Abstract
Superconducting samples of type (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , x=0.0,0.5,1.0,1.5,2.0, and 3.0 wt.% of the total sample’s mass were prepared by a single step solid-state reaction technique. The prepared samples were characterized using X-ray powder diffraction (XRD) and a scanning electron microscope (SEM). The electrical properties of the prepared samples were investigated using the electrical resistivity and I–V measurements. The Vickers microhardness (H v ) was measured at different applied loads (0.25–3.0 N) for studying the mechanical performance of the prepared samples. All prepared samples exhibited normal indentation size effect (normal ISE) and the H v number was load dependent. H v number increases as nano-Ag addition increased. The experimental data of H v was analyzed using different models; Mayer’s law, Hays–Kendall (H–K) approach, elastic/plastic deformation (EPD) model, proportional specimen resistance (PSR) model, and indentation induced cracking (IIC) model. In addition, the true microhardness (H o ) values were evaluated through different models. The obtained data has good agreement with the PSR model. Also, Young’s modulus (E), yield strength (Y), fracture toughness (K f ), and brittle index (B i ) were calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
(Cu0.5Tl0.5)-1223 superconducting phase is of a great importance in practical applications because it has the high superconducting transition temperature T c , the high critical current density J c , and the low anisotropy parameter [1]. (Cu0.5Tl0.5)-1223 has a tetragonal unit cell, which divided into two structural units [2]. The first unit is the charge reservoir layer CRL, (Cu0.5Tl0.5)Ba2O4−δ , where the presence of Cu in the CRL plays major role for lowering anisotropy parameter [1]. The second one consists of three CuO2-planes separated by Ca atoms, where the wave function of Ca atom develops correlation among the carriers in the CuO2 planes. This could improve the interplanar coupling of CuO2-planes [3].
Microhardness is mechanical property that measures how much the material will resist deformation by indentation and gives useful information about the material composition and structure. The most familiar method for determining microhardness is the Vickers indention method [4–10], where it based on produce indentions on small areas on the surface of the samples by pressing a hard indenter of specified geometry.
The weak links between the grains and the brittle nature inherent in HTSCs are the most serious difficulties for using these materials in technological applications [11]. It is well known that the Ag addition is an effective way in improving the physical and mechanical properties of HTSCs. Gul et al. [12] found that the addition of Ag2CO3 into the Bi-2223 phase enhanced the critical current density. Sallah et al. [13] reported that the addition of 5 wt.% Ag in different forms (Ag, Ag2O and AgNO3) on Tl-2212 increased the hardness. Foerster et al. [14] reported that the addition of 5 wt.% Ag2O into YBCO increased the hardness and elastic modulus. Sotelo et al. [15] reported that Ag addition to Bi-2212 and (Bi,Pb)-2212 samples improved the microstructure and the mechanical properties. Farbod et al. [16] found that increasing the size of nano-Ag from 30 to 700 nm addition to Y-123 improved the connectivity between grains and J c , and they decreased as the Ag size increases from 800 nm to 1 μm.
The addition of nanoparticles to HTSCs plays an important role for enhancing the flux pinning, critical current density, and mechanical properties. Addition of the proper amount of nanometer particles such as ZnO [17], MgO [18], and NiFe2O4 [19] to (Cu0.5Tl0.25Pb0.25)-1223, (Cu0.25Tl0.75)-1234, and (Bi,Pb)-2223 phases, respectively, acted as an effective source of pinning centers and improved the critical current density. Awad [18] found that nano-MgO addition up to 0.6 wt.% to (Cu0.25Tl0.75)-1234 phase increased the Vickers microhardness number. Mohammed et al. [20, 21] showed and found that the addition of nano-SnO2 up to 1.0 wt.% and nano-In2O3 up to x=0.1 wt.% to (Cu0.5Tl0.5)-1223 phase enhanced the mechanical properties.
This work is concerned with the effect of the nano-Ag addition on the mechanical properties of (Cu0.5Tl0.5)-1223 superconducting phase. For this study, superconducting samples of type (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , x=0.0,0.5,1.0,1.5,2.0, and 3.0 wt.%, were prepared and investigated using X-ray powder diffraction, scanning electron microscopy (SEM), electrical resistivity, I–V, and Vickers microhardness measurements.
