Abstract
A series of (Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ superconducting samples impregnated with different percentage additions of potassium salt {CoSiW11}x nanoparticles (x = 0.00, 0.01, 0.02, 0.04, 0.06, 0.08 and 0.12 wt.%) were prepared using a single-step solid-state reaction technique at ambient pressure. These samples were characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) to study their structures, construction of the synthesized samples and elemental composition, respectively. XRD studies indicate that the tetragonal structure of (Cu0.5Tl0.5)-1223 phase has not changed by the addition of {CoSiW11} nanoparticles the same as for the values of lattice parameters a and c, where they show a non-remarkable change. The relative volume fraction of (CuTl)-1223 phase record a maximum value for x = 0.02 wt.%. The formation of (CuTl)-1223 phase matches the presence of rectangular-shaped plates seen in SEM images. The enhancement of these plates after the addition of {CoSiW11} nanoparticles up to x = 0.02 wt.% agreed with the measurements of relative volume fraction. The mass percentage of all elements was measured using the EDX technique, which shows the distribution composition of both superconductor and nanoparticle compounds. Vibrational mode analyses of oxygen were accomplished via Fourier transform infrared (FTIR) spectroscopy in (CuTl)-1223 phase impregnated with {CoSiW11} nanoparticles. The Oδ oxygen modes observed are almost unaltered while the other oxygen modes of planar CuO2 are slightly softened and hardened. Superconducting transition temperature (Tc) and critical current density (Jc) were determined from the electrical resistivity and I–V measurements, respectively. The results indicate an increase in the Tc values from 125 K for the pure sample to 132 K for x = 0.02 wt.% impregnated samples, while a decrease in the Jc values is observed upon increasing ‘x’ value from x = 0.00 up to 0.12 wt.%. The suppression of these electrical superconducting parameters for x > 0.02 wt.% may be due to an increase of weak links' connectivity among the grain boundaries and growth of impurity phases that affect the formation of (CuTl)-1223 superconductor phase. The fluctuation-induced conductivity (FIC) analysis has been done in the light of the Aslamasov–Larkin (AL) theory to study the fluctuation conductivity Δσ above Tc as a function of reduced temperature for (CoSiW11)x/(CuTl)-1223 superconductor. The results show four non-identical fluctuation regions that are short-wave (Sw), two-dimensional (2D), three-dimensional (3D) and critical (Cr). The zero-temperature coherence length (\({\xi }_{c}(0)\)), the effective layer thickness of the two-dimensional system (d), inter-layer coupling (I), Fermi velocity (vF) and Fermi energy (EF) were evaluated as a function of ‘x’ addition from FIC analysis of the samples and then correlated to the superconductivity order parameters. The superconducting parameters were increased upon the addition of {CoSiW11} nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of high-temperature superconductors (HTSCs), many researches have made serious efforts to ameliorate their critical parameters and transport properties to enhance the technological applications and techniques [1]. (Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ phase is one of the most attractive superconductor phases of HTSCs cuprates due to its high values of superconducting transition temperature (Tc), the critical current density (Jc) the zero-temperature coherence length \([(0)\)] [2, 3]. An interesting property of this phase is the preparation at ambient pressure [4]; this is because it is a simple, time-saving and cost-effective process. Its unit cell structure is composed of an isolating charge reservoir layer, Cu0.5Tl0.5Ba2O4-δ, and three CuO2 planes interceded by Ca atoms [5].
The studies of excess conductivity are regarded as an important tool, focusing on the transport properties of HTSCs close to the transition region and beyond. The excess conductivity or fluctuation-induced conductivity (FIC), Δσ (T), is widely studied for various superconducting compounds [6, 7]. The Aslamazov–Larkin (AL) model fits well for thin films as does the Lawrence–Doniach (LD) model in bulk superconductors [8, 9]. A study of fluctuation-induced excess conductivity in the MgB2 sample by Rajput [10], with (AL) model and (LD) model indicates the three-dimensional nature of conduction of the carriers as opposed to the 2D character exhibited by the HTSCs and he found that the coherence length is ∼ 21 Å from (AL) model, same as found by other authors.
The incorporation of nanostructured materials in bulk HTSCs is an effective way to enhance the transport properties [11] and the additional pinning effects in the bulk polycrystalline samples result in the improvement of infield superconducting properties. Many attempts have been made to reduce the inter-grain voids and fill the pores and empty nano-scale spaces [12]. Additionally, several studies were carried out to investigate the influence of nanoparticle addition for various superconducting phases [7, 13,14,15,16,17]. For instance, it was observed that the low concentration of nano-ZnO addition in (Cu0.5Tl0.25Pb0.25)-1223 matrix improved the superconducting properties (Jc, Tc and the volume fraction), while high concentrations increased the grain-boundaries’ resistance due to agglomeration of nano-ZnO particles [13]. Mumtaz et al. [14] found that adding the nano-particle (DNPs)x to (Cu0.5Tl0.5)-1223 increased Tc for x = 0.5 wt.% and improved the inter-grain connectivity of (CuTl)-1223 phase. Furthermore, the addition of Ni nanoparticles into (Cu0.5Tl0.5)-1223 phase reduced the superconducting volume fraction, resulting in a shift in the 3D conductivity and a decrease in the interlayer coupling ‘I’ and ‘ξc(0)’ [8]. Mohammed et al. [7] displayed the effect of Ti substitution into (Cu0.5Tl0.5)-1223 phase, revealing that the increment of 3D width as Ti substitution increased, thus improving the mobility of free charge transporters in three dimensions and decreasing the value of the lattice parameter c.
