmor.10947
Flores DA, Giannini N, Abdala F (2003) Cranial ontogeny of Lutreolina crassicaudata (Didelphidae): a comparison with Didelphis albiventris. Acta Theriol 48:1–9. doi:10.1007/BF03194261
Flores DA, Giannini N, Abdala F (2006) Comparative postnatal ontogeny of the skull in the australidelphian metatherian Dasyurus albopunctatus (Marsupialia: Dasyuromorpha: Dasyuridae). J Morphol 267:426–440. doi:10.1002/jmor.10420
Flores DA, Abdala F, Giannini N (2010) Cranial ontogeny of Caluromys philander (Didelphidae, Caluromyinae): a qualitative and quantitative approach. J Mammal 91:539–550. doi:10.1644/09-MAMM-A-291.1
Flores DA, Abdala F, Giannini N (2013) Post-weaning cranial ontogeny in two bandicoots (Mammalia, Peramelomorphia, Peramelidae) and comparison with carnivorous marsupials. Zoology 116:372–384. doi:10.1016/j.zool.2013.07.003
Freedman L, Joffe AD (1967) Skull and tooth variation in the genus Perameles, Part 2: metrical features of P. nasuta. Rec Austral Mus 27:183–195
Freedman L, Rightmire GP (1971) Skull and tooth variation in Australian bandicoots (Peramelidae, Marsupialia): the genus Isoodon and multivariate comparisons with Perameles. J Roy Soc West Austral 54:21–31
Gardner A (2005) Order Paucituberculata. In: Wilson DE, Reeder DM (eds) Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press, Baltimore, pp 19–20
Gemmell NJ, Westerman M (1994) Phylogenetic relationships within the Class Mammalia: a study using mitochondrial 12S RNA sequences. J Mammal Evol 2:3–23. doi:10.1007/BF01464347
Giannini N (2014) Quantitative developmental data in a phylogenetic Framework. J Exp Zool doi:10.1002/jez.b.22588
Giannini NP, Goloboff P (2010) Delayed-response phylogenetic correlation, an optimization based method to test covariation of continuous characters. Evolution 64:1885–1898. doi:10.1111/j.1558-5646.2010.00956.x
Giannini NP, Abdala F, Flores DA (2004) Comparative postnatal ontogeny of the skull in Dromiciops gliroides (Marsupialia: Microbiotheriidae). Am Mus Novitates 3460:1–17. http://hdl.handle.net/2246/2770
Giannini NP, Segura V, Giannini MI, Flores D (2010) A quantitative approach to the cranial ontogeny of the Puma. Mammal Biol 75:547–554. doi:10.1016/j.mambio.2009.08.001
Goin FJ, Candela AM, Muizon C de (2003) The affinities of Roberthoffstetteria nationalgeographica (Marsupialia) and the origin of the polydolopine molar pattern. J Vertebr Paleontol 23:869–869. doi:10.1671/2383-11
Goin FJ, Sánchez-Villagra MR, Abello A, Kay RF (2007) A new generalized paucituberculatan marsupial from the Oligocene of Bolivia and the origin of ‘shrew-like’ opossums. Palaeontology 50: 1267–1276. doi:10.1111/j.1475-4983.2007.00706.x
Goin FJ, Candela AM, Abello A, Oliveira EV (2009) Earliest South American paucituberculatans and their significance in the understanding of ‘pseudodiprotodont’ marsupial radiations. Zool J Linn Soc 155:867–884. doi:10.1111/j.1096-3642.2008.00471.x
Goloboff PA, Mattoni CI, Quinteros AS (2006) Continuous characters analyzed as such. Cladistics 22:589–601. doi:10.1111/j.1096-0031.2006.00122.x
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24:774–786. doi:10.1111/j.1096-0031.2008.00217.x