2 Theoretical Background
The nonlinear behavior between the applied indentation test load (F) and indentation diagonal (d) is known as the indentation size effect (ISE) [22, 23]. There are several models to describe the ISE behavior such as Meyer’s law, Hayes–Kendall approach, elastic/plastic deformation, proportional specimen resistance, and the indentation-induced cracking models.
The Vickers microhardness number of different applied loads is calculated using the equation:
where F is the applied load in Newton (N), and d is the diagonal length in μm.
2.1 Mayer’s Law
Mayer’s law is the simplest way to describe the relationship between indentation test load and the resultant indentation diagonal [24–28] and is related by
where n is Mayer index and A is the standard hardness constant. For n<2, the material behaves like normal ISE. While for n>2, the material is reverse ISE. When n=2, the hardness is load independent.
2.2 Hayes–Kendall Approach
Hayes and Kendall [29] assumed that the resistance to deformation is Newtonian resistance pressure of the sample itself, the load dependence of indentation size is expressed as
where A 1 is load independent constant and W is a minimum test load necessary to initiate plastic deformation, and below which only elastic deformation occurs. Load independent, true microhardness, is evaluated from
2.3 Elastic/Plastic Deformation (EPD) Model
Bull et al. [30] consider that there is elastic recovery occurs instead of remaining indentation impression after the indenter is removed. The load dependence of indentation size is given by
where A 2 is constant and d 0 is correction in d due to elastic recovery of the tested sample. In this model, the true microhardness is given by
2.4 Proportional Specimen Resistance (PSR) Model
Li and Bradet [25] assumed that diagonal length depends on the applied load which is given by
where α and β are constant related to elastic and plastic properties for given material [25], respectively. The term αd has been attributed to specimen surface energy [31, 32], indenter edges acting as plastic hinges [33], and the proportional specimen resistance [25, 26]. Hence, the true microhardness is given by
2.5 Indentation-Induced Cracking (IIC) Model
Li and Bradt [34] considered that, at maximum penetration point during the loading half cycle, the applied load is balanced by the total specimen resistance composed of four components. These components are due to frictional component, elastic deformation which lead to normal ISE, plastic deformation and indentation cracking which lead to reverse ISE. In the case of indentation cracking, the Vickers microhardness may be given by
where λ 1, K 1 and K 2 are constants. The constant K 2 depends on the applied load F while K 1 is a geometrical conversion factor, depending on indenter geometry. For an ideally perfect plastic body λ 1=1 and the second term \(K_{2}(\frac{F^{5/3}}{d^{3}}) = 0\), while for the perfect brittle materials λ 1=0. The first term of Eq. (9) is equal to zero irrespective of the type of ISE. Equation (8) is modified empirically for brittle material as
where K and the exponent m are load independent constants. For normal ISE m>0.6, while for the reverse ISE m<0.6 [35].
The elastic modulus E is related to the Vickers microhardness H v by the following relation [6]:
where this relation was modified depending on the structure of the studied material by Awad et al. [36] for (Cu0.5Tl0.5)-1223 superconducting phase and expressed as
Also, the yield strength Y, the fracture toughness K f , and the brittleness index B i are related to the Vickers microhardness H v by the following relations [28, 37–39]:
3 Experimental Technique
Superconducting samples of type Cu0.5Tl0.5Ba2Ca2Cu3O10−δ added by nano-Ag (x=0.0,0.5,1.0,1.5,2.0, and 3.0 wt.%) were prepared by a single step solid-state reaction technique. Stoichiometric ratios of high purity oxides of Tl2O3, BaO2, CaO, and CuO were ground in agate mortar and sifted twice by a 85 μm sieve. Then nano-Ag (Aldrich, 20–40 nm) was added with different concentrations (0, 0.5, 1.0, 1.5, 2.0, and 3.0 wt.% of the total sample mass) to the sifted powder. The powder was again mixed for a long time to ensure the homogenous distribution of nano-Ag inside the sample. The powder was pressed into a disc of diameter of 1.5 cm and thickness of about 0.3 cm, and then the disc was wrapped in a silver foil to reduce the thallium losses during the preparation process. The samples were heated in sealed quartz tubes (1.5 cm diameter and 12 cm long) at a rate of 4 °C/min to 760 °C, followed by a rate of 2 °C/min to 850 °C, and finally held at this temperature for 5 hours, and after that they slowly cooled to room temperature.
