Abstract
Three new coordination polymers, namely [Zn(tdc)(impy)]n (1), [Zn(4,4′-sdb)(impy)]n·1.5n(H2O) (2) [Zn(D-cam)(impy)]n (3) (H2tdc = 2,5-thiophenedicarboxylic acid, 4,4′-H2sdb = 4,4′-sulfonyldibenzoate, D-H2cam = D(+)-camphoric acid, impy = 2,6-bis(1-imdazoly)pyridine), have been synthesized under hydrothermal conditions. Structural analysis indicates that these three compounds all display 2D layer structures. Compound 1 presents a 3-connected hcb topology with the point symbol of {63}, compound 2 presents another 3-connected topological network with the point symbol of {82.10}, and compound 3 presents a 4-connected sql topology with the point symbol of {44.62}. Their thermal and luminescent properties are also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, coordination polymers (CPs) constructed from metal ions and organic ligands have aroused great attention owing to their potential applications in luminescence, magnetism, gas storage, heterogeneous catalysis, and so on [1–5]. Against this background, great progress and development have been made in the construction of functional CPs [6–9]. However, the structure control and prediction remains a great challenge in the realm of crystal engineering owing to some unpredictable factors, such as organic ligands, temperatures, pH values, solvents, the ratio of metal ions and ligand, counter ions and so on, which can affect the self-assembly process of CPs [10–14]. Therefore, deep understanding the relationship between the structures of CPs and various external stimuli is still important.
Among the strategies to construct CPs, the mixed-ligand approach has been proven to be an effective method to synthesize new CPs with intriguing topological frameworks [15–17]. The mixed-ligand approach usually involves two different ligands: organic carboxylate ligand and N-containing ligand. The combination of O-donor and N-donor ligands with metal ions can easily obtain CPs with variety of topological architectures. In addition, the structures of CPs are sensitive to the carboxylate ligands with different backbones [15, 18]. In this work, we are interested in the construction of CPs based on the mixed multicarboxylates and N-containing ligands. By changing organic carboxylic acid ligand, we successfully obtained three new CPs, namely [Zn(tdc)(impy)]n (1), [Zn(4,4′-sdb)(impy)]2n·3n(H2O) (2) [Zn(D-cam)(impy)]n (3) (H2tdc = 2,5-thiophenedicarboxylic acid, 4,4′-H2sdb = 4,4′-sulfonyldibenzoate, D-H2cam = D(+)-Camphoric acid, impy = 2,6-bis(1-imdazoly)pyridine). Single crystal X-ary structure analysis reveals that these three compounds display different 2D layered structures.
2 Experimental
2.1 Materials and Instrumentation
All reagents and solvents employed in this work were commercially available and used without further purification. Elemental analyses (C, H and N) were determined with an elemental Vario EL III analyzer. Fluorescence spectra of the solid samples were performed on an Edinburgh Analytical instrument FLS920. Powder X-ray diffraction (PXRD) analyses were recorded on a PANalytical X’Pert Pro powder diffractometer with Cu/Kα radiation (λ = 1.54056 Å) with a step size of 0.05°.
2.2 Synthesis of [Zn(tdc)(impy)]n (1)
A mixture of Zn(NO3)2·6H2O (0.030 g, 0.1 mmol), H2tdc (0.017 g, 0.1 mmol), impy (0.021 g, 0.1 mmol), NaHCO3 (0.016 g, 0.2 mmol) and H2O (10 mL) was placed in a 23 mL Teflon–lined stainless steel reactor under autogenous pressure at 160 °C for 60 h and then cooled to the room temperature at a rate of 5 °C/h. Colorless block crystals were obtained in 34 % yield based on Zn(NO3)2·6H2O. Anal. calcd. for C17H11N5O4SZn (446.76): C, 45.66; H, 2.46; N, 15.66 %. Found: C, 45.61; H, 2.52; N, 15.62 %. IR (cm−1): 3422(w), 1712(m), 1675(s), 1587(m), 1509(m), 1389(m), 1378(m), 1109(w), 1025(w), 958(m), 893(m), 826(m), 778(w), 690(w), 674(w), 589(w), 562(w).
2.3 Synthesis of [Zn(4,4′-sdb)(impy)]n·1.5n(H2O) (2)
The synthesis of 2 is similar to that of 1, but with 4,4,-H2sdb (0.03 g, 0.1 mmol) in place of H2tdc. Colorless block crystals were obtained in 42 % yield based on Zn(NO3)2·6H2O. Anal. calcd. for C50H40N10O15S2Zn2 (1215.82): C, 49.35; H, 3.29; N, 11.51 %. Found: C, 49.36; H, 3.989; N, 11.53 %. IR (cm−1): 3535(m), 3245 (w), 2490 (w), 1711 (w), 1598 (s), 1520 (s), 1418(w), 1243 (m), 1169 (w), 876 (w), 839 (w), 784(w), 670(m).
