Abstract
To compare between the effect of partial substitution of lead ions (Pb4+ and Pb2+) at thallium (Tl) site in Tl0.8-xHg0.2PbxBa2Ca2Cu3O9−δ superconductor; two different compounds, lead (II) oxide (PbO) and lead (IV) oxide (PbO2), were used for the synthesis of the superconducting samples. Samples with nominal compositions Tl0.8-xHg0.2PbxBa2Ca2Cu3O9−δ, with x \(=\) 0.00, 0.05, 0.10, 0.15, and 0.20, were synthesized via solid state reaction technique. The x-ray diffraction (XRD) results showed that the partial substitution of both lead ions has not affected the tetragonal structure. Moreover, the volume fraction was increased from 75.95% to 90.38% and 89.41% as x increased up to 0.20 for PbO and PbO2 substitutions, respectively. The scanning electron microscopy (SEM) images demonstrated better grain connectivity and rectangular-shaped plates, supporting the phase formation of (Tl,Hg)-1223. The energy-dispersive x-ray (EDX) analysis revealed good agreement between the nominal and real compositions. Moreover, the elemental composition and oxidation states were proved by x-ray photoelectron spectroscopy (XPS). Both the superconducting transition temperature (Tc) and critical current (Jc) showed enhancement at x \(=\) 0.5 and 0.1 for PbO and PbO2 substituted samples, respectively. Vickers microhardness (Hv) measurements were used to examine the mechanical properties of the composites under a range of applied loads (0.49–9.8 N). All the prepared composites revealed the normal indentation size effect. The proportional sample resistance model proved to be the best model for interpreting the experimental data for the prepared samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thallium-based and mercury-based high temperature superconductors (HTSC’s) are the best among all other cuprates due to their high superconducting transition temperature (Tc) 125 K and 135 K at normal pressure, respectively [1,2,3]. Accordingly, HgBa2Ca2Cu3Oy (Hg-1223) and TlBa2Ca2Cu3Oy (Tl-1223) phases are desirable materials for the use in practical applications, such as electric communication systems, superconducting power cables, and superconducting magnetic energy storage systems [4]. However, there are difficulties in the preparation of Tl-based and Hg-based superconductors, related to thallium and mercury oxide’s toxicity and their instability at high temperatures [5, 6]. Moreover, the primary disadvantages of using HTSC’s in practical applications are the pinning capacity and the weak links across the lacking of flux granular networks at applied magnetic fields and high temperatures [7]. Even though the two phases are almost structurally identical, the oxygen occupancy in the Hg–O and Tl–O planes distinguishes Hg-the 1223 phase from the Tl-1223 phase. Hg-1223 is an under-doped phase because it is typically oxygen deficient. However, Tl-1223 has almost a full occupancy of oxygen sites, making it an over-doped phase. Accordingly, the partial replacement of Hg2+ by Tl3+ ions in the Hg-1223 phase is expected to decrease the oxygen deficiency in the Hg–O plane and thus enhance the superconducting properties of the Hg–O plane. This substitution produced some very intriguing results because it covered the entire density range, from regions that were under-doped to optimum-doped to over-doped [8]. Chang et al. [9] reported the enhancement of the volume fraction of the Tl1−xHgxBa2Ca2Cu3O9 superconducting phase as well as the increase in Tc from 120 to 130 K. Moreover, the intergrain critical current density Jc was improved in Hg1−xTlxBa2Ca2Cu3O9−δ by the increase in Tl-content from x = 0.5 to x = 0.8 [10].
In HTSC’s, the partial chemical substitution is a powerful technique for enhancing physical characteristics and producing new effective flux pinning sites to achieve greater Tc and Jc [11,12,13,14]. Aksyah et al. [11] reported a study on the impact of Se substitution at the Tl-site of the Tl1−xSex (Ba, Sr) CaCu2O7 superconducting phase. The results showed better superconducting behavior with an improvement in the intergranular characteristic. That was attributed to the smaller ionic radius of Se4+ exhibiting better superconducting behavior. Asyraf et al. [12] reported the effects of Bi and Si substitution on (Tl0.5Bi0.5−xSix) Sr2CaCu2O7 superconductors. Si substitution enhanced the zero-transition temperature (Tc-zero), however, it suppressed the onset transition temperature (Tc-onset) and susceptibility transition temperature (Tcχ’). The peak temperature (Tp) was shifted to higher temperatures, indicating the enhancement of flux pinning and intergranular coupling. Mumtaz et al. [13] investigated the influence of different substituted elements (thallium, lithium, sodium, and potassium) on (Cu0.5Tl0.25M0.25) Ba2Ca2Cu3O10−δ. It has been demonstrated that substituting atoms of lower electronegativity and smaller ionic sizes (i.e., Na+1, Li+1) at Tl sites in the charge reservoir layer results in a significant enhancement in superconductivity. In comparison with pure Tl-1223, the substituted phases of Tl-1223 have been intensively investigated for structural domain stability by substitution studies of Sr2+ for Ba2+ and Pb+4 or Bi+3 for Tl+3 [14].
The substitution of lead in several superconducting systems resulted in a significant improvement in superconducting parameters [15,16,17,18,19,20,21,22]. In the Hg-based superconductor, Pb doping supported the stability of the Hg-1223 phase and improved its thermal stability [15]. Moreover, the Tc of Tl-1223 was enhanced by Pb doping [16], whereas the Pb doping in Bi2−xPbxSr2CaCu2O8 decreased Tc significantly [17]. Pb substitution promotes grain growth, which is responsible for strong grains connectivity and superconducting phase homogeneity of the YBa2−xPbxCu3O7−δ system [18]. Doping of Nd and Pb in Bi2−x(Pb, Nd)xSr2Ca2Cu3O10+δ showed an enhancement in Tc [19]. Pb substitution at the Tl-site of (Tl1-xPbx)Sr2CaCu2O7 enhanced Tc up to 90 K for x \(=\) 0.5 and showed improvement in the formation of Tl-1212 phase with Tc = 89 K for x \(=\) 0.6 [20]. Also, the replacement of Pb in Tl0.6Pb0.4(Ba, Sr)CaCu2O7 increased the onset transition temperature to 118 K [21]. AbuHlaiwa et al. [22] reported the enhancement of Tc and grain size for x = 0.06 Pb content in (Cu, Tl)-1223 phase when PbF2 is used for substitution.