On the other hand, polyoxometalates (POMs) are inorganic nanoparticles of metal oxides clusters, which are considered as highly agreeable materials regarding their structural properties, reactivity and relevance. Thus, they are useful for many applications such as in medicine, catalysis, and materials science [18,19,20,21,22,23,24]. To the best of our knowledge, very few studies have been carried on the influence of POM nanoparticles on superconductor properties. El Ghouch et al. [25, 26] investigated the addition of {FeSiW11} and {MnSiW11} POM nanoparticles on (Bi,Pb)-2223 superconductor phase. The reports revealed that the addition of {MnSiW11}and {FeSiW11} nanoparticles enhanced the volume fraction, Tc and Jc of (Bi,Pb)-2223 phase.
The present work aims at investigating the effect of the phase formation and inter-grains connectivity, as well as Tc, Jc and the excess conductivity of the (Cu0.5Tl0.5)-1223 phase. For this purpose, seven samples of (CoSiW11)x/(Cu0.5Tl0.5)-1223, 0.00 ≤ x ≤ 0.12 wt.%, were prepared using a conventional solid-state reaction technique. The different samples were structurally characterized using powder X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) analysis. Also, the dc-electrical resistivity, critical current density and excess conductivity measurements were performed to obtain the transport properties of (CoSiW11)x/(Cu0.5Tl0.5)-1223 superconductor phase samples.
2 Materials and methods
2.1 Materials
The chemicals used in the preparation of POM nanoparticles are cobalt nitrate ((Co(NO3)2·6H2O, ≥ 99%), sodium metasilicate (Na2SiO3·5H2O, ≥ 95%) and sodium tungstate (Na2WO4·2H2O, ≥ 95%), purchased from SIGMA-ALDRICH and HIMEDIA, respectively, and used without further purification. Moreover, distilled water, acquired and prepared in the lab via RO water distiller, was used as a solvent. As for the superconductor phase, the analytical grade of calcium oxide powder (CaO, ≥ 95%) was used from HIMEDIA, while thallium (III) oxide (Tl2O3, 99%), barium peroxide (BaO2, ≥ 95%), copper (II) oxide (CuO, 98%) were used from SIGMA-ALDRICH.
2.2 Synthesis of POM nanoparticles and characterizations
The {CoSiW11} nanoparticles were synthesized according to Müller et al. [27]. Typically, 5 mL distilled water was used to dissolve a solution of 0.146 g cobalt nitrate (Co(NO3)2·6H2O, 0.5 mmol), and then the obtained solution was heated until boiling. A sample of 1.61 g of the solid (K8[α-SiW11O39]·13H2O, 0.5 mmol) was added in small amounts over 10 min to the boiling solution. The process of preparing (K8[α-SiW11O39]·13H2O) is reported in the literature [27]. After filtering this solution, a 0.2-g of potassium chloride (KCl, 2.7 mmol) was prepared by dissolving in 1 mL distilled water and added to the final solution. The mixture was then placed at 278 K for 2 days in the refrigerator. The [CoIISiW11(H2O)O39]·14H2O{CoSiW11} nanoparticles were filtered out and finally air-dried.
XRD was used to investigate the structural properties of the nanoparticles with Cu–Kα radiation on a Bruker D8 Advance powder diffractometer. Transmission electron microscopy (TEM), JEM-100CX, was used to determine the crystalline size and the surface morphology of {CoSiW11}, which proceeded at 80 kV. Besides, FTIR spectroscopy was used to characterize the nanoparticles in the range 2000–550 cm−1, using a Nicolet iS5 spectrometer.
2.3 Preparation of superconductor samples
(Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ superconductor samples impregnated with different percentages of {CoSiW11} POM nanoparticles varied from x = 0.00 wt.% to 0.12 wt.% were prepared by a single-step solid-state reaction method. First, stoichiometric quantities of BaO2, CaO, CuO and Tl2O3* were weighted and then blended well. The obtained mixture was grinded in an agate mortar into a fine powder and then sifted using a sieve (60 μm) to obtain a homogenous mixture. The POM nanoparticles with different wt.% amounts were added into the mixed compound. Afterward, the powder was compressed to 13 mm-diameter discs under 11 tons (for 2 min) and then the obtained discs were wrapped with a silver foil, preventing the mass loss of thallium during the heating process. The sample was then introduced in a sealed quartz tube of length 10 cm and diameter 3 cm and put in a computerized box furnace (Gallenkamp, FSL-340-110w). The heating process started with a rate of 4 ºC/min up to 740 ºC and 2 ºC/min up to 845 ºC, which was left for 4 h. Finally, a cooling rate of about 2 ºC/min was applied to decrease its temperature back to room temperature.
2.4 Crystal structure and morphology characterization
The prepared samples (CoSiW11)x/(Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ were structurally characterized using XRD, Bruker D8 Advance powder diffractometer. The results were carried out at room temperature with Cu–Kα radiation and 2θ ranging from 4 to 70º. From the patterns obtained, lattice parameters and the type of structure were determined for the superconductor samples using interplanar distance and miller indices of the crystal planes for the major phase. The measurements of FTIR spectroscopy were taken using Nicolet iS5 spectrometer with a wavenumber ranging from 700 to 400 cm−1. The spectra browse the influence of nanoparticle’s addition on the oxygen phonon modes in (CuTl)-1223 phase unit cell. The microstructure and grain size of (CoSiW11)x/(Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ superconducting samples were examined using SEM, AIS 2300 C, proceeded with resolution power of 30 μm at 20 kV × 1.5 K. The elemental composition analysis was investigated by EDX with Apollo X detector type, accelerated with a voltage of 20 kV.