Gordon G, Hulbert AJ (1989) Peramelidae. In: Walton DW, Richardson DJ (eds) Fauna of Australia. Australian Government Publishing Service, Sydney, pp 1–42
Goswami A, Milne N, Wroe S (2011) Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc Roy Soc B 278:1831–1839. doi:10.1098/rspb.2010.2031
Goswami A, Polly PD, Mock OB, Sánchez Villagra MR (2012) Shape, variance and integration during craniogenesis: contrasting marsupial and placental mammals. J Evol Biol 25:862–872. doi:10.1111/j.1420-9101.2012.02477.x
Gregory WK (1922) On the “habitus” and “heritage” of Caenolestes. J Mammal 3:106–14. doi:10.2307/1373300
Groves CP (2005) Order Peramelemorphia. In: Wilson DE, Reeder DB (eds) Mammal Species of the World: A Taxonomic and Geographic Reference. Johns Hopkins University Press, Baltimore, pp 38–42
Groves CP, Flannery T (1990) Revision of the families and genera of bandicoots. In: Seebeck JH, Brown PR, Wallis RL, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty & Sons, Baulkham Hills, New South Wales, pp 1–11
Hershkovitz, P (1995) The staggered marsupial third lower incisor: hallmark of cohort Didelphimorphia, and description of a new genus and species with staggered i3 from the Albian (Lower Cretaceous) of Texas. Bonn Zool Beitr 45:153–169
Horovitz I, Sánchez-Villagra MR (2003) A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics 19:181–212. doi:10.1111/j.1096-0031.2003.tb00363.x
Hughes RL, Hall LS, Archer M, Aplin K (1990) Observations on placentation and development in Echymipera kalubu. In: Seebeck JH, Brown PR, Wallis RL, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty & Sons, Baulkham Hills, New South Wales, pp 259–270
Jolicoeur P (1963) The multivariate generalization of the allometry equation. Biometrics 19:497–499. doi:10.2307/2527939
Kingsmill E (1962) An investigation of criteria for estimating age in the marsupials Trichosurus vulpecula Kerr and Perameles nasuta Geoffroy. Austral J Zool 10:597–616. doi:10.1071/ZO9620597
Kirsch JAW, Waller PF (1979) Notes on the trapping and behavior of the Caenolestidae (Marsupialia). J Mammal 60:390–395. doi:10.2307/1379811
Kirsch JAW, Reig OA, Springer MS (1991) DNA hybridization evidence for the Australian affinity of the American marsupial Dromiciops australis. PNAS 88:10465–10469.
Koyabu DD, Werneburg I, Morimoto N, Zollikofer CPE, Forasiepi AM, Endo H, Kimura J, Ohdachi SD, Son NT, Sánchez-Villagra MR (2014) Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat Comm 5:3625. doi:10.1038/ncomms4625
Krajewski C, Buckley L, Westerman M (1997) DNA phylogeny of the marsupial wolf resolved. Proc Roy Soc Lond 264:911–917. doi:10.1098/rspb.1997.0126
Laurin M (2004) The evolution of body size, Cope’s rule and the origin of amniotes. Syst Biol 53:594–622. doi:10.1080/10635150490445706
Lavedèze S, Muizon C de (2010) Evidence of early evolution of Australidelphia (Metatheria, Mammalia) in South America: phylogenetic relationships of the metatherians from the late Palaeocene of Itaboraí (Brazil) based on teeth and petrosal bones. Zool J Linn Soc 159:746–784. doi:10.1111/j.1096-3642.2009.00577.x
Luckett PW, Hong N (2000) Ontogenetic evidence for dental homologies and premolar replacement in fossil and extant caenolestids (Marsupialia). J Mammal Evol 7:109–127. doi:10.1023/A:1009406632509