The present study focuses on comparing the effect of Pb chemical substitutions of thallium (Tl) by lead (Pb) in (Tl0.8Hg0.2)-1223 phase using two different phases of PbO and PbO2. A series of superconducting samples of type Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ with x \(=\) 0.00, 0.05, 0.10, 0.15, and 0.20 were prepared. The structural, electrical, and mechanical properties of the prepared samples have been studied.
2 Experimental Techniques
Samples with nominal compositions of Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ, with x \(=\) 0.00, 0.05, 0.10, 0.15, 0.20, were prepared by the solid-state reaction technique using appropriate weights of highly pure oxides Tl2O3, HgO, BaO2, CaO, CuO, PbO (Phase A) and PbO2 (Phase B). The weights were determined using a sensitive balance, and the powders were mixed and ground in an agate mortar before being sieved twice through a 60 μm sieve to achieve a fine homogeneous mixture. The powder was pressed into a disc-shaped pellet using a hydraulic press at a pressure of 10 tons/cm2. The samples were wrapped in silver foil and placed in a sealed quartz tube in order to reduce thallium and mercury evaporation during preparation. The samples were heated at 993 K at a rate of 4 K/min in a furnace with programmable sintering control. The temperature is raised again to 1133 K with a heating rate of 2 K/min and held at this temperature for 5 h before being cooled to room temperature at a rate of 2 K/min.
The crystal structure, lattice properties, and phase purity of the samples were investigated using X-ray diffraction patterns (XRD). Bruker D8 focus diffractometer with Cu Kα radiation was utilized at a variety of diffraction angles \(15^\circ \le\) 2θ \(\le\) 80°, with λ = 1.5406 Å. The microstructure morphology of the samples was characterized using SEM equipped with a JEOL scanning electron microscope, model JSM-IT200 operated at 20 kV with a resolution of 5 µm. The chemical composition of the samples was identified via energy-dispersive x-ray (EDX) by a detector-type JEOL scanning electron microscope, model JSM-IT200 accelerated at 20 kV. The KRATOS X SAM 800 spectrometer was utilized for x-ray photoelectron spectroscopy (XPS) examination of the surface to obtain significant quantitative and chemical state information. The temperature dependence of electrical resistivity is investigated via a four-probe technique from room temperature down to zero resistivity temperature. The transport critical current density (Jc) was measured at liquid nitrogen temperature (77 K) using the homemade conventional four-probe technique. The Vickers microhardness (HV) measurements were performed using MHVD-1000IS tester with applied loads ranging from 0.49 up to 9.8 N at a dwell time of 20 s. Five readings were recorded for each load to calculate the average HV.
3 Results and Discussion
3.1 X-Ray Powder Diffraction (XRD)
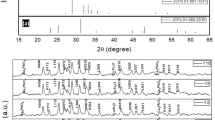
X-ray powder diffraction patterns for phases A and B are shown in Fig. 1a, b, respectively. For both phases, XRD patterns are well fitted to the basis of the (Tl, Hg)-1223 tetragonal structure with space group P4/mmm [8]. Few peaks with a low intensity related to the (Tl, Hg)-1212 superconducting phase, which are typically created during the solid-state reaction technique used to prepare the (Tl, Hg)-1223 phase [23]. In addition, various non-superconducting impurities, such as BaHgO2, BaCuO2, and CaHgO2 are formed. The formation of such impurities is attributed to the change in the partial pressure of oxygen during the preparation process [24]. Since the samples were treated in closed quartz with the presence of air instead of vacuum, the vapor pressure increased during the heating process. Furthermore, the porosity between the grains is higher in pure sample and at low lead concentrations, which increases the formation of the BaCuO2 impurity phase as confirmed by the SEM images which are analyzed below [25]. It’s worth mentioning that the Pb substitution in both phases does not affect the peaks positions of the Bragg’s diffractions, therefore the tetragonal structure is preserved under the Pb substitution. This result was confirmed by similarity of X-ray patterns and executing the Rietveld refinement for all the composite samples, using the MAUD program [26], as shown in the graphs of Fig. 2a, c. The refinement analysis findings revealed a satisfying excellent match in peak fitting between the measured and computed patterns.
The relative volume fractions for superconducting phases (Tl, Hg)-1223, (Tl, Hg)-1212, as well as the obtained impurities, for both phases A and B, were calculated via MAUD analysis and listed in Table 1. For both phases, the volume fractions were enhanced with lead substitution contents. In phase A, the relative volume fraction of (Tl, Hg)-1223 increases from 75.95 to 90.38%. However, in phase B, the volume fraction increases from 75.95 to 89.41%. Therefore, both types of lead ions replacement enhances the formation of (Tl, Hg)-1223 superconducting phase with no effect on the tetragonal crystal structure.