2.5 Electrical measurement
Electric resistivity measurements were performed using the standard four-probe technique for the prepared samples. The working medium represents a closed cryogenic refrigeration system using liquid nitrogen as a coolant; the results were carried out with the variation of temperature from room temperature down to lower ones (77 K ≤ T ≤ ~ 300 K), allowing to determine Tc and zero superconducting transition temperature (T0). Temperature sensor Platinum–Iridium with 100 Ω at 0 ºC was used to monitor the temperature of the samples. The cryostat system employed in this measurement is a homemade cryostat connected with liquid nitrogen flow. The dimensions of the samples used in this measurement were about 1.2 × 0.5 × 0.4 cm3. The sample was supplied by a constant current of 120 mA by two copper terminals and two copper wires 7 mm apart were connected to the sample using conductive silver paint to measure the voltage. The obtained results were recorded on the PC using CASSY LAB software through CASSY interface. Finally, the critical current density (Jc) was measured also using the four-probe technique at 77 K. The coolant medium used is a liquid nitrogen flow. The current was supplied to the measured samples using a high current power supply source (LD Didactic GmbH) up to Imax = 20 A in steps of 100 mA for each measurement with \(\le\) 1% stabilization at full load, while the voltage was recorded using a LEYBOLD DIDACTIC 532 13 microvoltmeter. The samples with dimensions about 1.2 × 0.4 × 0.4 cm3 were connected to the copper wires using conductive silver paint.
3 Results and discussions
3.1 POM nanoparticles
The XRD pattern of {CoSiW11} nanoparticles is depicted in Fig. 1. It shows sharp diffraction peaks around 8.8°, 15.6°, 16.6°, 25.2°, 29°, 34.1° and 39.5°. This result confirms that the compound has high crystallinity, belonging to the tetragonal structure of P \(\stackrel{-}{4}\) m2 space group without other detectable phases and possessed a Keggin structure [28, 29]. The average crystallite size (D) depends on \(\lambda\), \(\theta\) and the full width at half maximum (\(\beta\)). It was determined using Scherrer’s equation
The constant \(k\) represents the factor of the tetragonal structure. The high value of D extracted from XRD may be due to the presence of water molecules in the prepared POM [30, 31]. In accordance, Zonnevijlle et al. [32] studied the preparation and characterization of Heteropolytungstates. They listed the structural properties for all polytungstate series concerning the number of cations in the final compound. Thus, the values of lattice parameters (a and c) of our polyoxometalate were conducted and listed in Table 1, as well as those of D.
Figure 2 presents the spectrum of FTIR for {CoSiW11} nanoparticles. The transmission spectrum points out to a strong absorption band ranging from 694 to 1000 cm−1, revealing the characteristic vibrational peaks for Keggin structure. The peak observed at 1630 cm−1 is assigned for the deformation vibrations of water molecule δ(H–O–H). The presence of anti-symmetric expansion vibration of νas(Si–O), anti-symmetric expansion vibration of νas(W–O), symmetric expansion vibration of νs(W–O–W) and anti-symmetric expansion vibrations of the νas(W–O–W) bridges [33] correspond to wavenumbers 1000, 955, 899 and 790 cm−1, respectively. Compared with the spectrum of Keggin precursor K8[α-SiW11O39]·13H2O, namely mono-lacunary, we have observed a nominal change in the peak position from 800 to 790 cm−1 and an overlap of most of the peaks at the same wavenumber. Furthermore, the spectrum recorded the absence of other anti-symmetric expansion vibrations of νas(W–O–W) bridges at 720 cm−1. It may be related to the change from idealized Cs symmetry to a group symmetry of pseudo-Td point [34]. Al-Oweini et al. [20, 35,36,37] conducted similar results. In all their studies, they confirmed the purity, chemical bonding and the functional groups of our prepared POM nanoparticles.
Typical TEM image and particle size distribution for {CoSiW11} nanoparticles are depicted in Fig. 3. The microstructure formed exhibits uniform and compact structure of single molecules that are almost of similar and uniform particle size with an average of 26.75 nm. The aggregation of nanoparticles shown in this image represents the irregular particulate nature of the POM [38]. The presence of a large amount of tungsten prevents the electron beam from entering the spherical assemblies and has thus produced internal dark core spherical fragments [39].
3.2 XRD measurements
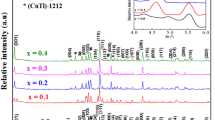
The prepared samples were analyzed structurally using XRD measurements. The XRD patterns of (CoSiW11)x/(Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ phase for all samples are shown in Fig. 4. The diffractogram indicates the formation of a tetragonal crystal structure with P4/mmm space group, confirming the formation of (Cu0.5Tl0.5)-1223 superconductor phase. Also, a characteristic peak appeared at 2θ = 5.1° corresponding to (001) crystal plane of the major phase. Moreover, a few additional peaks of small intensity have been observed in XRD scans, revealing the presence of unknown impurities and the formation of other superconductor and non-superconductor phases like (CuTl)-1234, (CuTl)-1212 and BaCuO2, respectively. Thus, no change in the peak positions of the major prepared phase is observed. Several ways were used to identify the presence of the compounds: comparing the obtained peaks with literature and using the MAUD program through CIF files. Also, JCPDS 00-038-1402 and JCPDS 00-042-0352 standards can give a help about the peaks of the resulting structures. The cell parameters and the relative volume fraction of (CuTl)-1223 phase were determined using the least-squares method and by comparing the peaks’ intensity, respectively [40]. Their values are listed in Table 2. The values of a and c remain approximately constant for all samples, which verify that the impregnated {CoSiW11} nanoparticles remain at the inter-grain boundaries without entering to the matrix of (CuTl)-1223 superconductor phase. The relative volume fraction of (Cu0.5Tl0.5)-1223 superconducting phase was calculated using the following formula:
where I is the peak intensity of the present phases above the baseline. It increases for x = 0.02 wt.% to reach its optimum value (92.70%), then decreases till x = 0.04 wt.% and after that enhances with the enhancement of nanoparticles’ concentration to x = 0.12 wt.%. In general, (CuTl)-1223 superconductor phase is stabilized by the addition of {CoSiW11} nanoparticles. Consequently, this enhancement would be related to the improvement of the connectivity between the grains after the addition of nanoparticles, which acts as pinning centers that fix the vortices [41]. For x > 0.02 wt.%, the rate of formation of other phases suppresses that of our prepared phase which may be attributed to the change within the reaction kinetics or within the viscosity of the transient liquid formation [42, 43]. Srour et al. [44] conducted a similar research upon studying the addition effect of BaSnO3 nanoparticles on (Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ phase, where the volume fraction was suppressed after a high addition of BaSnO3 nanoparticles and retarded the formation of the (CuTl)-1223 phase.
