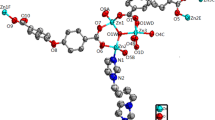
Abstract
A new coordination polymer, namely, {[Zn(DTPP)(H2O)2]·H2O}n (H2DTPP = 3-(3,5-di(2H-tetrazol-5-yl)phenoxy)pyridine, compound 1), was successfully assembled by using a new semirigid tripodal nitrogen-containing heterotopic ligand and characterized by single-crystal X-ray diffraction, elemental analysis, infrared spectroscopy, TG, and luminescence. X-ray single-crystal diffraction analysis revealed that compound 1 crystallizes in an orthorhombic crystal system with space groups of Ibca. It is a 2D coordination network with (4·82) topology, and the 2D networks are further connected by hydrogen bonds between coordinated water molecules and tetrazole N atoms to form a 3D supramolecular framework. Moreover, compound 1 exhibits intense blue emission centered at 345 nm upon excitation at 276 nm and has the ability to sense Fe3+ via photoluminescence quenching.
Graphical Abstract
A new coordination polymer synthesized from a new semirigid tripodal nitrogen-containing heterotopic ligand, {[Zn(DTPP)(H2O)2]·H2O}n, was characterized.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As new crystalline materials have diverse structures and extensive applications in gas adsorption and separation, catalysis, luminescent sensing, and so on [1,2,3,4,5,6], the construction of coordination polymers (CPs) has still attracted much attention. In general, new CPs can be obtained by regulating their building blocks (such as organic ligands, metal ions, and counter anions) or/and assembly conditions (such as temperature, pH, concentration, and solvent). Among them, organic ligands play a main role in the formation of new CPs with desirable structures and properties. Tripodal nitrogen-containing ligands are a group of excellent ligands that have been applied to construct various coordination supramolecules, including CPs and metal-organic cages [7,8,9,10,11,12]. However, the most frequently used tripodal nitrogen-containing ligands are homotopic, and the three coordinating groups are identical. However, those based on heterotopic tripodal nitrogen-containing ligands have been relatively less explored [13].
Recently, we focused on the construction of new CPs based on a series of rigid tripodal nitrogen-containing ligands containing two kinds of donor groups, such as tetrazole-imdazole [13,14,15,16,17], tetrazole-pyridine [18, 19], and pyrazole-imidazole ligands [20, 21]. In this study, a new tetrazole-pyridine ligand, 3-(3,5-di(2H-tetrazol-5-yl)phenoxy)pyridine (H2DTPP), was selected to construct new CPs. A Zn(II) CP with a 2D network structure was successfully obtained and well characterized by single-crystal X-ray diffraction, elemental analysis, and infrared spectroscopy. Additionally, the thermal stability and photoluminescence properties of the selected compounds were investigated in detail.
Experimental
Materials and Methods
The ligand H2DTPP was purchased from Shanghai Kylpharm Co., Ltd., through its customized service. All the other reagents were of analytical grade quality and were obtained from Guangzhou Chemical Reagent Factory without further purification. Infrared (IR) spectroscopy was performed on a Nicolet FT-IR-170SX spectrometer with KBr pellets in the 400−4000 cm−1 region. Powder X-ray diffraction (PXRD) patterns were obtained by an Ultima IV diffractometer with a scan speed of 12°/min at 40 kV and 40 mA with a Cu-target tube and a graphite monochromator. Elemental analysis (C, H, and N) was performed with a Perkin-Elmer 240 C elemental analyzer. Thermogravimetric analysis (TGA) was performed on a NETZSCH STA 449 C thermogravimetric analyzer at a heating rate of 10 °C/min under a nitrogen atmosphere. The luminescent spectra for H2DTPP and compound 1 were determined by using an Edinburgh FLS-900 spectrophotometer with a 150 W xenon lamp as the light source at room temperature.
Synthesis of Compound 1, {[Zn(DTPP)(H 2 O) 2 ]·H 2 O} n
A mixture of H2DTPP (0.03 mmol, 9.2 mg), Zn(NO3)2·6H2O (0.06 mmol, 17.8 mg), methanol (1.5 mL), DMF (1.5 mL), and H2O (3 mL) was stirred for 5 min. Then, the solution was sealed in a conical flask and left for 8 days to obtain pale yellow crystals in solution. The crystals were filtered and washed with deionized water and dried in air (yield: 53% based on H2DTPP). Anal. Calcd. for C104H104N72O32Zn8: C, 36.77; H, 3.09%; N, 29.68%; Found: C, 36.69%; H, 3.12%; N, 29.76%. IR (KBr pellet, cm−1): 3222(s), 1620(m), 1574(m), 1438(s), 1243(s), 1120(w), 1060(w), 934(m), 887(m), 790(m), 691(m).