The lattice parameters a and c for all prepared samples were determined using the least square method using Miller indices (hkl) and inter-planar distances (dhkl) [27]. For the unsubstituted sample, the lattice parameters were a = 3.843 Å and c = 15.848 Å. These values are comparable to those reported by Metskhvarishvili et al. [5] for the Tl-1223 phase. The variation of a and c with Pb-content, for A and B phases, is shown in Fig. 3a, b. Compared to the pure sample, the values of the lattice parameters show an alteration as Pb content increases. For phase A, it is obvious that the lattice parameter “a” has no discernible change at low dopant and then increases as Pb content increases. In this case, the elongation is most likely caused by the expansion of the Cu–O plane as a result of the addition of electrons into the antibonding orbital caused by the partial replacement of Tl3+ ion by Pb2+ ions[28]. The lattice parameter “c” shows a greater value for all Pb substituted samples. This behavior is assumed because the ionic radius of Pb2+ (1.19 Å) is larger than that of Tl3+ (1.05 Å) and Hg2+ (1.02 Å) [22, 29, 30]. On the other hand, for phase B, the lattice parameter “a” increases till x \(= 0.10\) and then decreases, while the lattice parameter “c” shows a decreasing trend owing to the smaller ionic radius of Pb4+ (0.915 Å) compared to Tl3+ and Hg2+ ionic radii [20]. Moreover, c/a ratio for substituted samples increases from 4.124 to 4.136 and then decreases to reach 4.092 as the lead content increases up to x \(=\) \(0.20\) in the substituted samples using PbO. While for all PbO2 samples, c/a ratio decreases from 4.121 to 4.03 as lead content increases. This might be owing to the ionic radii mutuality of Pb and Tl ions, which compensates through the unit cell structure rather than the interstitial site [31].
3.2 Size, Surface Morphology, and Elemental Composition
Figures 4 and 5 show the SEM micrographs of Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ for both phases A and B, respectively, with x \(=\) 0.00, 0.05, 0.10, 0.15 and 0.20. Obviously, the rectangular-shape plates morphology guarantees the formation of the (Tl, Hg)-1223 phase. A similar result was reported by Abdeen et al. [32] for the (Tl, Hg)-1223 phase. Furthermore, the irregular forms contained within the grains correspond to impurity phases like (Tl, Hg)-1212 and other non-superconducting phases as demonstrated by relative volume fraction estimation in XRD results. For both phases, when Pb content increased, the (Tl, Hg)-1223 phase formation enhanced, the grain size increased, and the voids among superconducting plates decreased [22, 33].
EDX spectra of Tl0.8-xHg0.2PbxBa2Ca2Cu3O9−δ superconductor phase, for x = 0.00, 0.10, and 0.20 of phases A and B, are shown in Figs. 6a–c and 7a–c, respectively. The peaks positions and intensities are similar for different regions, indicating the high homogeneity of the tested samples. Moreover, the peaks corresponding to Pb appeared in Pb-substituted samples with intensity proportional to the number of substitutions. These data show that the Pb in (Tl, Hg)-1223's microstructure has been well substituted in the host lattice of the main phase. Figures 6d and 7 (d) show the atomic percentage of thallium and lead in both phases. Lead replaces thallium in the two phases, demonstrating a decline in thallium's atomic percentage and an increase in lead's atomic percentage with the increase in x. The variation of the atomic % of oxygen indicated in the insets of Figs. 6d and 7d. In both phases, the oxygen % varies with x, demonstrating that PbO and PbO2 in (Tl, Hg)-1223 influence the oxygen concentration. The role of oxygen in the formation and behavior of cuprates HTSC’s is well understood. This is evident from the fact that all HTSC’s feature square-planar CuO2 planes, which give rise to superconductivity [34]. Additionally, the non-monotonic changes in the superconducting properties may potentially be caused by the variations in the oxygen concentrations [35]. Table 2 gives the mean real composition and the nominal composition of O, Tl, Hg, Ba, Ca, and Cu with Pb-content for Tl0.65Hg0.2Pb0.15Ba2Ca2Cu3O9−δ which assures that the real stoichiometric are substantially identical to the starting material's nominal stoichiometric. While the smaller stoichiometric ratios of Hg and Tl are due to the Hg and Tl vaporization on the surface during the solid-state process [36,37,38].
3.3 X-Ray Photoelectron Spectroscopy (XPS)
X-ray photoemission spectroscopy is a well-known surface-sensitive technique for identifying elemental compositions and the electronic state of the elements present in the superconductor phase. The survey spectra of phases A and B are shown in Fig. 8a, b. The Pure sample confirms the presence of Tl, Ba, Ca, Cu, and O with small amounts of carbon. For doped samples, the presence of Pb in addition to the primary elements is also evident in the survey spectra. This supports the results of the EDX investigation. The sample's interaction with ambient air during the XPS examination is what caused the presence of carbon (C) [39].
Figure 9a, b illustrates the Tl-4f XPS spectra of all samples for further investigation of Tl oxidation states. For the non-substituted sample, the spectra exhibit two main peaks centered at 118.25 eV and 122.73 eV, corresponding to Tl-4f7/2 and Tl-4f5/2, respectively. Due to the Pb substitution, the signal is shifted non-monotonically toward lower binding energy at low dopant and higher binding energy at high dopant in both phases. The peak shift on Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ might be explained by the reduction in the valence of Tl with Pb substitutions [40]. The peak at 118.25 eV clearly indicates the Tl2+ oxidation state, whereas the peak at 119.9 eV is associated to the Tl1+ oxidation state [41, 42]. These observations suggested that Pb substitution reduces the oxidation state of Tl from Tl3+ in the pure sample to Tl1+ and Tl2+ in doped samples.
Due to the variable valence of Pb ions, it is essential to investigate their true valence in superconducting samples. Figure 10a, b shows the Pb-4f XPS spectrum of Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ, for x = 0.10, 0.15, and 0.20 for phases A and B, respectively. Pb-4f spectral lines have two different peaks at approximately 137.8 eV and 142.7 eV, which belong to Pb 4f7/2 and Pb 4f5/2, respectively. The spin–orbit splitting was determined to be 4.9 eV in all samples. The XPS spectra of Pb-4f7/2 and Pb-4f5/2 can be fitted into two peaks suggesting that the valence states of lead in these compounds are + 2 and + 4 [43]. The higher binding energy peaks of Pb-4f7/2 and Pb-4f5/2 correspond to the characteristic peaks of Pb4+, while their lower binding energy peaks correspond to the characteristic peaks of Pb2+. As shown in Fig. 10a, b, the intensity of the Pb2+ and Pb4+ spectra increased as the Pb concentration increased in phases A and B, respectively. All the superconducting samples contain Pb2+ and Pb4+ as oxidation states, as indicated by the binding energy of the Pb-4f peaks [44]. However, in phase A, the intensity of the Pb2+ spectra increased as Pb concentrations increased, whereas in phase B, the intensity of the Pb4+ spectra as Pb concentrations increased.