3.3 Scanning electron microscope and energy dispersive X-ray
Two SEM micrographs and EDX spectra were taken at two different positions of (CoSiW11)x/(Cu0.5Tl0.5)-1223 superconductor phase with x = 0.00, 0.02 and 0.12 wt.% and are shown in Figs. 5a−c, aʹ–cʹ, respectively. The pure (CuTl)-1223 sample demonstrates rectangular-shaped plates, indicating the formation of (Cu0.5Tl0.5)-1223 phase [45] as shown in Fig. 5a, aʹ. There is also a little number of spherical grains and irregular shapes that refer to the formation of the superconducting (Cu0.5Tl0.5)-1212 phase [46], (Cu0.5Tl0.5)-1234 phase and BaCuO2 non-superconductor composite.
Since the nanoparticles were prepared separately and mixed in (CuTl)-1223 matrix to obtain (CoSiW11)x/(Cu0.5Tl0.5)-1223 superconductor composite, the position where these nanoparticles can reside is only between grains and/or on the superconductor grain surfaces. It is clearly noticed that the population of inter-grain connectivity and voids observed in Fig. 5a, aʹ were reduced after the inclusion of {CoSiW11} nanoparticles, particularly, for x = 0.02 wt.%. These enhancements among the grains may be due to the presence of nanoparticles at the grain boundaries and the healing-up of voids and microcracks. As a result, this improved the intergranular coupling and inter-grain connectivity by reducing the inter-grain weak links. In contrast, the degradation of the grains presented in Fig. 5c, cʹ could have resulted from the agglomeration of impregnated nanoparticles at high concentrations (x = 0.12 wt.%). This could affect the formation rate of (CuTl)-1223 superconductor phase and thus decrease the relative volume fraction.
The EDX spectra were taken at different regions of the specimen, which show the presence of all elements in the final prepared compound. The position of the peaks for Tl, Ba, Ca, O and Cu does not change with the addition of nanoparticles, and the peaks corresponding to W, Si and Co elements appear at x ≥ 0.02 wt.%. The real atomic percentages for all compositions are recorded in Table 3. These percentage values of Ca, Tl, Ba, O and Cu elements are close to the stoichiometric composition of the (Cu0.5Tl0.5)-1223 superconductor matrix. Moreover, the enhancement in the atomic percentage of tungsten affirms the regular addition of {CoSiW11} nanoparticles in the prepared phase. Thus, XRD, SEM, EDX and FTIR data show that {CoSiW11} nanoparticles occupy the interstitial spaces among the grains and do not affect the crystal structure of (CuTl)-1223 matrix. Moreover, Fig. 5 is a good representation for showing the grains of superconductor phase and the distribution of {CoSiW11} nanoparticles (in the added nanoparticle’s samples), where both SEM images and EDX spectra showed similar results for the same sample as well as the nanoparticles were embedded between the grains.
3.4 FTIR measurements
The FTIR absorption spectra of (CoSiW11)x/(CuTl)-1223 phase for x = 0.00, 0.01, 0.02, 0.04, 0.08 and 0.12 wt.% in the wavenumber ranging from 700 to 400 cm−1are shown in Fig. 6a–f. The interesting oxygen phonon modes in the unit cell of (CuTl)-1223 superconductor phase are apical oxygen atoms, CuO2 planner oxygen atoms and Oδ atoms in the charge reservoir layer [47, 48], which are related to the wavenumber bands from 400 to 540 cm−1, 541 to 600 cm−1 and from 670 to 700 cm−1, respectively. Concerning the (Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ superconductor pure sample, the oxygen phonon modes were observed around i) 419, 429, 439, 448, 458, 468 and 495 cm−1 for apical oxygen of type Tl–OA–Cu(2), ii) 525 and 544 cm−1 for apical oxygen of type Cu(1)–OA–Cu(2), iii) 552 and 576 cm−1 for Cu(2)–Op–Cu(2) planar CuO2 and finally iv) 669 and 693 cm−1 for Oδ atoms.
Among all the impregnated samples with {CoSiW11} nanoparticles, it is obvious from Fig. 6b–f that Oδ modes stayed almost unaltered (669 and 693 cm−1). On the other hand, the slightly softening in most peak positions of both types of apical oxygen modes and also slightly hardening of planar oxygen mode could be attributed to the stresses and strain resulting from the relaxation and compression that would affect the length of the bond between the atoms of (CuTl)-1223 phase matrix. Moreover, the unsystematic change in the peak positions for many vibrational modes may be related to c-axis lengths, observed by XRD results, especially for nanoparticles addition concentrations in the range 0.04 ≤ x ≤ 0.12 wt.%. As a conclusion, the impregnated nanoparticles did not interfere with the composition nor changed the stoichiometry of the inter-grain material [44]. From the absorption spectra, the intensity of the Oδ vibrational modes for the impregnated samples was enhanced relative to the pure sample, confirming the existence of oxygen with a higher population through the charge reservoir layer in the doped compound [49]. In addition, a merge may have happened between the peak expected from POM nanoparticles at 694 cm−1 and that of Oδ atoms in superconductor samples at 693 cm−1.