Luteyn JL (1999). Páramos: A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature. New York Botanical Garden Press, New York
Lyne AG (1964) Observations on the breeding and growth of the marsupial Perameles nasuta Geoffroy, with notes on other bandicoots. Austral J Zool 12:322–339. doi:10.1071/ZO9640322#sthash.oG7tuTG7.dpuf
Lyne AG, Mort PA (1981) A comparison of skull morphology in the marsupial bandicoot genus Isoodon: its taxonomic implication and notes on a new species, Isoodon arnhemensis. Austral Mammal 4:107–133
Manly BFJ (1997) Randomization, Bootstrap, and Monte Carlo Methods in Biology, 2nd ed. Chapman & Hall, London
Marshall LG (1980) Systematics of the South American marsupial family Caenolestidae. Fieldiana Geol (n ser) 5:1–145. doi:10.5962/bhl.title.3314
Martin GM (2007) Dental anomalies in Dromiciops gliroides (Microbiotheria, Microbiotheriidae), Caenolestes fuliginosus and Rhyncholestes raphanurus (Paucituberculata, Caenolestidae). Rev Chil Hist Nat 80:393–406. doi:10.4067/S0716-078X2007000400001
Martin GM (2011) Geographic distribution of Rhyncholestes raphanurus Osgood, 1924 (Paucituberculata: Caenolestidae), an endemic marsupial of the Valdivian temperate rainforest. Austral J Zool 59:118–126. doi:10.1071/ZO11038
Martin GM (2013) Intraspecific variability in Lestoros inca (Paucituberculata, Caenolestidae), with reports on dental anomalies and eruption pattern. J Mammal 94:601–617. doi:10.1644/12-MAMM-A-180.1
Maunz M, German Z (1996) Craniofacial heterochrony and sexual dimorphism in the short-tailed opossum (Monodelphis domestica). J Mammal 77:992–1005. doi:10.2307/1382780
Medina CE, Zeballos H, López E (2012) Diversidad de mamíferos en los bosques montanos del valle de Kcosnipata, Cusco, Perú. Mastozool Neotrop 19:345–351. http://www.sarem.org.ar/wp-content/uploads/2012/11/SAREM_MastNeotrop_19-1_08_Medina.pdf
Meredith RW, Westerman M, Springer MS (2008) A timescale and phylogeny for “bandicoots” (Peramelemorphia: Marsupialia) based on sequences for five nuclear genes. Mol Phylogen Evol 47:1–20. doi:10.1016/j.ympev.2008.01.002
Munemasa M, Nikaido M, Donnellan S, Austin CC, Okada N, Hasegawa M (2006) Phylogenetic analysis of diprotodontian marsupials based on complete mitochondrial genomes. Genes Genet Syst 81:181–191. doi:10.1266/ggs.81.181
Myers P, Patton J (2008) Genus Lestoros Oehser, 1934. In: Gardner A (ed) The Mammals of South America, Vol. 1. University of Chicago Press, Chicago, pp 124–126
Nilsson MA, Arnason U, Spencer PBS, Janke A (2004) Marsupial relationships and a timeline for marsupial radiation in south Gondwana. Gene 340:189–196. doi:10.1016/j.gene.2004.07.040
Ojala-Barbour RC, Pinto M, Brito J, Albuja L, Lee TE Jr, Patterson BD (2013) A new species of shrew-opossum (Paucituberculata: Caenolestidae) with a phylogeny of extant caenolestids. J Mammal 94:967–982. doi:10.1644/13-MAMM-A-018.1
Osgood WH (1921) A monographic study of the American marsupial, Caenolestes. Field Mus Nat Hist Zool Ser 14:1–156
Osgood WH (1924) Review of living caenolestids with description of a new genus from Chile. Field Mus Nat Hist Zool Ser 14:165–73. doi:10.5962/bhl.title.2955
Osgood WH (1943) The mammals of Chile. Field Mus Nat Hist Zool Ser 30:1–268.