The effect of metal atoms on Cu–O bonding has been the primary focus of research into oxide superconductors [45]. The XPS spectra of the Cu2p orbital for the Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ samples of phases A and B are shown in Fig. 11a, b, respectively. The main peak at 934 eV and the satellite peak at 942.5 eV corresponds to Cu2p3/2, whereas the next two peaks are attributed to Cu2p1/2 [46]. The satellite peaks are the result of the "shake-up" process, which occurs when excess electrons are excited to higher energy levels [47, 48]. The peak separation between the two primary peaks of Cu 2p1/2 and Cu 2p3/2 was shown to be about 20 eV, which is consistent with values published in the literature [38, 41]. Due to the asymmetry of the peaks, they can be deconvoluted into two separate peaks that correspond to Cu2+ and Cu3+ (higher binding energy peak), respectively. A balanced Cu oxidation states (Cu2+ and Cu3+) is required for achieving maximum TC and enhancing the superconductivity of the phases by maintaining optimal hole doping in the Cu–O plane [49].
3.4 Electrical Resistivity Measurements
The temperature dependence of the electrical resistivity for Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ, with x \(=\) 0.00, 0.05, 0.10, 0.15, and 0.20, for phases A and B samples, is demonstrated in Fig. 12a, b, respectively. All the samples show a metallic-like behavior above Tc, followed by a zero-resistance region as the temperature decreases. A small curvature is noticed above Tc, caused by thermodynamic fluctuations due to the formation of the Cooper pairs above Tc. Tc is calculated from the crest of the first-order derivative of the electrical resistivity dρ/dT as a function of T for both phases, as in the insets of Fig. 12. The pure sample Tl0.8Hg0.2Ba2Ca2Cu3O9−δ has a Tc of 105.4 K. For phase A, Tc rises to 108.2 K for x = 0.05 and then falls to 101.6 K as the Pb substitution increases to x = 0.1. However, in phase B, Tc drops to 103.9 K at low Pb doping and subsequently rises to 108.4 K when x \(=\) 0.1. For x ≥ 0.15, Tc becomes invariant with Pb content for both phases. The decrease in Tc is mostly the result of disorientation between grains, as shown in the SEM images [50]. Moreover, the decrease in Tc may be attributable to an increase in oxygen concentration, as demonstrated by the EDX data. The hole filling mechanism is linked to the variation of Tc as Pb concentration increased [51]. For optimum hole doping, the greatest value of Tc is expected. The increase in Tc to 108.4 K (\(x = 0.1\)) in phase B, could be attributed to an increase in the c-axis, which causes an increase in the CuO layer [52].
The room temperature electrical resistivity ρn, the residual electrical resistivity ρo, the resistivity temperature coefficient α, the superconducting transition temperature Tc, zero resistivity temperature T0, and the transition width ΔT (Tc—T0) are listed in Table 3. ρn and ρo are obtained from the fitting of resistivity data in the temperature range from room temperature down to 2Tc according to the equation [53]:
The normal-state resistivity is regarded as a measure of the disorder of cations, so that, the sample with the highest Tc has the lowest disorder. The residual resistivity is widely known to be caused by the impurity scattering in the CuO2-plane. Accordingly, samples with higher ρo values indicate that impurity scattering in the CuO2-plane is high [32]. The transition width (ΔT) for substituted samples has increased as a result of replacing lead with thallium, which acted to increase the effectiveness of the high phase and the number of nucleation sites. Furthermore, this replacement may cause further gaps in the copper oxide layers, which contributes to the electron pair's survival and continuity [54].
3.5 I-V Results
The capacity to carry the current of the prepared superconducting samples is examined using measurements of the transport critical current density (Jc). The E–J curves are used for the determination of Jc for Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ, 0.00 ≤ x ≤ 0.20, are shown in Fig. 13a, b for PbO and PbO2 substituted samples, respectively.
E–J characteristic curve of the samples can be described using the relation [55]:
where γ and n are material constants. n is known as the transition order, describing the homogeneity of superconductors [56]. E–J characteristic curve can be interpreted through three stages: First, the current is insufficient to produce vortex pair unbinding, implying that V (I) = 0. As a result, the system has no resistance [57]. The free vortices below Tc are formed in the second part of the curve, when the voltage values are low, due to a limited penetration depth that is resolved by a thermally supported flux flow [58]. The voltage drop arises at a particular magnitude of applied current Ic in the nonlinear region, and it is characterized by flux creep (Lorentz force FL = Pinning force FP). The last section shows an increase in the linear curve, which is produced by vortex pair unbinding induced by strong Lorentz force (FL > FP), as mentioned in flux flow explanations [59]. The values of Jc for all samples were obtained using a criterion of 1 μV/cm for both phases and the values are listed in Table 4. Jc and Tc can be affected by variations in porosity, oxygen derivation, crystalline, grain shape, and relative volume fraction [56]. The critical current density Jc increases as x increases from 0.00 to 0.05 for PbO2 doped materials and then falls as x increases further. The rise in the volume fractions of (Tl, Hg)-1223 phase might explain the increase in Jc. The decrease in Jc for x > 0.05 might be attributable to an increase in the grain boundary resistance and a drop in flux pinning inside the samples [55]. For PbO samples Jc increases as Pb content increases till x = 0.15 and then decreases with further increase. Therefore, the effect of PbO is more significant than that of PbO2 in improving the flux pinning and critical current density of the prepared samples. This is most likely owing to lattice defects caused by the partial replacement of Pb2+ with Tl3+ which is more sufficient than the replacement of Pb4+ with Tl3+, which can promote flux pinning and grain coupling [32]. These results are comparable with the study reported by Cigáň et al.[60] for (Tl0.6Pb0.5)(Sr0.8Ba0.2)2Ca2Cu3O8+δ–xLaO1.5 superconducting phase.