The prepared samples were characterized by X-ray powder diffraction (XRD) using X’Peret Phillips powder diffractometer with CuK α radiation (λ=1.54056 Å) in the range 4∘≤2θ≤70∘. The grain size and microstructure morphology of the samples were identified using Jeol scanning electron microscope JSM-5300, operated at 30 kV.
Vickers microhardness measurements of the studied samples were performed in atmospheric air using manual Vickers microhardness tester IN-412A at room temperature. The applied load was varied from 0.25 to 3.0 N for a loading time of 59 seconds, and the diagonals of indentation were measured with an accuracy of ±0.1 μm. An average value of microhardness number (H v ) was calculated by taking 4 readings at different locations on the specimen surface.
4 Results and Discussion
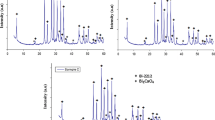
The XRD patterns for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , with 0.0≤x≤3.0 wt.% are shown in Fig. 1. The major peaks of the samples with 0.0≤x≤2.0 wt.% are well indexed by the tetragonal unit cell of (Cu0.5Tl0.5)-1223 phase with space group P4/mmm [40]. Only small amount of impurities such as (Cu0.5Tl0.5)-1212 and Ba2Cu3O5.9 are appeared in XRD patterns for the same values of x. On the other hand, the major peaks of the sample with x=3.0 wt.% are well indexed by the tetragonal unit cell of (Cu0.5Tl0.5)-1212 phase with space group P4/mmm [41]. In addition, a small amount of the BaCuO2 appeared as an impurity. This means that a phase change from (Cu0.5Tl0.5)-1223 phase to (Cu0.5Tl0.5)-1212 phase occurred under our preparation conditions. It is noticed that there are no peaks corresponding to impurity phases containing Ag or Ag-rich compounds in XRD patterns. This indicates that nano-Ag does not enter the crystal structure of this phase, but just occupies interstitial positions between the superconducting grains up to x=2.0 wt.%. The phase change from (Cu0.5Tl0.5)-1223 phase to (Cu0.5Tl0.5)-1212 phase, for x=3.0 wt.%, indicates that nano-Ag affects the crystal structure of the phase. A similar result was observed by Azambuja et al. [42] who found that the Cu atoms might be substituted by Ag atoms in Y-123 added by Ag, and Mellekh et al. [43] who found for Y-123 phase added by nano-Al2O3 that Al could occupy Y and Cu(1) sites and there was no peak corresponding to Al2O3 or Al-based compounds in XRD patterns. Also, Awad et al. [44] recorded the change of (Bi,Pb)-2223 phase added by nano-SnO2 to (Bi,Pb)-2212 phase at a high nano-SnO2 addition. The calculated volume fraction of (Cu0.5Tl0.5)-1223 phase estimated from the peaks intensities of the same particular reflections is shown in the inset of Fig. 1. It is clear that the volume fraction of (Cu0.5Tl0.5)-1223 increases slightly as the nano-Ag addition increases up to x≤1.5 wt.%, and then it decreases for x>1.5 wt.%. This indicates that the high concentration of nano-Ag addition retards the formation rate of the (Cu0.5Tl0.5)-1223 phase.
Typical SEM micrographs for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , with x=0.0,0.5,1.5, and 3.0 wt.% are shown in Figs. 2(a), (b), (c), and (d), respectively. The surface morphology of the samples exhibit rectangular-shape plates which refer mainly to (Cu0.5Tl0.5)-1223 phase [45]. Little numbers of irregular shaped and spherical grains, which correspond to the superconducting (Cu0.5Tl0.5)-1212 phase [46] and the nonsuperconducting impurity phases Ba2Cu3O5.9 [46] and BaCuO2 [45], respectively. It is obvious that the numbers of irregular shaped and spherical grains decrease while the rectangular-shaped plates increase and their alignments are improved up to x=1.5 wt.%. This indicates that the low nano-Ag addition enhances the phase formation of (Cu0.5Tl0.5)-1223 phase. Similar results were observed by Şakiroğlu et al. [47] for the (Bi,Pb)-2223 phase substituted by Ag. SEM micrograph for x=3.0 wt.%, Fig. 2(d), shows an increase in the number of irregular-shaped plates, indicating the increase of the (Cu0.5Tl0.5)-1212 phase formation.