3.5 Resistivity results
The temperature-dependent resistivity measurements of (CoSiW11)x/(CuTl)-1223 samples (x = 0.00, 0.01, 0.02, 0.04, 0.06, 0.08 and 0.12 wt.%) in the temperature range from 77 to 300 K are shown in Fig. 7. From these curves, all samples show a metallic-like behavior above Tc, followed by a single-step transition. This indicates the presence of good grain connectivity and displays a transition to the superconducting state below Tc. The inset of Fig. 7 displays the first-order derivative of the electrical resistivity (dρ/dT) as a function of T for the pure sample (x = 0.00 wt.%), where Tc corresponds to the maximum of this derivative, and the single peak indicates that the sample is single phase. The determined values of Tc and T0 are tabulated in Table 4. It was observed that Tc increases to its highest value (132 K) for x = 0.02 wt%, and then it behaves with an unsystematic variation. It decreases until x = 0.04 wt.% gets its lower value at 115.6 K, followed by an increase for x = 0.06 wt.% and then a decrease for x = 0.08 wt.% and finally an increase for x = 0.12 wt.% to reach Tc = 128.8 K. This non-monotonic change of these superconducting parameters between x = 0.04 and 0.12 wt.% could be associated with the multivalence of W that leads to the change in the oxygen content or the inhomogeneous distribution and/or agglomeration of {CoSiW11} nanoparticles at the grain boundaries [50]. It may also be due to the trapping of mobile carriers raised by oxygen vacancy disorder [51, 52]. The enhancement of Tc and T0 up to x = 0.02 wt.% could be due to the improvement of volume fraction of (CuTl)-1223 superconductor high Tc phase as determined from XRD results and SEM micrographs as well as due to the reduction in the weak links among the grains. The increase in Tc to 132 K (x = 0.02 wt.%) may be due to the increase of the c-axis which leads to an increase in the CuO layer [53]. On the other hand, the linear part of the resistivity curves follows the linear equation, Matthiessen rule, which gives the normal state resistivity (ρn) as
where β is the resistivity temperature coefficient and ρ0 is the residual resistivity [54]. The values of β, ρ0, ρroom (at 300 K), and the superconducting transition width (∆T = Tc − T0) for all samples, were determined and listed in Table 4. ∆T values are calculated to examine the purity of the prepared samples, where the low value of ΔT recorded for x = 0.02 wt.% agrees with the calculated volume fraction in XRD measurements. The variation of ρ0 and ρroom for (CoSiW11)x/(CuTl)-1223 as a function of the addition concentration x is also listed in Table 4. The normal state resistivity ρroom is noticed as a measure of the disorder of {CoSiW11} nanoparticles at grain boundaries; hence the increase in ρroom for x > 0.08 is due to the enhancement of the secondary phases, which increases the number of scattering centers and reduces the inter-granular coupling [55]. The lower value of ρ0 corresponds to the higher value of Tc, indicating the decrease of carrier-impurities scattering and that the samples are of high quality with fewer defects [56]. In Table 4, the decrease of β values in the samples with high Tc values may be due to the increase in the carrier-concentrations.
3.6 I–V measurements
The ability of the prepared superconducting samples to hold current is analyzed using the transport critical current density measurements at a low temperature (77 K). The variation of the electric field (E) as a function of the current density (J) for (CoSiW11)x/(CuTl)-1223 phase with x = 0.00, 0.01, 0.02, 0.06 and 0.12 wt.% is shown in Fig. 8. The obtained values of Jc are tabulated in Table 5. The behavior of the curves can be interpreted as follows: The current in the first portion is not large enough to cause vortex pair unbinding, which implies that V(I) = 0. Thus, the system is resistanceless [57]. In the second portion of the curve, where the values of voltage are low, the free vortices below Tc are created due to a finite penetration depth which is resolved by a thermally assisted flux flow. In the nonlinear portion, the voltage drop appears at a certain value of applied current Ic. This mechanism is described by flux creep, where the Lorentz force (FL) is equal to the Pinning force (FP). The last portion exhibits the increase in the linear curve; this is due to the vortex pair unbinding caused by the strong Lorentz force, (FL > FP), discussed by flux flow explanations. The variation of porosity, oxygen deficiency, crystallinity, grain morphology, the relative volume fraction and many other factors may affect Jc as well as Tc.
It is seen from Table 5 that the values of Jc decrease with the increase of nanoparticle concentration x from x = 0.01 up to 0.12 wt.%. The suppression in the Jc values, despite the improvement of Tc and phase formation, especially for x = 0.02 wt.%, may be due to the increment of the grain boundary resistance or the presence/growth of minor superconductor and non-superconductor phases impurities. But the high retrogression of Jc for x = 0.06 and 0.12 wt.% could be due to large agglomeration of {CoSiW11} nanoparticles among the grains, decrement of volume fraction and the increasing growth of impurity content. The obtained curves are investigated through the following relation:
where γ and n are material dependent constants. The n values, index of transition, describe the homogeneity of a superconductor and can be calculated from the slope of ln(E) in terms of ln(J). It is important to note that the higher the n values, the more homogeneous the prepared samples and the higher their quality. The highest n value of 21.299 observed in the x = 0.02 wt.% sample reflects these aforementioned properties and is consistent with the lowest residual resistivity value ρ0 = 0.015 (mΩ cm) and the highest Tc value of 132 K observed in this sample. Also, it is related to the pinning potential U according to the following equation [58]:
The values of n and U are also listed in Table 5. There is the proportionality of n and U with respect to the addition content of x (wt.%). Thus, with the addition of {CoSiW11} nanoparticles, the superconductor homogeneity becomes greater.