Palma RE (2003) Evolution of American marsupials and their phylogenetic relationships with Australian metatherians. In: Jones M, Dickman C, Archer M (eds) Predators with Pouches: the Biology of Carnivorous Marsupials. CSIRO Publishing, Collingwood, Australia, pp 21–29
Palma RE, Spotorno AE (1999) Molecular systematic of marsupials based on the rRNA 12S mitochondrial gene: the phylogeny of Didelphimorphia and the living fossil microbiotheriid Dromiciops gliroides Thomas. Mol Phylogen Evol 13:525–535. doi:10.1006/mpev.1999.0678
Patterson BD (2008) Order Paucituberculata Ameghino, 1894. In: Gardner AL (ed) Mammals of South America. Vol. 1. Marsupials, Xenarthrans, Shrews, and Bats. University of Chicago Press, Chicago, pp 119–120
Patterson BD, Gallardo MH (1987) Rhyncholestes raphanurus. Mammal Species 286:1–5
Pearson OP (1995) Annotated keys for identifying small mammals living in or near Nahuel Huapi National Park or Lanin National Park, southern Argentina. Mastozool Neotrop 2:99–148. http://www.sarem.org.ar/wp-content/uploads/2014/05/SAREM_MastNeotrop_2-2_02_Pearson.pdf
Porto A, Shirai, LT, de Oliveira FB, Marroig G (2013) Size variation, growth strategies, and the evolution of modularity in the mammalian skull. Evolution 67:3305–3322. doi:10.1111/evo.12177
Prevosti F, Turazzini CF, Chemisquy MA (2010) Morfología craneana en tigres dientes de sable: alometría, función y filogenia. Ameghiniana 47:239–256. http://www.ameghiniana.org.ar/index.php/ameghiniana/article/view/167
Retief JD, Krajewski C, Westerman M, Winkfein RJ, Dixon GH (1995) Molecular phylogeny and evolution of marsupial protamine PI genes. Proc Roy Soc Lond B 259:7–14. doi:10.1098/rspb.1995.0002
Rougier GW, Apesteguía S, Gaetano LC (2011) Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479: 98–102. doi:10.1038/nature10591
Rougier, GW, Wible JR, Beck RMD, Apesteguía S (2012) The Miocene mammal Necrolestes demonstrates the survival of a Mesozoic nontherian lineage into the late Cenozoic of South America. Proc Natl Acad Sci USA 109(49):20053–20058. doi:10.1073/pnas.1212997109
Sánchez-Villagra MR (2013) Why are there fewer marsupials than placentals? On the relevance of geography and physiology to evolutionary patterns of mammalian diversity and disparity. J Mammal Evol 20:279–290. doi:10.1007/s10914-012-9220-3
Segura V (2014) Ontogenia craneana postnatal en cánidos y félidos neotropicales: funcionalidad y patrones evolutivos. Dissertation, Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina.
Segura V, Prevosti F (2012) A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131:79–92. doi:10.1007/s00435-012-0145-4
Segura V, Prevosti F, Cassini G (2013) Cranial ontogeny in the Puma lineage, Puma concolor, Herpailurus yagouaroundi, and Acinonyx jubatus (Carnivora: Felidae): a three dimensional geometric morphometric approach. Zool J Linn Soc 169:235–250. doi:10.1111/zoj.12047
Shirai L, Marroig G (2010) Skull modularity in neotropical marsupials and monkeys: size variation and evolutionary constraint and flexibility. J Exp Zool 314B:663–683. doi:10.1002/jez.b.21367
Smith RJ (1981) On the definition of variables in studies of primate dental allometry. Am J Phys Anthropol 55:323–329. doi:10.1002/ajpa.1330550306
Smith KK (1997) Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution 51:1663–1678
Solari S, Muñoz-Saba Y, Rodríguez-Mahecha JV, Defler TR, Ramírez-Chaves HE, Trujillo F (2013) Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozool Neotrop 20:301–365. http://www.sarem.org.ar/wp-content/uploads/2013/12/SAREM_MastNeotrop_20-2_08_Solari.pdf
Springer MS, Westerman M, Kavanagh JR, Burk A, Woodburne MO, Kao DJ, Krajewski C (1998) The origin of the Australasian marsupial fauna and the phylogenetic affinities of the enigmatic monito del monte and marsupial mole. Proc R Soc B 265:2381–2386. doi:10.1098/rspb.1998.0587
Strait SG (1993) Molar morphology and food texture among small bodied faunivorous mammals. J Mammal 74:391–402. doi:10.2307/1382395