3.6 Vickers Microhardness
The Vicker’s microhardness of both phases is calculated by using the relation [61]:
where F is the applied load in Newton (N) and d is the average diagonal length in μm. All readings were taken under a dwell time of 20 s. Figure 14a, b illustrates the variation of HV with the test loads (F) for PbO substitution (Phase A) and PbO2 substitution (Phase B). The experimental HV curves clearly show a nonlinear decrease for applied loads up to 2.94 N, then it maintains saturation when the applied load exceeds this value. The indenter’s penetration depth and the contribution of different layers in the resistance to indenter movement provide an explanation for this behavior [62]. At smaller applied loads, the indenter only penetrates the sample's surface, leading to a smaller penetration depth. However, indenting deeper into the specimen at higher loads results in a greater penetration depth. Thus, the effect of the inner layers should be considered, and it contributes well at higher loads. The dependence of the microhardness on the applied load is known as the indentation size effect (ISE).
The microhardness results of PbO substituted samples exhibit practically a decreasing behavior as x increases. The drop in HV values can be related to bond distortion, a decrease in the contact area between superconducting grains, and impurity phases production with an uneven grain orientation distribution [35, 63]. However, changes in the unit cell size may modify the distribution of PbO, which could also reduce HV [64]. For PbO2 substituted samples, HV is enhanced for x \(\ge 0.15\). The improvement is due to improved grain connectivity [35] and crack propagation resistance among the grains [65].
To examine the ISE, Meyer proposed that the applied load F is related to the indentation diagonal (d) according to the general relation [66]:
where \(A^{\prime }\) is the force per unit indentation depending on the material under investigation. The exponent n reflects the strength of the ISE; for n = 2, there is no dependence of HV on the applied load, therefore the sample gives a constant microhardness number whatever the applied load is. For n \(<\) 2, it was suggested that the increase rate in F is less than that of d2, thus HV decreases with the increase in F leading to the normal indentation size effect (NISE). On contrary, for n \(>\) 2, the increase in F dominates the increase in d2, leading to the increase in HV as F increases. This explains the reverse indentation effect (RISE).
The Meyer index can be obtained by graphing \(\mathit{ln} F\) against \(ln d\), as illustrated in Fig. 15a, b. The result is a straight line of slope equals n and intersects the y-axis at \(A^{\prime }\). The extracted values of \(A^{\prime }\) and n are listed in Table 5. The values of n for all samples were less than 2, which indicates the presence of NISE.
In order to explain the microhardness behavior, proportional sample resistance model (PSR) and modified proportional sample resistance model (MPSR) approaches were introduced. The PSR and MPSR models are considered to be the best for describing the majority of high-temperature superconductors prepared by single step solid state reaction. These models have been thoroughly investigated and found to accurately describe the behavior of these superconductors under a variety of conditions [67,68,69].
3.6.1 Proportional Sample Resistance Model (PSR)
The proportional sample resistance (PSR) model was introduced by Li and Bradt [70]. This model is used to describe the mechanical identification of the material showing the ISE behavior for numerous materials. Furthermore, the PSR model is based on the energy dispersion linked to the development of vacancies, residual fractures, and defects on the surface sample. Accordingly, the load dependence on the indentation is expressed as follows [71]:
where α defines the load dependency of the microhardness and consists of two components, the elastic resistance of the tested sample and the friction resistance at the indenter-sample interface. Table 5 shows that every sample has the typical ISE characteristic owing to positive values for α parameter, demonstrating the development of both elastic and plastic deformations. β is a load-independent parameter that is used to estimate real microhardness. Figure 16a, b depicts the plot of F/d against d, from which the values of α and β are obtained using the linear fitting and listed in Table 5. Accordingly, the PSR- microhardness values are given using the following formula [72]:
3.6.2 Modified Proportional Sample Resistance Model (MPSR)
The PSR model was modified to include the impact of the machining-induced plastically deformed surface on the microhardness data to take the following form [73] (Fig. 17):
The minimum applied load for the impression length is represented by the α2. The PSR model's definitions of α3 and α4 are equivalent on a physical level [74]. For PbO and PbO2 superconducting samples, a plot of the applied load versus indentation length is illustrated in Fig. 17, and the MPSR model's parameters (α2, α3, and α4) are listed in Table 5. The positive values of α2 for all samples in the two phases indicate that the Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ phase exhibits elastic and plastic deformation [72]. According to the positive values of α4 parameter, all the samples exhibit the ISE nature [75].
The following equation was used to determine the MPSR load-dependent microhardness (HMPSR):
Figures 18 and 19a–e show a comparison between the experimental HV and the theoretical values calculated from the different models for the Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ phase, with 0.00 ≤ x ≤ 0.20 for A and B phases, respectively. Figures 18 and 19a–e indicate that under high loads, both the PSR and MPSR models agree well with the experimental data, whereas at low applied loads, the PSR Model has the lowest divergence. This finding may be attributable to the low values of the required minimum applied load α2 because of the precise finishing of all prepared samples. The PSR model is also applicable to both phases because, as observed in SEM pictures, reducing the space between grains increases contact surfaces between grains, increasing surface energy, thus, resists the indenter’s movement through the samples.