3.7 Excess conductivity analysis
Figure 9a–f presents the normalized excess conductivity Δσ/σroom versus the reduced temperature t in ln–ln plot for (CoSiW11)x/(CuTl)-1223 phase for x = 0.00, 0.01, 0.02, 0.06, 0.08 and 0.12 wt.%. It is clearly observed that the plotted curves are divided into four unlike regions (Sw, 2D, 3D and Cr).
According to Aslamazov–Larkin (AL) model [8], the excess conductivity \(\Delta \sigma\) is defined as follows:
where \({\rho }_{\mathrm{m}}\left(T\right)\) is the measured resistivity for each temperature \(T\). The values of \({\uprho }_{\mathrm{n}}\left(T\right)\) were determined from the linear fit of dc-resistivity curves for T ≥ 2Tc.
According to AL model, the normalized excess conductivity is given as follows:
where \(t=\frac{T-{T}_{c}}{{T}_{\mathrm{c}}}\) is the reduced temperature and Z is the conductivity exponent [59], which has the following values:
On the other hand, the constant \(C\) is the temperature-independent amplitude and is given by the following equations in 3D and 2D, respectively [3, 4]:
and
where ζc(0) parameter represents the zero-temperature coherence length along the c-axis while the effective layer thickness of the 2D is \(d\).
The interlayer coupling (I), Fermi velocity (vF) and Fermi energy (EF) were calculated as follows:
where kB is the Boltzmann’s constant and m* = 10.m0 is the electron effective mass [60]. The four different regions are linearly fitted and also the conductivity exponent Z is calculated from the slope. At the junction of various thermally activated processes, three crossover temperatures are found, i.e., TG = T3D−Cr, T2D−3D and TSw−2D. The TG is the crossover temperature of Ginzburg–Landau regime with three-dimensional conductivity regimes, the T2D−3D is the crossover temperature of 3D conductivity regime with 2D conductivity regimes and the TSw−2D is the crossover temperature of 2D conductivity regime critical fluctuations conductivity region.
The crossover temperatures are determined from the intersection between the linear parts of the different regions. The crossover temperatures for the samples are listed in Table 6.
Concerning the first region Sw, which corresponds to high temperature, the excess conductivity reduces sharply with ZSw-values starting from –2.867 to −3.113, denoting the prevalent role of short-wave fluctuation [61]. A crossover between the mean-field region and the short-wave fluctuations is detected at a temperature TSw–2D above Tc by decreasing the temperature. The existence of the 2D and 3D fluctuations are clearly observed in the graphs with the conductivity exponents varying as −0.972 ≤ Z2D ≤ −1.151 and −0.487 ≤ Z3D ≤ −0.606, respectively. In the 2D region, the charge carriers move in the conducting CuO2 planes resulting from the layered structure which characterizes the HTSCs, while in the 3D region, they move between the molecular planes and they are more influenced by thermal fluctuations. This means that the charge carriers tend to displace more freely in the global crystal before they make pairs. In the critical region, the conductivity exponent varies as -0.294 ≤ ZCr ≤ −0.37. Hence, the order parameter fluctuations become comparable in magnitude to the order parameter value [62].
The values of ζc(0), d and I are calculated according to Eqs. (8)–(10), respectively; the values of vF and EF are calculated from Eqs. (11) and (12) and all values are listed in Table 7. The increase in the coherence length along the c-axis and the effective layer thickness, with increasing the concentration of {CoSiW11} nanoparticles, confirm that the inter-planar coupling is enhanced in our samples. The values of vF and EF are increased with increasing {CoSiW11} concentration; this confirms that the addition of {CoSiW11} promotes the Josephson junction-like coupling between CuO2 planes. Moreover, the values of I are less than one, which confirms the low coupling between CuO2 planes and the crossover between 3 and 2D regions [63].
The variance of the microscopic parameters measured from the FIC study is following the experimental finding in the FTIR measurements, where the higher oxygen population in the charge reservoir layer increases the density of the charging carriers in the CuO2 conducting planes. The optimal density of mobile charging carriers in the conducting CuO2 planes results in an improvement in the material’s grain size, while also increasing the inter-grain connectivity and explaining the overall enhancement of superconductivity after introducing nanoparticles {CoSiW11} to the (CuTl)-1223 superconductor.
4 Conclusion
The effect of {CoSiW11} nanoparticles’ addition on the superconducting properties as well as the phase formation of (CuTl)-1223 matrix was thoroughly studied. We synthesized (CoSiW11)x/(CuTl)-1223 phase with 0.00 ≤ x ≤ 0.12 wt.% successfully by a single-step solid-state reaction technique at normal pressure. The lower nano-addition, 0.0 ≤ x ≤ 0.02 wt.%, increased the volume fraction of (Cu0.5Tl0.5)-1223 superconductor major phase up to its maximum value for x = 0.02 wt.%; then it was reduced with the further increase of x, as indicated by XRD results. The lattice parameters remain almost constant, thus indicating that the nanoparticles did not enter the unit cell; however, they exist only at the grain boundaries. The SEM micrographs show the formation of (CuTl)-1223 phase, which was enhanced after the addition of {CoSiW11} nanoparticles for x = 0.02 wt.%, thus confirming the results of relative volume fraction. Moreover, the elemental analyses of the samples, recorded by EDX, show that the mass percentage of all elements is closer to the stoichiometric composition of the host superconductor (Cu0.5Tl0.5)-1223 matrix as well as that of nanoparticles’ compound. The latter indication was also confirmed in the FTIR results, where the position of Oδ modes did not change and the fact that both types of the apical oxygen modes and the planar oxygen modes are slightly softened and hardened, respectively. Furthermore, the superconducting transition temperature, Tc, increases up to its maximum value for x = 0.02 wt.%, then decreases with a further increase of x, as indicated in electrical resistivity results. Although most of the superconducting parameters were improved after the addition of {CoSiW11} nanoparticles, the critical current density gave wondrous results. The values of Jc decrease with the increase of nanoparticle’s addition up to x = 0.12 wt.%. Finally, the conductivity exponents obtained from the plot of the excess conductivity Δσ/σroom with a reduced temperature t were about − 0.3, − 0.5, − 1.0 and − 3, corresponding to the fluctuation’s regions Cr, 3D, 2D and Sw, respectively. Correspondingly, the values coincide with the theoretical predictions. Moreover, the enhancement of the inter-planar coupling was confirmed from the increase in the coherence length along the c-axis, and the effective layer thickness as the added concentration of {CoSiW11} nanoparticles increased. Furthermore, the Josephson junction, similar to the coupling between CuO2 planes, was also promoted by the increase of vF and EF values. Finally, all the results obtained under our preparation conditions revealed that the sample with x = 0.02 wt.% had the best superconducting parameters.