The variation of both superconducting transition temperatures T c which are determined from electrical resistivity measurements and the critical current density J c , which are determined from I–V measurements at 77 K, with x is shown in Fig. 3 [48]. Both T c and J c increase as nano-Ag increases up to x=1.5 wt.% then decreases for a further increase in x. The increases of T c with x could be explained due to the increase in the volume fraction of (Cu0.5Tl0.5)-1223. The decrease of T c for x>1.5 wt.% may be due to the phase change from the (Cu0.5Tl0.5)-1223 phase to the (Cu0.5Tl0.5)-1212 phase or the trapping of mobile holes [43].
The increase in J c may be attributed to the increase in the volume fractions of (Cu0.5Tl0.5)-1223 phase or the diffusion of nano-Ag in the samples over the pore surfaces, grain boundaries, and twin boundaries. This can increase flux pinning and strong coupling between grains, leading to increase the critical current density. A similar result was observed by Gul et al. [12] and Sotelo et al. [15]. The decrease in J c , for x>1.5 wt.%, could be attributed to the phase change from (Cu0.5Tl0.5)-1223 phase to (Cu0.5Tl0.5)-1212 phase. Also, the excess of nano-particles induces large agglomerations between the grains and not through the boundaries and voids, which deteriorate the intergranular critical current density [16]. Similar results were observed by Ghattas et al. [49] for the (Bi,Pb)-2223 phase added by Al2O3 nanoparticles.
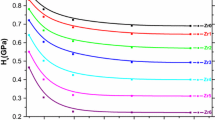
The measured Vickers microhardness number H v for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , with x=0.0,0.5,1.0,1.5,2.0, and 3.0 wt.% superconducting samples was plotted as function of applied loads F (N) in Fig. 4. It is observed that the variation of H v with F can be divided into two regions. The first region is the load dependent region (from 0.25 to 1.0 N), where H v decreases nonlinearly with increasing the applied load. The second region is the load independent region (for F>1.0 N) where H v tends to attain saturation. This type of non-linear behavior is known as indentation size effect (ISE) [21, 22]. Kölemen et al. [50] explained the ISE behavior qualitatively on the basis of indentation depth of indenter. At small loads, the indenter penetrates surface layer only so the surface effect is more pronounced. But at higher loads where the penetration depth increases, the effect of inner layers becomes more prominent, and there is a slight change in the values of microhardness with load. This nonlinear behavior was observed by Awad et al. [36] for (Cu0.5Tl0.5)-1223 substituted by Pr and Uzun et al. [51] for Al-Si alloys. It is clear that the H v number increases by increasing the nano-Ag addition for each load as shown in Fig. 5. This indicates that the addition of nano-Ag particles enhances the mechanical properties of (Cu0.5Tl0.5)-1223. This enhancement can be attributed to the reduce of the porosity, which provides a plastic-flow region and may be resist crack propagation. Also, similar result was observed by Foerster et al. [14] for YBCO added with Ag2O in micro-scale and Sotelo et al. [52] for Bi-2212 added with Ag. In addition, a similar result was observed by Liu et al. [53] who found that the small amount of nano-SiC on Sn–Ag–Cu alloys improved the microhardness. They attributed the increase in microhardness to the increase in the grain connectivity and the crack resistance propagation among the grains. In order to describe the ISE behavior of the materials, several relationships between the applied test load and the indentation diagonal were mentioned before. The Meyer’s law correlates the applied load and the resultant indentation diagonal by (2). Figure 6 shows the plot between lnF versus lnd for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ with 0.0≤x≤3.0 wt.%. This plot gives the values of Mayer’s index (n) and the constant (A) from slop and the intersection, respectively and their values are listed in Table 1. It is obvious that n for all samples is less than 2, which indicates the presence of normal ISE. The constant A can be interpreted as the load needed to initiate unit indentation. The values of A are very small to be acceptable for the ceramic materials, which are characterized by its high microhardness [36]. According to the Hayes–Kendall approach, there exists a minimum applied test load, W, necessary to initiate plastic deformation is given by Eq. (3). Figure 7 represents the plot between F versus d 2 for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, which gives the values of W and the constant A 1 from intersection and the slop, respectively. The values of the fitting parameters W and A 1 are also listed in Table 1. It is noticed that the lowest applied load (0.25 N), which gives plastic deformation is lower than the calculated values of W. This means that the Hayes–Kendall approach failed to describe the behavior of our microhardness data.