4 Conclusion
The effect of partial replacement of Tl3+ ions by Pb2+ and Pb4+ in the Tl0.8−xHg0.2PbxBa2Ca2Cu3O9−δ phase was investigated through XRD, SEM, EDX, XPS, electrical resistivity, I-V measurements, and Vickers microhardness test. According to the XRD analysis, the volume fraction of (Tl, Hg)-1223 phase increased from 82.83 to 89.15% and 87.91%, as compared to the pure sample and the two substitutes, PbO and PbO2, respectively. Therefore, both types of lead ion replacement enhanced the formation of (Tl, Hg)-1223 superconducting phase with no effect on the tetragonal crystal structure. The observations from SEM demonstrated the sample’s surface structure and grain size in both substitutions. EDX results confirmed that the Pb atoms doped into the (Tl, Hg)-1223 phase were replaced by Tl atoms and entered the crystal structure. Moreover, the XPS studies stated that the superconductivity of the (Tl, Hg)-1223 was controlled by the electron transfer mechanism by PbO and PbO2 doping. The enhancement of Tc up to its maximum value for x = 0.05 PbO and x = 0.1 PbO2 could be related to the hole filling mechanism and increase in the CuO layer. I–V measurements showed that PbO has a greater impact on the flux pinning and critical current density of the produced samples than PbO2. Finally, microhardness tests were carried out to investigate how Pb atoms alter the material's mechanical characteristics. The results of the Vickers microhardness analysis were in excellent agreement with those of the PSR model.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
E.K. Al-Shakarchi, A.I. Al-Janabi, J. Supercond. Nov. Magn. 33, 379 (2020). https://doi.org/10.1007/s10948-019-05220-7
Z.Z. Sheng, A.M. Hermann, Nature 332, 55 (1988). https://doi.org/10.1038/332055a0
A. Schilling, M. Cantoni, J.D. Guo, H.R. Ott, Nature 363, 56 (1993). https://doi.org/10.1038/363056a0
M.A. Rahman, M.Z. Rahaman, M.N. Samsuddoha, Am. J. Phys. Appl. 3, 39 (2015)
I. Metskhvarishvili, T.E. Lobzhanidze, G.N. Dgebuadze, B.G. Bendeliani, M.R. Metskhvarishvili, M. Sh. Rusia, V.M. Gabunia, and K. Komakhidze, SG and SSR Approach in the Preparation of Precursors Influence on Superconducting Properties of Tl-1223 Superconductors (In Review, 2020). https://doi.org/10.21203/rs.3.rs-68452/v1
N.S. Abed, S.J. Fathi, K.A. Jassim, S.H. Mahdi, J. Phys. Conf. Ser. 1003, 012098 (2018). https://doi.org/10.1088/1742-6596/1003/1/012098
M. Anas, G.A. El-Shorbagy, J. Low Temp. Phys. 194, 183 (2019). https://doi.org/10.1007/s10909-018-2081-2
A.I. Abou-Aly, N.H. Mohammed, M. Roumié, A. El Khatib, R. Awad, S.A. Nour El Dein, J. Supercond. Nov. Magn. 22, 495 (2009). https://doi.org/10.1007/s10948-009-0447-z
I.C. Chang, J.Z. Liu, M.D. Lan, P. Klavins, R.N. Shelton, Chin. J. Phys. 34, 497 (1996)
A.K. Pandey, G.D. Verma, O.N. Srivastava, Physica C 306, 47 (1998). https://doi.org/10.1016/S0921-4534(98)00287-1
J. Nur-Akasyah, M.G. Ranjbar, R. Abd-Shukor, Ceram. Int. 47, 31920 (2021). https://doi.org/10.1016/j.ceramint.2021.08.078
J.M. Zubair-Asyraf, A.B.P. Ilhamsyah, R. Abd-Shukor, Cryogenics 105, 103011 (2020). https://doi.org/10.1016/j.cryogenics.2019.103011
M. Mumtaz, N.A. Khan, F. Ashraf, J. Supercond. Nov. Magn. 24, 1547 (2011). https://doi.org/10.1007/s10948-010-1051-y
R. Shipra, J.C. Idrobo, A.S. Sefat, Supercond. Sci. Technol. 28, 115006 (2015). https://doi.org/10.1088/0953-2048/28/11/115006
H.M. Shao, C.C. Lam, P.C.W. Fung, X.S. Wu, J.H. Du, G.J. Shen, J.C.L. Chow, S.L. Ho, K.C. Hung, X.X. Yao, Phys. C 246, 207 (1995). https://doi.org/10.1016/0921-4534(95)00153-0
T.-M. Chen, J.S. Ho, Phys. C 282–287, 915 (1997). https://doi.org/10.1016/S0921-4534(97)00559-5
J. Kane, K.-W. Ng, D. Moecher, Phys. C 294, 176 (1998). https://doi.org/10.1016/S0921-4534(97)01696-1
S. Ezzatpour, L. Sharifzadegan, F. Sarvari, H. Sedghi, Phys. C Supercond. Appl. 549, 150 (2018). https://doi.org/10.1016/j.physc.2018.02.023
A.K. Jassim, F.S. Abed, J. Non-Oxide Glasses 11, 41 (2019)
J. Nur-Akasyah, A.B.P. Ilhamsyah, R. Abd-Shukor, Ceram. Int. 46, 18413 (2020). https://doi.org/10.1016/j.ceramint.2020.04.210
M.G. Ranjbar, M. Ghoranneviss, R. Abd-Shukor, Appl. Phys. A 124, 456 (2018). https://doi.org/10.1007/s00339-018-1838-4