References
M.H. Pu, W.H. Song, B. Zhao, X.C. Wu, Y.P. Sun, J.J. Du, J. Fang, Phys. C 361, 181 (2001)
H. Ihara, K. Tanaka, Y. Tanaka, A. Iyo, N. Terada, M. Tokumoto, F. Tateai, M. Kawamura, K. Ishida, S. Miyashita, T. Watanabe, Phys. B 284–288, 1085 (2000)
H. Ihara, K. Tanaka, Y. Tanaka, A. Iyo, N. Terada, M. Tokumoto, M. Ariyama, I. Hase, A. Sundaresan, N. Hamada, S. Miyashita, K. Tokiwa, T. Watanabe, Phys. C 341–348, 487 (2000)
M. Mumtaz, S.M. Hasnain, A.A. Khurram, N.A. Khan, J. Appl. Phys. 109, 023906 (2011)
H. Ihara, K. Tokiwa, K. Tanaka, T. Tsukamoto, T. Watanabe, H. Yamamoto, A. Iyo, M. Tokumoto, M. Umeda, Phys. C 282–287, 957 (1997)
I. Qasim, M. Waqee-ur-Rehman, M. Mumtaz, G. Hussain, K. Nadeem, K. Shehzad, J. Magn. Magn. Mater. 403, 60 (2016)
N.H. Mohammed, Phys. C 485, 95 (2013)
L.G. Aslamasov, A.I. Larkin, Phys. Lett. A 26, 238 (1968)
Y. Jia, P. Cheng, L. Fang, H. Luo, H. Yang, C. Ren, L. Shan, C. Gu, H.-H. Wen, Appl. Phys. Lett. 93, 032503 (2008)
S. Rajput, S. Chaudhary, J. Mater. 2013, 1 (2013)
G.H. Lee, R. Abd-Shukor, AIP Conf. Proc. 1528, 292 (2013)
K.T. Lau, S.Y. Yahya, R. Abd-Shukor, J. Appl. Phys. 99, 123904 (2006)
M.M. Elokr, R. Awad, A. Abd El-Ghany, A. Abou Shama, A. Abd El-wanis, J. Supercond. Nov. Magn. 24, 1345 (2011)
M. Mumtaz, Z. Iqbal, M.R. Hussain, L. Ali, M. Waqee-ur-Rehman, M. Saqib, J. Supercond. Nov. Magn. 31, 1315 (2018)
N. Hassan, N.A. Khan, J. Alloy. Compd. 471, 39 (2009)
N.H. Mohammed, A.I. Abou-Aly, I.H. Ibrahim, R. Awad, M. Rekaby, J. Alloy. Compd. 486, 733 (2009)
N.A. Khan, M. Irfan, Phys. C 468, 2341 (2008)
L. Youssef, G. Younes, R. Al-Oweini, J. Taibah Univ. Sci. 13, 274 (2019)
F. Yahya, H. El-Rassy, G. Younes, R. Al-Oweini, Int. J. Environ. Anal. Chem. 99, 1375 (2019)
R. Al-Oweini, B.S. Bassil, M. Itani, D.B. Emiroğlu, U. Kortz, Acta Cryst. C 74, 1390 (2018)
M. Natali, I. Bazzan, S. Goberna-Ferrón, R. Al-Oweini, M. Ibrahim, B.S. Bassil, H. Dau, F. Scandola, J.R. Galán-Mascarós, U. Kortz, A. Sartorel, I. Zaharieva, M. Bonchio, Green Chem. 19, 2416 (2017)
A. Bijelic, C. Molitor, S.G. Mauracher, R. Al-Oweini, U. Kortz, A. Rompel, ChemBioChem 16, 233 (2015)
R. Al-Oweini, A. Sartorel, B.S. Bassil, M. Natali, S. Berardi, F. Scandola, U. Kortz, M. Bonchio, Angew. Chem. Int. Ed. 53, 11182 (2014)
R. Al-Oweini, S. Aghyarian, H. El-Rassy, J. Sol–Gel Sci. Technol 61, 541 (2012)
N.E. Ghouch, R. Al-Oweini, R. Awad, Mater. Res. Express 6, 116001 (2019)
N.E. Ghouch, R. Al-Oweini, R. Awad, Appl. Phys. A 12, 1 (2019)
A. Müller, L. Dloczik, E. Diemann, M.T. Pope, Inorg. Chim. Acta 257, 231 (1997)
T. Huang, N. Tian, Q. Wu, Y. Yan, W. Yan, Mater. Chem. Phys. 165, 34 (2015)
N. Tian, M. Zhu, Q. Wu, W. Yan, A.B. Yaroslavtsev, Mater. Lett. 115, 165 (2014)
F.J. Berry, G.R. Derrick, M. Mortimer, Polyhedron 68, 17 (2014)
M.J. da Silva, L.C. de Andrade-Leles, R. Natalino, S.O. Ferreira, N.C. Coronel, Catal. Lett. 148, 1202 (2018)
Frans. Zonnevijlle, C. M. Tourne, and G. F. Tourne, Inorg. Chem. 21, 2742 (1982).