The Elastic/Plastic Deformation (EPD) model proposed that there exists a correction in measured indentation diagonal due to elastic recovery d 0, which is given from Eq. (5). Figure 8 shows the plot between F 1/2 versus d for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, giving the values of d 0 and the constant A 2 from intersection and the slope, respectively. It is obvious that the calculated values of d 0, Table 1, are greater than the values of indentation, which correspond to the lowest load (0.25 N) experimentally. So, this model failed to describe the behavior of our microhardness data.
The Proportional Specimen Resistance (PSR) model modified the Hays–Kendall approach by replacing the load independent constant W in Eq. (3) by the term αd to obtain Eq. (7). Figure 9 displays the plot between F/d versus d for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, which gives the values of α and β from intersection and the slope, respectively. The values of the fitting parameters α and β are listed in Table 1. The constant β is suggested to be a measure of true hardness [54], which increases with increasing nano-Ag addition. This result is in agreement with the measured microhardness values H v in the given range of the applied load.
Indentation-induced cracking (IIC) model can describe the type of ISE from calculating m values in Eq. (10). Figure 10 shows the plot between ln(H v ) versus ln(F 5/3/d 3) for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, which gives the values of K and m from intersection and the slope, respectively. The values of the fitting parameters (K and m) are listed in Table 1. It is obvious that the values of m are greater than 0.6 for all samples which confirm the normal ISE for all prepared samples. The values of K show nonsystematic variation.
In order to determine the consistency of the mentioned models with our experimental true microhardness data (obtained as the average of the last two points in the plateau region) for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, the theoretical true microhardness, H o calculated according to Eqs. (4), (6), (8), and (10) and percentage deviation for different models are calculated and listed in Table 2. We can see that the minimum percentage deviations are obtained for PSR model in the given range of the applied load. Also, Fig. 11 shows the variation of the experimental and theoretical true microhardness for different models with x for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ ensured this result. So, the PSR model is the most suitable model to give a good explanation for the true microhardness for all the studied samples in the plateau region.
The elastic modulus E, the yield strength Y, the fracture toughness K f and the brittleness index B i for (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ , 0.0≤x≤3.0 wt.%, superconducting samples are calculated according to Eqs. (12) to (15) and listed in Table 3. E is an important parameter for superconducting material in determining critical grain size above which microcracking will occur due to anisotropic thermal stresses that arise during processing [55]. The values of E for the prepared samples increase with an increased nano-Ag addition. This could be explained as the Ag addition may reside at grain boundaries and impedes dislocation motion (strain), which enhances the elastic modulus. Similar results were observed by Solunke et al. [56] for the Bi-2212 phase added with Ag. Also, the yield strength Y (stress at which the plastic deformation become noticeable) and the fracture toughness K f (the ability of material to resist crack) increase with an increased nano-Ag addition. This may be due to the presence of the ductile Ag between (Cu0.5Tl0.5)-1223 grains, which may provide a plastic flow region and relaxes undesirable residual stress resulting from the grain anisotropy of the superconductors [57]. Also, the addition of Ag causes a reduction in the number of pores and cracks in the microstructure which improve the mechanical properties [58–60]. Similar results were observed with the Ag addition on the Y-123 phase by Foerster et al. [14] and Joo et al. [57]. The brittleness index, B i , decreases with increasing the nano-Ag addition up to x=1.5 wt.%, then increased for further increase in x indicating that the samples become more brittle and more hard.
5 Conclusion
In this work, the (nano-Ag) x Cu0.5Tl0.5Ba2Ca2Cu3O10−δ superconducting phase has been successfully prepared by using a single step solid-state reaction technique. The effect of nano-Ag addition on the phase formation, microstructure electrical, and mechanical properties of this phase has been studied. The addition of nano-Ag to Cu0.5Tl0.5Ba2Ca2Cu3O10−δ superconducting phase up to x=1.5 wt.% improved the volume fraction, superconducting transition temperature and critical current density. With further increase in x (x>1.5 wt.%), a reverse trend was observed. The Vickers microhardness H v and the elastic parameters (E,Y,K f , and B i ) improved with increasing the nano-Ag addition. Furthermore, the PSR model successfully explained the experimental results in the saturation regime.