Szalay FS (1982) A new appraisal of marsupial phylogeny and classification. In: Archer M (ed) Carnivorous Marsupials. Royal Society of New South Wales, Crow Nest, pp 621–640
Szalay FS (1994) Evolutionary History of the Marsupials and an Analysis of Osteological Characters. Cambridge University Press, New York
Szalay FS, Sargis EJ (2001) Model-based analysis of poscranial osteology of marsupials of Palaeocene of Itaboraí (Brazil) and the phylogenetics and biogeography of Metatheria. Geodiversitas 23: 139–302
Tarnawski BA, Cassini GH, Flores DA (2014) Allometry of the postnatal cranial ontogeny and sexual dimorphism in Otaria byronia (Otariidae). Acta Theriol 59:81–97. doi:10.1007/s13364-012-0124-7
Tarnawski BA, Cassini GH, Flores DA (2014) Skull allometry and sexual dimorphism in the ontogeny of the southern elephant seal (Mirounga leonina). Can J Zool 92:19–31. doi10.1139/cjz-2013-0106
Temple-Smith PD (1987) Sperm structure and marsupial phylogeny. In: Archer M (ed) Possums and Opossums: Studies in Evolution. Surrey Beatty & Sons, Sydney, pp 171–194
Timm RM, Patterson BD (2008) Genus Caenolestes O. Thomas 1895. In: Gardner AL (ed), Mammals of South America. Vol. 1. Marsupials, Xenarthrans, Shrews, and Bats. University of Chicago Press, Chicago, pp 120–124
Travouillon KJ, Archer M, Hand SJ, Muirhead J (2014) Sexually dimorphic bandicoots (Marsupialia: Peramelemorphia) from the Oligo-Miocene of Australia, first cranial ontogeny for fossil bandicoots and new species descriptions. J Mammal Evol. doi:10.1007/s10914-014-9271-8
Tyndale-Biscoe CH (2005) Life of Marsupials. CSIRO Publishing, Sydney
Tyndale-Biscoe H, Renfree MB (1987) Reproductive Physiology of Marsupials. Cambridge University Press, New York
Warton DI, Weber NC (2002) Common slope tests for bivariate structural relationships. Biometrics 44:161–174. doi:10.1002/1521-4036(200203)44:2<161::AID-BIMJ161>3.0.CO;2-N
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line fitting methods for allometry. Biol Rev 81:259–291. doi:10.1017/S1464793106007007
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40:243–261
Wilson L (2011) Comparison of prenatal and postnatal ontogeny: cranial allometry in the African striped mouse (Rhabdomys pumilio). J Mammal 92:407–420. doi:10.1644/10-MAMM-A-209.1
Wilson L (2013) Allometric disparity in rodent evolution. Ecol Evol 3:971–984
Wilson L, Sánchez-Villagra MR (2010) Diversity trends and their ontogenetic basis: an exploration of allometric disparity in rodents. Proc Roy Soc B 277:1227–1234
Wroe S, Milne N (2007) Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution 61:1251–1260
Acknowledgments
We thank curators who allowed the examination of the material under their care: Bruce Patterson and Bill Stanley of the Field Museum of Natural History (FMNH, Chicago), Kris Helgen, Darrin Lunde, and Linda Gordon of the Smithsonian Institution (USMNH, Washington, D.C.), Hugo López and Catalina Cárdenas of the Instituto de Ciencias Naturales Universidad Nacional de Colombia (ICN, Bogotá), and Sergio Solari of the Instituto de Biologia, Universidad de Antioquia (CTUA, Medellín). We thank Valentina Segura and Francisco Prevosti for discussion about character optimization on confidence intervals and for providing raw data of L. culpaeus. Guillermo Cassini performed the R script for bivariate comparisons. GMM thanks E. Watkins and M. Simeon for their economic support. This work was supported by an external fellowship of the Consejo Nacional de Investigaciones Cientificas y Técnicas of Argentina (CONICET) to DAF, and the projects PICT2008-1798 to NPG and PICT2012-1583 to DAF, of the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (ANPCyT).
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 28 kb)
Rights and permissions
About this article
Cite this article
Flores, D.A., Abdala, F., Martin, G.M. et al. Post-Weaning Cranial Growth in Shrew Opossums (Caenolestidae): A Comparison with Bandicoots (Peramelidae) and Carnivorous Marsupials. J Mammal Evol 22, 285–303 (2015). https://doi.org/10.1007/s10914-014-9279-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-014-9279-0