X−ray Crystallography
Single-crystal X-ray diffraction data for compound 1 were recorded on a Rigaku SuperNova Dual Atlas diffractometer with graphite-monochromated Cu Ka radiation (λ = 1.54184 Å) at 100 K. Data reduction, scaling, and absorption corrections were performed using CrysAlisPro 1.171.41.119a [22]. Empirical absorption correction using spherical harmonics was implemented in the SCALE3 ABSPACK scaling algorithm. Using Olex2 [23], structural solution and refinement based on F2 were performed with the SHELXS-2018 and SHELXL-2018 program packages [24, 25], respectively. Anisotropic atomic displacement parameters were applied to all non-hydrogen atoms during refinement. The hydrogen atoms were added geometrically. The details of the crystal parameters, data collection, and refinement for the title compound are shown in Table 1, and selected bond lengths and angles are displayed in Table 2.
Results and Discussion
Description of the Crystal Structure
Single-crystal X-ray diffraction revealed that compound 1 crystallizes in an orthorhombic system with the space group Ibca. The asymmetric unit contains one Zn(II) ion, one DTPP2- ligand, two coordinated water molecules, and one uncoordinated water molecule. The Zn(II) ion adopts a five-coordinate triangular bipyramidal coordination mode, coordinating with two N atoms from two different tetrazole groups and one N atom from the pyridine group on the triangular plane and coordinating with two coordinated water molecules in the axial direction (Fig. 1). The Zn–N bond lengths range from 2.008(3) to 2.041(3) Å, and the Zn–O bond distances are 2.153(3) and 2.161(3) Å (Table 2), all of which are comparable to those observed for other related Zn(II) CPs based on tetrazole-based and pyridyl-based ligands [26, 27]. The DTPP2- ligand adopts a μ3-coordinate mode in which it binds to three Zn(II) ions with two tetrazole groups and one pyridyl group (Fig. 1). Although the coordination modes of the two tetrazolyl groups in the DTPP2- ligand are the same, the dihedral angles between the two tetrazole groups and the central benzene ring are different: 11.71(14)° and 7.65(14)°, respectively.
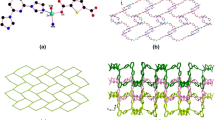
The combination of Zn(II) ions and DTPP2- ligands gives rise to a 2D coordination network extending along the ac plane, as shown in Fig. 2. From a topological perspective, both Zn(II) ions and DTPP2- can be seen as three connected nodes; thus, the overall 2D network can be simplified as a (4·82) topological network. Such topology is usually observed in 2D coordination polymers [28,29,30].
Furthermore, the adjacent 2D networks are connected by various O–H···N hydrogen bonds between the coordinated H2O molecule and the uncoordinated N atoms on the tetrazole group (Table 3), resulting in a 3D supramolecular framework, as shown in Fig. 3a and Fig. 3b. Small pores along the b direction were observed, as shown in Fig. 3c. It exhibits a percent effective free volume of 10.9% (a total potential solvent volume of 805 Å3 out of every unit cell volume of 7353 Å3), as calculated by PLATON software [31].
Phase Purity and Thermal Stability
The phase purity of compound 1 was determined by powder X-ray diffraction (PXRD). As shown in Fig. 4, the position of the diffraction peak obtained from the experimental pattern is basically consistent with that from the simulated pattern, indicating that compound 1 has a high phase purity. The dissimilarity in reflection intensity between them may be attributed to the preferred orientation of the compound 1 sample during data collection.
The thermal stability of compound 1 was measured by thermogravimetric analysis (TGA) from room temperature to 800 °C in a nitrogen atmosphere at a rate of 10 °C min−1. As displayed in Fig. 5, the weight loss of 4.5% from room temperature to 110 °C may be due to the release of uncoordinated water molecules (Calc. 4.2%). The secondary weight loss of 9.0% from 110 to 150 °C may be attributed to the removal of two coordinated water molecules (Calc. of 8.4%). A sharp weight loss is observed above 270 °C, indicating decomposition of the coordination framework.