2.4 Synthesis of [Zn(D-cam)(impy)]n·(3)
The synthesis of 3 is similar to that of 1, but with D-H2cam (0.02 g, 0.1 mmol) in place of H2tdc. Colorless block crystals were obtained in 48 % yield based on Zn(NO3)2·6H2O. Anal. calcd. for C21H22N5O4Zn (473.83): C, 53.18; H, 4.64; N, 12.66 %. Found: C, 53.21; H, 4.63; N, 12.59 %. IR (cm−1): 3130(m), 2966(m), 1593(s), 1520(s), 1446(m), 1383(vs), 1358(s), 1271(w), 1233(w), 1112(m), 1088(s), 947(m), 810(w), 730(m), 658(m), 631(w).
2.5 X-ray Crystallography
Suitable single crystals of 1–3 were carefully selected under an optical microscope and glued to thin glass fibers. Structural measurements were performed with a computer–controlled mercury CCD diffractometer with graphite–monochromated Mo–Kα radiation (λ = 0.71073 Å) at T = 293(2) K. Absorption corrections were made using the SADABS program [19]. The structures were solved by direct methods and refined by full–matrix least–square methods on F 2 by using the SHELXL–97 program package [20]. All non–hydrogen atoms were refined anisotropically. The H atoms attached to their parent atoms of organic ligands were geometrically placed and refined using a riding model. Crystal data, as well as details of data collection and refinements of 1–3 are summarized in Table 1, selected bond lengths and angles are given in Table 2.
3 Results and Discussion
3.1 Description of Compound 1
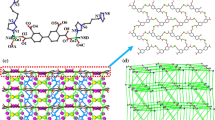
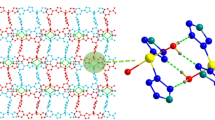
Single crystal X–ray structural analyses reveal that compound 1 is a 2D layer with 3-connected hcb topology. The asymmetric unit of 1 contains one Zn(II) ion, one tdc2− ligand and one impy ligand. As shown in Fig. 1a, Zn1 is coordinated by two carboxylate oxygen atoms (O2 and O3a) from two different tdc2− ligands and two nitrogen atoms (N5 and N5b) from two different impy ligands, displaying a tetrahedral coordination geometry. The Zn–O and Zn–N distances are in the range of 1.948(4)–1.979(4) Å, 1.991(5)–2.006(5) Å, respectively, which are similar to other Zn(II)-based compounds [21]. Two impy ligands bridge two Zn(II) ions together, forming a closed [Zn2(impy)2] cyclic unit (Fig. S1a). Then, these cyclic units are further connected together by the tdc2− ligands in bis-monodentate mode, generating a 2D layered structure (Fig. 2b). From a topological viewpoint, if the Zn(II) ions are viewed as 3-connected nodes, and tdc2− and impy ligands are viewed as connectors, this 2D sheet can be described as a 3-connected hcb topology with the point symbol of {63} (Fig. 1c). Finally, these 2D sheets are stacked parallel to each other, forming a 3D supramolecular framework via relatively weak van der Waals interactions (Fig. 1d).
3.2 Description of Compound 2
Compound 2 crystallizes in monoclinic C2/c space group with the asymmetric unit containing one Zn(II) ion, one 4,4′-sdb2− ligand and one impy ligand. As shown in Fig. 2a, each Zn(II) ion is five-coordinated by three carboxylate oxygen atoms (O1, O5b and O6b) from two different 4,4′-sdb2− ligands and two nitrogen atoms (N1 and N5b) from two different impy ligands, displaying a slightly distorted square pyramidal geometry. The Zn–O and Zn–N distances locate in the range of 1.986(2)–2.300(4) Å, 2.023(3)–2.031(3) Å, respectively. Like compound 1, two impy ligands also connect two Zn(II) ions into a closed [Zn2(impy)2] cyclic unit (Fig. S1b). Each 4,4′-sdb2− ligand links two different Zn(II) ions using its two carboxylate groups in chelating and monodentate mode, respectively. As a result, the [Zn2(impy)2] cyclic units are linked by 4,4′-sdb2− ligands to form a 2D layered structure (Fig. 2b). The lattice water molecules are bounded to the 2D layer via hydrogen bonds of O1w-H1wa…O5, O2w-H2wa…O2 and O2w-H2wb…O1 (H1w…O5 = 2.944 Å and ∠O1w-H1wa…O5 = 161°, O2w…O2 = 2.998 Å and ∠O2w-H2wa…O2 = 168°, O2w…O1 = 2.839 Å and ∠O2w-H2wb…O = 128°). Topological speaking, if the Zn(II) ions are simplified as 3-connected nodes, and 4,4′-sdb2− and impy ligands are viewed as connectors, this 2D sheet represents another 3-connected topological network with the point symbol of {82.10} (Fig. 2c). Finally, these 2D sheets are stacked in a –ABAB- sequence and hold together by van der Waals interactions to form a 3D supramolecular framework (Fig. 2d).