References
Ihara, H.: In: Yamafuji, K., Morishita, T. (eds.) Advances in Superconductivity VII, p. 225. Springer, Tokyo (1995)
Khan, N.A., Khurram, A.A., Mazhar, M.: Physica C 23, 407 (2004)
Khan, N.A., Javaid, A., Khurram, A.A., Haider, N.: Physica C 425, 90 (2005)
Uzan, O., Köleman, U., Çelebi, S., Güçlü, N.: J. Eur. Ceram. Soc. 25, 969 (2005)
Muralidhar, M., Reddy, K.N., Babu, V.H.: Phys. Status Solidi A 126, 115 (1991)
Veerender, C., Dumke, V.R., Nagabhooshanam, M.: Phys. Status Solidi A 144, 299 (1994)
Tampieri, A., Celotti, G., Guicciardi, S., Melandri, C.: Mater. Chem. Phys. 42, 188 (1995)
Ionescu, M., Zeimetz, B., Dou, S.X.: Physica C 306, 213 (1998)
Khalil, S.M.: Phys. Status Solidi A 178, 731 (2000)
Khalil, S.M.: J. Phys. Chem. Solids 62, 457 (2001)
Kohno, O., Ikeno, Y., Sadakaat, N., Goto, K.: Jpn. J. Appl. Phys. 27, L77 (1988)
Gul, I.H., Amin, F., Abbasi, A.Z., Anis-ur-Rehman, M., Maqsood, A.: Physica C 499, 139 (2006)
Salleh, F.M., Yahya, A.K., Imad, H., Abd-Shukor, R.: Mater. Sci. Eng. B 98, 17 (2003)
Foerster, C.E., Lima, E., Rodrigues, P., Serbena, F.C., Lepienski, C.M., Cantao, M.P., Jurelo, A.R., Obradors, X.: Braz. J. Phys. 38(3A) (2008)
Sotelo, A., Madre, M.A., Diez, J.C., Rasekh, Sh., Angurel, L.A., Martínez, E.: Supercond. Sci. Technol. 22, 034012 (2009)
Farbod, M., Batvandi, M.R.: Physica C 471, 112 (2011)
Eloker, M.M., Awad, R., Abd El-Ghany, A., Abou Shama, A., Abd El-wanis, A.: J. Supercond. Nov. Magn. 24, 1345 (2011)
Awad, R.: J. Supercond. Nov. Magn. 21, 461 (2008)
Kong, W., Abd-Shukor, R.: J. Supercond. Nov. Magn. 23, 257 (2010)
Mohammed, N.H., Abou-Aly, A.I., Ibrahim, I.H., Awad, R., Rekaby, M.: J. Alloys Compd. 486, 733 (2009)
Mohammed, N.H., Abou-Aly, A.I., Ibrahim, I.H., Awad, R., Rekaby, M.: J. Supercond. Nov. Magn. 24, 1463 (2011)
Tickoo, R., Tandon, R.P., Bamzai, K.K., Kotru, P.N.: Mater. Chem. Phys. 80, 446 (2003)
Gong, J., Wu, J., Guan, Z.: Mater. Lett. 38, 197 (1999)
Mott, B.W.: Microindentation Hardness Testing. Butterworths, London (1956)
Li, H., Bradt, R.C.: J. Mater. Sci. 28, 917 (1993)
Li, H., Han, Y.H., Bradt, R.C.: J. Mater. Sci. 29, 5641 (1994)
Jain, A., Razdan, A.K., Kotru, P.N., Wanklyn, B.M.: J. Mater. Sci. 29, 3847 (1994)
Tabor, D.: The Hardness of Metals. Oxford University. Press, Oxford (1951)
Hays, C., Kendall, E.G.: Metallography 6, 275 (1973)
Bull, S.J., Page, T.F., Yoffe, E.H.: Philos. Mag. Lett. 59, 281 (1989)
Frohlich, F., Grau, P., Grellmann, W.: Phys. Status Solidi A 42, 79 (1977)
Oliver, W.C., Hutchings, R., Pethica, J.B.: In: Blau, P.J., Lawn, B.R. (eds.) Microindentation Techniques in Materials Science, Engineering, p. 90. ASTM, Philadelphia (1986)
Ma, Q., Clarke, D.R.: J. Mater. Res. 10, 853 (1995)
Li, H., Bradt, R.C.: J. Mater. Sci. 31, 1065 (1996)
Sangwal, K.: Mater. Chem. Phys. 63, 145 (2000)
Awad, R., Abou Aly, A.I., Kamal, M., Anas, M.: J. Supercond. Nov. Magn. 24, 1947 (2011)