Z. Li, W. Li, X. Li, F. Pei, X. Wang, H. Lei, J. Inorg. Biochem. 101, 1036 (2007)
M. Sadakane, D. Tsukuma, M.H. Dickman, B.S. Bassil, U. Kortz, M. Capron, W. Ueda, Dalton Trans. 26, 2833 (2007)
R. Al-Oweini, H. El-Rassy, J. Mol. Struct. 919, 140 (2009)
R. Al-Oweini, B.S. Bassil, T. Palden, B. Keita, Y. Lan, A.K. Powell, U. Kortz, Polyhedron 52, 461 (2013)
R. Al-Oweini, B.S. Bassil, J. Friedl, V. Kottisch, M. Ibrahim, M. Asano, B. Keita, G. Novitchi, Y. Lan, A. Powell, U. Stimming, U. Kortz, Inorg. Chem. 53, 5663 (2014)
T.A.G. Duarte, A.C. Estrada, M.M.Q. Simões, I.C.M.S. Santos, A.M.V. Cavaleiro, M.G.P.M.S. Neves, J.A.S. Cavaleiro, Catal. Sci. Technol. 5, 351 (2015)
M. Saeidifar, H. Mirzaei, N. Ahmadi-Nasab, H. Mansouri-Torshizi, J. Mol. Struct. 1148, 339 (2017)
W. Abdeen, A. El-Tahan, R. Awad, A.I. Abou-Aly, E.M. El-Maghraby, A. Khalaf, Appl. Phys. A 122, 574 (2016)
K. Kocabaş, M. Gökçe, M. Çiftçioğlu, Ö. Bilgili, J. Supercond. Novel Magn. 23, 397 (2010)
M. Imran, M. Z. Khan, M. Waqee-Ur-Rehman, A. Ullah, S. Ahmed, K. Nadeem, and M. Mumtaz, J. Low. Temp. Phys. (2020)
M. Me, Barakat and K. Habanjar, J Adv Ceram 6, 100 (2017)
A. Srour, R. Awad, W. Malaeb, M.M.E. Barakat, J. Low Temp. Phys. 189, 217 (2017)
R. Awad, A.I. Abou-Aly, S. Isber, W. Malaeb, J. Phys. 43, 474 (2006)
P. Badica, A. Iyo, A. Crisan, Y. Ishiura, A. Sundaresan, H. Ihara, Supercond. Sci. Technol. 15, 964 (2002)
H. Salamati, P. Kameli, Solid State Commun. 125, 407 (2003)
V.P.S. Awana, S.K. Malik, W.B. Yelon, C.A. Cardoso, O.F. de Lima, A. Gupta, A. Sedky, A.V. Narlikar, Phys. C 338, 197 (2000)
M. Mumtaz, N.A. Khan, S.M. Hasnain, F. Ashraf, J. Supercond. Nov. Magn 25, 2291 (2012)
N.E. Ghouch, R. Al-Oweini, R. Awad, J. Low Temp. Phys. 200, 62 (2020)
A. Mellekh, M. Zouaoui, F. Ben-Azzouz, M. Annabi, M. Ben-Salem, Solid State Commun. 140, 318 (2006)
J. Zhang, F. Liu, G. Cheng, J. Shang, J. Liu, S. Cao, Z. Liu, Phys. Lett. A 201, 70 (1995)
K.A. Jasim, L.A. Mohammed, J. Phys. Conf. Ser. 1003, 012071 (2018)
Y. Slimani, E. Hannachi, M.K. Ben Salem, A. Hamrita, M.B. Salem, F.B. Azzouz, J. Supercond. Nov. Magn. 28, 3001 (2015)
A. Nishida, C. Taka, I. Shigeta, Phys. C 392–396, 349 (2003)
A.K. Ghosh, S.K. Bandyopadhyay, P. Barat, P. Sen, A.N. Basu, Phys. C 255, 319 (1995)
T.M. Katona, S.W. Pierson, Phys. C 270, 242 (1996)
S.E. Mousavi Ghahfarokhi, M. Zargar Shoushtari, Phys. B Condens. Matter 405, 4643 (2010)
A.K. Pradhan, S.B. Roy, P. Chaddah, C. Chen, B.M. Wanklyn, Phys. Rev. B 50, 7180 (1994)
I. Van Driessche, A. Buekenhoudt, K. Konstantinov, E. Bruneel, S. Hoste, Appl. Supercond. 4, 185 (1996)
L. Reggiani, R. Vaglio, A.A. Varlamov, Phys. Rev. B 44, 9541 (1991)
C.J. Lobb, Phys. Rev. B 36, 3930 (1987)
A.I. Abou-Aly, R. Awad, I.H. Ibrahim, W. Abdeen, Solid State Commun. 149, 281 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasser, A., Srour, A., El Ghouch, N. et al. Investigation of the physical properties of (Cu0.5Tl0.5)Ba2Ca2Cu3O10-δ impregnated with mono cobalt(II)-substituted Undecatungstosilicate Nanoparticles. Appl. Phys. A 126, 951 (2020). https://doi.org/10.1007/s00339-020-04083-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04083-3