3.3 Description of Compound 3
Single crystal X-ray diffraction analysis reveals that compound 3 crystallizes in monoclinic system, P21/c space group and features a 2D layered structure with 4-connected sql topology. The asymmetric unit of 3 consists of one Zn(II) ion, one D-cam2− ligand and one impy ligand. As shown in Fig. 3a, each Zn(II) is tetrahedrally coordinated by two carboxylate oxygen atoms (O1 and O3a) from two different D-cam2− ligands and two nitrogen atoms (N1 and N5b) from two different impy ligands. The Zn–O and Zn–N distances fall in the range of 1.961(4)–2.109(8) Å, 2.024(4)–2.043(4) Å, respectively. Each D-cam2− ligand links two Zn(II) ions with its two carboxylate groups in uniform monodentate mode. As a result, Zn(II) ions are connected by D-cam2− ligands to form a 1D chain (Fig. 3b). Adjacent 1D chains are further bridged together by the impy ligands, forming the final 2D layered structure (Fig. 3c). From a topological viewpoint, this 2D sheet of 3 can be simplified into a 4-connected sql tological net with the point symbol of {44.62} by viewing Zn(II) ions as 4-connected nodes, D-cam2− and impy ligands as linkers, respectively (Fig. 3d). Under the effect of van der Waals interactions, these 2D sheets are stacked parallel to each other, generating a 3D supramolecular framework (Fig. 3e).
3.4 Powder X-ray Diffraction Patterns and Thermal Analysis
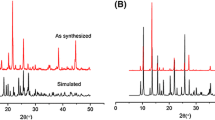
In order to confirm the purity of the bulk samples, PXRD experiments for compounds 1–3 were performed. As shown in Fig. S2, the peak positions of the lines of the experimental and simulated patterns are in good agreement, indicating the pure phased of these compounds. In addition, thermal analysis experiments were also done to characterize the thermal stabilities of compounds 1–3 (Fig. S3). Compound 1 is thermal stable under 300 °C and then continuously losses weight to afford ZnO residue at 800 °C (obsd: 18.45 %, calcd: 18.13 %). The TGA curve of compound 2 displays two mains steps of weight loss: the first step occurring in the range of 80–110 °C corresponds to the departure of free lattice water molecules (obsd: 4.82 %, calcd:4.44 %). The second step occurs from 300 to 680 °C corresponding to the decomposition of framework, affording the final residue of ZnO (obsd: 13.98 %, calcd: 13.32 %). For compound 3, it has no obvious weight loss in the temperature range of 30–364 °C. After 364 °C, it continuously losses weight until 760 °C, affording the ZnO residue (obsd:17.62 %, calcd: 17.09 %).
3.5 Photoluminescent Property of Compounds 1–3
Considering that luminescent CPs based on d10 metal centers are of current research interest due to their potential applications in photochemistry, electroluminescent displays, chemical sensors and so on [22–24]. Hence, the solid state luminescencent properties of compounds 1–3 are investigated at room temperature (Fig. 4). The main emission peaks of compounds 1–3 were observed at 413 nm (λ ex = 320 nm), 411 nm (λ ex = 315 nm), 408 nm (λ ex = 312 nm), respectively. Acoording to previously reported literatures, H2tdc ligand has a maximum emission band at 382 nm (λ ex = 320 nm), 4,4′-H2sdb has two emission bands at 328 nm and 341 nm (λ ex = 280 nm), and D-H2cam has no emissions in the range of visible light [25, 26]. In order to ascertain the luminescence origin, the luminescent property of free impy ligand was also investigated at the same conditions. The free impy ligand shows a luminescent emission band maxima at 405 nm (λ ex = 310 nm). Notably, the emission spectra of compounds 1–3 are similar to that of the free impy ligand, which indicates that the luminescence of compounds 1–3 may be mostly derived from the impy intraligand charge transfer [27]. In addition, the corresponding decay lifetimes for compounds 1–3 are 2.87, 2.34, 2.72 ns, respectively.
4 Conclusion
In summary, three new Zn(II) CPs constructed from different dicarboxylic acid and auxiliary impy ligands have been successfully synthesized and structurally characterized. The results reveal that the backbones of the dicarboxylate ligands play an important role in determining the structural diversity. This work also demonstrates that mixed-ligand strategy is a powerful method towards the synthesis of functional CPs.