H. AbuHlaiwa, H. Basma, M. Rekaby, M. Roumie, R. Awad, J. Low Temp. Phys. 198, 26 (2020). https://doi.org/10.1007/s10909-019-02245-z
R. Awad, A.I. Abou-Aly, I.H. Ibrahim, W. Abdeen, Solid State Commun. 146, 92 (2008). https://doi.org/10.1016/j.ssc.2007.12.029
A.I. Abou-Aly, R. Awad, M. Kamal, M. Anas, J. Low Temp. Phys. 163, 184 (2011). https://doi.org/10.1007/s10909-010-0339-4
A. Abou Aly, I. Ibrahim, R. Awad, A. El-Harizy, A. Khalaf, J. Supercond. Novel Magn. 23(7), 1325–1332 (2010). https://doi.org/10.1007/s10948-010-0776-y
L. Lutterotti, Acta Crystallogr. Sect. A Found. Crystallogr. 56(s1), s54–s54 (2000). https://doi.org/10.1107/S0108767300021954
A. Srour, R. Awad, W. Malaeb, M.M.E. Barakat, J. Low Temp. Phys. 189(3–4), 217–229 (2017). https://doi.org/10.1007/s10909-017-1806-y
A. Kamar, A. Srour, M. Roumié, W. Malaeb, R. Awad, A. Khalaf, Appl. Phys. A 127, 579 (2021). https://doi.org/10.1007/s00339-021-04707-2
R. Awad, N.S. Aly, I.H. Ibrahim, A.I. Abou-Aly, A.I. Saad, Phys. C 341–348, 685 (2000). https://doi.org/10.1016/S0921-4534(00)00650-X
M.M.E. Barakat, D. El-Said Bakeer, A.-H. Sakr, J. Taibah Univ. Sci. 14(1), 640–652 (2020). https://doi.org/10.1080/16583655.2020.1761676
A. Laheeb, K. Mohammed, A. Jasim, Ibn AL-Haitham J. Pure Appl. Sci. 31(3), 26–32 (2018). https://doi.org/10.30526/31.3.2024
A.I. Abou-Aly, R. Awad, I.H. Ibrahim, W. Abdeen, J. Alloy. Compd. 481, 462 (2009). https://doi.org/10.1016/j.jallcom.2009.02.156
J. Nur-Akasyah, Int. J. Electrochem. Sci. 16, 2 (2021). https://doi.org/10.20964/2021.10.14
J.L. Tallon, Oxygen in high-Tc cuprate superconductors, in Frontiers in Superconducting Materials. ed. by A.V. Narlikar (Springer-Verlag, Berlin/Heidelberg, 2005), pp.295–330. https://doi.org/10.1007/3-540-27294-1_7
N. El Ghouch, R. Al-Oweini, K. Habanjar, R. Awad, J. Phys. Chem. Solids 151, 109807 (2021). https://doi.org/10.1016/j.jpcs.2020.109807
J.L. Jorda, Th. Hopfinger, M. Couach, P. Pugnat, C. Bertrand, Ph. Galez, J. Supercond. 11, 87 (1998). https://doi.org/10.1023/A:1022602517307
R.J. McNeely, J.A. Belot, T.J. Marks, Y. Wang, V.P. Dravid, M.P. Chudzik, C.R. Kannewurf, J. Mater. Res. 15, 1083 (2000). https://doi.org/10.1557/JMR.2000.0156
Z.L. Du, P.C.W. Fung, J.C.L. Chow, Y.Y. Luo, Q.Y. Li, J. Supercond. 9, 43 (1996). https://doi.org/10.1007/BF00728423
G. Greczynski, L. Hultman, Progress Mater. Sci. 107, 100591 (2020). https://doi.org/10.1016/j.pmatsci.2019.100591
T. Suzuki, M. Nagoshi, Y. Fukuda, S. Nakajima, M. Kikuchi, Y. Syono, M. Tachiki, Supercond. Sci. Technol. 7, 817 (1994). https://doi.org/10.1088/0953-2048/7/11/007
P.E. Lippens, L. Aldon, J. Olivier-Fourcade, J.C. Jumas, A. Gheorghiu de la Rocque, C. Sénémaud, J. Phys. Chem. Solids 60, 1745 (1999). https://doi.org/10.1016/S0022-3697(99)00022-0
K. Tanaka, A. Iyo, N. Terada, K. Tokiwa, S. Miyashita, Y. Tanaka, T. Tsukamoto, S.K. Agarwal, T. Watanabe, H. Ihara, Phys. Rev. B 63, 064508 (2001). https://doi.org/10.1103/PhysRevB.63.064508
X. Zheng, L. Zhang, X. Wang, Y. Qing, J. Chen, Y. Wu, S. Deng, L. He, F. Liao, Y. Wang, J. Geng, J. Sun, G. Li, L. Liu, J. Lin, Inorg. Chem. Front. 7, 3561 (2020). https://doi.org/10.1039/D0QI00828A
S. Kambe, Y. Murakoshi, R. Sekine, M. Kawai, K. Yamada, S. Ohshima, K. Okuyama, Phys. C 190, 139 (1991). https://doi.org/10.1016/S0921-4534(05)80228-X
P. Kulkarni, S. Mahamuni, M. Chandrachood, I.S. Mulla, A.P.B. Sinha, A.S. Nigavekar, S.K. Kulkarni, J. Appl. Phys. 67, 3438 (1990). https://doi.org/10.1063/1.345330
S. Marik, A.J. Dos santos-Garcia, C. Labrugere, E. Morán, O. Toulemonde, M.A. Alario-Franco, 1212-Molybdo-Cuprates; effect of oxygenation in the structure, properties and electronic states. MRS Proc. (2014). https://doi.org/10.1557/opl.2014.414
J. Jiang, X. Liu, J. Han, K. Hu, J. Chen, Processes 9, 680 (2021). https://doi.org/10.3390/pr9040680
Z.H. Gan, G.Q. Yu, B.K. Tay, C.M. Tan, Z.W. Zhao, Y.Q. Fu, J. Phys. D: Appl. Phys. 37, 81 (2004). https://doi.org/10.1088/0022-3727/37/1/013
V. Gayathri, E.P. Santanu Bera, T.G. Amaladass, R.P. Kumary, A. Mani, Phys. Chem. Chem. Phys. 23(22), 12822–12833 (2021). https://doi.org/10.1039/D1CP01262B
M. Mahtali, S. Chamekh, J. Supercond. Nov. Magn. 24, 351 (2011). https://doi.org/10.1007/s10948-010-1008-1