McClintock, F.A., Argon, A.S.: Mechanical Behaviour of Materials, p. 455. Addison-Wesley, Reading (1996)
Farber, B.Y., Sidorov, N.S., Kulakov, V.I., Lunin, A.Y., Izotov, A.N., Emel’chenko, G.A., Bobrov, V.S., Fomenko, L.S., Natsik, V.D., Lubenets, S.V.: Superconductivity 4, 2296 (1991)
Nihara, K., Movna, R., Hasselman, D.P.H.: J. Mater. Sci. 1, 13 (1982)
Ihara, H., Tokiwa, K., Tanaka, K., Tsukamoto, T., Watanabe, T., Yamamoto, H., Iyo, A., Tokumoto, M., Umede, M.: Physica C 282–287, 957 (1997)
Ganguli, A.K., Subbanna, G.N., Rao, C.N.R.: Physica C 156, 116 (1988)
Azambuja, P., Júnior, P.R., Jurelo, A.R., Serbena, F.C., Foerster, C.E., Costa, R.M., Souza, G.B., Lepienski, C.M., Chinelatto, A.L.: Braz. J. Phys. 39(4) (2009)
Mellekh, A., Zouauoui, M., Ben Azzouz, F., Annabi, M., Ben Salem, M.: Solid State Commun. 140, 318 (2006)
Awad, R., Abou-Aly, A.I., Abdel gawad, M.M.H., G-Eldeen, I.: J. Supercond. Nov. Magn. 25, 739 (2011)
Awad, R., Abou-Aly, A.I., Isber, S., Malaeb, W.: J. Phys. Conf. Ser. 43, 474 (2006)
Badica, P., Iyo, A., Crisan, A., Ishiura, Y., Sundaresan, A., Ihara, H.: Supercond. Sci. Technol. 15, 964 (2002)
Şakiroğlu, S., Kocabaş, K.: J. Supercond. Nov. Magn. 24, 1321 (2011)
Abdeen, W., Mohammed, N.H., Awad, R., Mahmoud, S.A., Hasebbo, M.: J. Supercond. Nov. Magn. 26, 623 (2013)
Ghattas, A., Annabi, M., Zauaoui, M., Ben Azzouz, F., Ben Salem, M.: Physica C 468, 31 (2008)
Kölemen, U., Uzun, O., Yilmazlar, M., Güçlü, N., Yanmaz, E.: J. Alloys Compd. 415, 300 (2006)
Uzun, O., Karaaslan, T., Gogebakan, M., Keskin, M.: J. Alloys Compd. 376, 149 (2004)
Sotelo, A., Mora, M., Madre, M.A., Diez, J.C., Angurel, L.A., De la Fuente, G.F.: J. Eur. Ceram. Soc. 25, 2947 (2005)
Liu, P., Yao, P., Liu, J.: J. Electron. Mater. 37, 874 (2008)
Kölemen, U.: J. Alloys Compd. 425, 429 (2006)
Zewen, W., Wanqi, J.: Mater. Sci. Eng. A 452–453, 508 (2007)
Solunke, M.B., Modi, K.B., Lakhani, V.K., Zankat, K.B., Sharma, P.U., Reddy, P.V., Shah, S.S.: Indian J. Pure Appl. Phys. 45, 764 (2007)
Joo, J., Jung, S.-B., Nah, W., Kim, J.-Y., Kim, T.S.: Cryogenics 39, 107 (1999)
Sakai, N., Seo, S.J., Inoue, K., Miyamoto, T., Murakami, M.: Physica C 335, 107 (2000)
Nariki, S., Sakai, N., Tomita, M., Murakami, M.: Physica C 378–381, 779 (2002)
Diko, P., Fuchs, G., Krabbes, G.: Physica C 363, 60 (2001)
Acknowledgements
The authors of the present study wish to express their thanks to the superconductivity and metallic glass lab, Physics Department, Faculty of Science, Alexandria University, Alexandria, Egypt, for aiding with the experimental procedures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdeen, W., Mohammed, N.H., Awad, R. et al. Influence of Nano-Ag Addition on the Mechanical Properties of (Cu0.5Tl0.5)-1223 Superconducting Phase. J Supercond Nov Magn 26, 3235–3245 (2013). https://doi.org/10.1007/s10948-013-2192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-013-2192-6