5 Supplementary Material
CCDC Nos. 1033061-1033063 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html [or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (internat.) +44 1223/336 033; E-mail: deposit@ccdc.cam.ac.uk].
References
D.J. Tranchemontagne, J.L. Mendoza-Cortes, M. O’Keeffe, O.M. Yaghi, Chem. Soc. Rev. 38, 1257 (2009)
H.X. Zhang, H.R. Fu, H.Y. Li, J. Zhang, X.H. Bu, Chem. Eur. J. 19, 11527 (2013)
M.C. Das, S.C. Xiang, Z.J. Zhang, B.L. Chen, Angew. Chem. Int. Ed. 50, 10510 (2011)
B.L. Chen, L.B. Wang, F. Zapata, G.D. Qian, E.B. Lobkovsky, J. Am. Chem. Soc. 130, 6718 (2008)
S. Bureekaew, H. Sato, R. Matsuda, Y. Kubota, R. Hirose, J. Kim, K. Kato, M. Takata, S. Kitagawa, Angew. Chem. Int. Ed. 122, 7826 (2010)
X. Zhang, Y.Y. Huang, Y.G. Yao, J. Mol. Struct. 1024, 146 (2012)
H.N. Miras, J. Yan, D.L. Longa, L. Cronin, Chem. Soc. Rev. 41, 7403 (2012)
B.J. Burnett, P.M. Barron, W. Choe, CrystEngComm 14, 3839 (2012)
Y.Q. Lan, H.L. Jiang, S.L. Li, Q. Xu, Adv. Mater. 23, 5015 (2011)
X. Zhang, J.X. Yang, J. Zhang, J.K. Cheng, M.L. Sun, Y.G. Yao, Inorg. Chem. Comm. 14, 986 (2011)
X.L. Tang, W. Dou, J.A. Zhou, G.L. Zhang, W.S. Liu, L.Z. Yang, Y.L. Shao, CrystEngComm 13, 2890 (2011)
J.K. Sun, W. Li, L.X. Cai, J. Zhang, CrystEngComm 13, 1550 (2011)
P.X. Yin, J. Zhang, Y.Y. Qin, J.K. Cheng, Z.J. Li, Y.G. Yao, CrystEngComm 13, 3536 (2011)
Z.Z. Xue, T.L. Sheng, Q.L. Zhu, D.Q. Yuan, Y.L. Wang, C.H. Tan, S.M. Hu, Y.H. Wen, Y. Wang, R.B. Fu, X.T. Wu, CrystEngComm 15, 8139 (2013)
F. Guo, F. Wang, H. Yang, X.L. Zhang, J. Zhang, Inorg. Chem. 51, 9677 (2012)
S.M. Chen, T.T. Lian, Solid State Sci. 2012, 14 (1055)
J. Chen, C.P. Li, M. Du, CrystEngComm 2011, 13 (1885)
F. Guo, B.Y. Zhu, G.L. Xu, M.M. Zhang, X.L. Zhang, J. Zhang, J. Solid State Chem. 199, 42 (2013)
G.M. Sheldrick, SADABS (University of Göttingen, Göttingen, 1996)
G.M. Sheldrick, SHELXS 97, program for solution of crystal structures (University of Göttingen, Göttingen, 1997)
G.B. Yang, Z.H. Sun, Inorg. Chem. Comm. 29, 94 (2013)
E.C. Yang, Z.Y. Liu, T.Y. Liu, L.L. Li, X.J. Zhao, Dalton Trans. 40, 8132 (2011)
J. Pan, F.L. Jiang, D.Q. Yuan, X.C. Shan, M.Y. Wu, K. Zhou, Y.L. Gai, X.J. Li, M.C. Hong, CrystEngComm 15, 5673 (2013)
L. Liu, C. Huang, Z.C. Wang, D.Q. Wu, H.W. Hou, Y.T. Fan, CrystEngComm 15, 7095 (2013)
L. Zhou, C.G. Wang, X.F. Zheng, Z.F. Tian, L.L. Wen, H. Qu, D.F. Li, Dalton Trans. 42, 12375 (2013)
D.Q. Wu, W. Meng, L. Zhang, L. Liu, H.W. Hou, Y.T. Fan, Inorg. Chim. Acta 405, 318 (2013)
Z.Y. Chen, Z.E. Lin, T. Stips, S. Dehnen, Inorg. Chem. Comm. 14, 137 (2011)
Acknowledgments
This work was supported by Grants from the Sci-technical Support Project of Hebei Province (14227115D)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Zhou, P., Dong, Y. et al. Structural Diversity of a Series of 2D Zn(II) Coordination Polymers Tuned by Different Dicarboxylic Acids Ligands. J Inorg Organomet Polym 25, 650–656 (2015). https://doi.org/10.1007/s10904-014-0134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-014-0134-9