A.H. Ali, A.K.D. Ali, K.A. Jasim, IOP Conf. Ser. Mater. Sci. Eng. 871(1), 012079 (2020). https://doi.org/10.1088/1757-899X/871/1/012079
K.A. Jasim, L.A. Mohammed, J. Phys. Conf. Ser. 1003, 012071 (2018). https://doi.org/10.1088/1742-6596/1003/1/012071
A.T. Ulgen, T. Turgay, C. Terzioglu, G. Yildirim, M. Oz, J. Alloy. Compd. 764, 755 (2018). https://doi.org/10.1016/j.jallcom.2018.06.142
M.A. Omar, S.J. Fathi, Res. Jet J. Anal. Invent. 2, 94 (2021)
W. Abdeen, N.H. Mohammed, R. Awad, S.A. Mahmoud, M. Hasebbo, J. Supercond. Novel Magn. 26, 623 (2013). https://doi.org/10.1007/s10948-012-1803-y
A. Nasser, A. Srour, N. El Ghouch, W. Malaeb, R. Al-Oweini, R. Awad, Appl. Phys. A 126, 951 (2020). https://doi.org/10.1007/s00339-020-04083-3
T.M. Katona, S.W. Pierson, Phys. C 270, 242 (1996). https://doi.org/10.1016/S0921-4534(96)00521-7
J.M. Repaci, C. Kwon, X.G. Jiang, Bull. Am. Phys. Soc. 40, 445 (1995). https://doi.org/10.1103/PhysRevB.54.R9674
A. Jukna, Materials 15, 4260 (2022). https://doi.org/10.3390/ma15124260
A. Cigáň, G. Plesch, M. Škrátek, M. Kopčok, J. Maňka, P. Jurdák, A. Koňakovský, Open Phys. 9, 213 (2011). https://doi.org/10.2478/s11534-010-0042-8
Y. Zalaoglu, E. Bekiroglu, M. Dogruer, G. Yildirim, O. Ozturk, C. Terzioglu, J. Mater. Sci. Mater. Electron. 24, 2339 (2013). https://doi.org/10.1007/s10854-013-1098-1
B. Sahoo, D. Behera, J. Mater. Sci. Mater. Electron. 30, 12992 (2019). https://doi.org/10.1007/s10854-019-01661-x
M. Barakat, J. Supercond. Novel Magn. 30, 2945 (2017). https://doi.org/10.1007/s10948-016-3791-9
H. AbuHlaiwa, H. Basma, M. Rekaby, R. Awad, Appl. Phys. A 125, 1 (2019). https://doi.org/10.1007/s00339-019-2972-3
K. Sangwal, B. Surowska, Mater. Res. Innov. 7, 91 (2003). https://doi.org/10.1080/14328917.2003.11784768
H.C. Ling, M.F. Yan, J. Appl. Phys. 64, 1307 (1988). https://doi.org/10.1063/1.341851
W. Abdeen, N.H. Mohammed, R. Awad, S.A. Mahmoud, M. Hasebbo, J. Supercond. Nov. Magn. 26, 3235 (2013). https://doi.org/10.1007/s10948-013-2192-6
M.H. El Makdah, N. El Ghouch, M.H. El-Dakdouki, R. Awad, M. Matar, Appl. Phys. A 129, 265 (2023). https://doi.org/10.1007/s00339-023-06547-8
M. Anas, Chem. Phys. Lett. 742, 137033 (2020). https://doi.org/10.1016/j.cplett.2019.137033
H. Li, R.C. Bradt, J. Mater. Sci. 28, 917 (1993). https://doi.org/10.1007/BF00400874
J. Petrík, Arch. Metall. Mater. 61, 1819 (2016). https://doi.org/10.1515/amm-2016-0294
M. Rekaby, N.H. Mohammed, M. Ahmed, A.I. Abou-Aly, Appl. Phys. A 128, 261 (2022). https://doi.org/10.1007/s00339-022-05394-3
A. Srour, W. Malaeb, M. Rekaby, R. Awad, Phys. Scripta 92, 104002 (2017). https://doi.org/10.1088/1402-4896/aa86ce
S. Celik, O. Ozturk, E. Coşkun, M. Sarıhan, E. Asikuzun, K. Ozturk, C. Terzioglu, J. Mater. Sci. Mater. Electron. 24, 2218 (2013). https://doi.org/10.1007/s10854-013-1082-9
Y. Zalaoglu, T. Turgay, A.T. Ulgen, U. Erdem, M.B. Turkoz, G. Yildirim, J. Mater. Sci. Mater. Electron. 31, 22239 (2020). https://doi.org/10.1007/s10854-020-04724-6
Acknowledgements
This work was done in the Faculty of Science, Beirut Arab University, at the Specialized Materials Science Laboratory, Physics Department, in collaboration with the Faculty of Science at Alexandria University in Alexandria, Egypt.
Funding
The study that was submitted by the authors was not funded by any organization.
Author information
Authors and Affiliations
Contributions
RA: Suggested the point of the research. RK: Prepared the samples. MA and KH characterized the sample using XRD, EDX and XPS and analyzed the data. They prepared figures 1-10 RK and KH: Measured the electrical resistivity and IV. They prepared figures 11 and 12 RK and MA: measured the VM and analyzed the data. They prepared figures 13-20 All authors participated in writing and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicting interests to disclose that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khattar, R.F., Habanjar, K., Awad, R. et al. Comparative Study of Structural, Electrical, and Mechanical Properties of (Tl, Hg)-1223 High Temperature Superconducting Phase Substituted by Lead Oxide and Lead Dioxide. J Low Temp Phys 211, 166–192 (2023). https://doi.org/10.1007/s10909-023-02968-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10909-023-02968-0