Fluorescence Properties
Zn(II) CPs generally exhibit photoluminescent properties [32, 33]. Therefore, the photoluminescence of compound 1 was evaluated. As shown in Fig. 6, compound 1 exhibited strong blue luminescence with a maximum emission band at 345 nm when excited at 276 nm in the solid-state at room temperature. For comparison, the photoluminescence of H2DTPP was also measured. The results showed an emission band centered at 350 nm when excited at 248 nm. Compound 1 only exhibited a slight blueshift of 5 nm compared with that of H2DTPP. In addition, the shapes of the emission peaks for compound 1 and H2DTPP are similar. Thus, the emission of compound 1 may be attributed to intraligand transitions.
Properties of Metal Ion Sensing
The ability of compound 1 to sense metal ions was investigated. The selected metal ions were added to the aqueous suspension of compound 1, and their photoluminescence spectra were recorded. The results showed that the emission of the compound 1 suspension strongly quenched after the addition of Fe3+, Cu2+, and Ag+, indicating that compound 1 may have the ability to sense these ions and may be most sensitive to Fe3+ (Figs. 7a and S1). The sensitivity of the compound 1 suspension for detection in water was further determined. The photoluminescence intensities of the compound 1 suspensions with different Fe3+, Cu2+, and Ag+ concentrations are shown in Figs. 7b and S2-S4. As shown in Fig. 7b, the luminescence intensity at 345 nm decreased as the concentration of Fe3+ increased. The linear relationship between I0/I and the Fe3+ concentration is in the range of 5×10−4–1×10−3 M. Consequently, the LOD is calculated to be 1.30×10−5 M. These LODs are more moderate than those of some reported MOF sensors [34, 35]. For Cu2+ and Ag+, the LODs are 5.64×10−2 and 5.06×10−2, respectively, which are higher than those for Fe3+.
a Luminescence spectra of compound 1 in aqueous solutions containing various cations (0.5 M). b Luminescence spectra of compound 1 with various concentrations of Fe3+ ions in aqueous solutions. c The PXRD of compound 1 before and after immersing in aqueous solutions containing Fe3+, Cu2+, and Ag+. d Compare the UV‒vis spectra of Fe3+, Cu2+, and Ag+ with the excitation and emission spectra of compound 1
The quenching of the fluorescence emission of MOFs by metal ions may be due to structural collapse, energy transfer, competition, etc. [35,36,37,38,39]. The PXRD pattern of compound 1 after immersion in solutions containing 0.1 M Fe3+, Cu2+, and Ag+ ions showed that the crystal structures were retained (Fig. 7c); thus, quenching was not caused by structural collapse. In addition, the FT-IR spectra (Fig. S5) of the ion-treated sample remained unchanged, further revealing that compound 1 has a stable crystal structure. To determine whether energy transfer and/or competition occurred. The liquid UV‒Vis spectra of Fe3+, Cu2+, and Ag+ were measured. The results showed that they have a broad absorption between 250 and 350 nm for Cu2+ and Ag+ and between 250 and 375 nm for Fe3+. These bands covered the majority of the absorption band of compound 1. Consequently, these metal ions competed to absorb the energy of the light source when excited light passed, resulting in quenching [39]. For the Fe3+ ion, there is also some overlap between the absorption spectrum of Fe3+ and the emission spectrum of compound 1; therefore, energy transfer from compound 1 to the Fe3+ ion also contributed to fluorescence quenching.
Conclusions
In conclusion, we successfully constructed and structurally characterized a new Zn(II) CPs synthesized from a new tripodal N-containing heterotopic ligand. The compound displays a 2D network structure with a (4·82) topology and exhibits blue photoluminescent emission. In addition, it showed the ability to sense Fe3+ via photoluminescence quenching. This work indicates that a tripodal N-containing heterotopic ligand with two pyridyl and tetrazolyl groups is an effective organic ligand for the construction of new CPs.
Data Availability
Crystallographic data of the compound 1 (CSD 2339096) was deposited at the Cambridge Crystallographic Data Centre and could be obtained free of charge upon application to CCDC, 12 Union Road, Cambridge CB21EZ, UK [fax: (+ 44) 1223-336-033; email: deposit@ccdc.cam.ac.uk].
References
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM (2013) Science 341:1230444
Chen G, Liu G, Pan Y, Liu G, Gu X, Jin W, Xu N (2023) Chem Soc Rev 52:4586–4602
Siu B, Chowdhury AR, Yan Z, Humphrey SM, Hutter T (2023) Coord Chem Rev 485:215119
Fan Y, Zheng H, Labalme S, Lin W (2023) J Am Chem Soc 145:4158–4165
Iliescu A, Oppenheim JJ, Sun C, Dincǎ M (2023) Chem Rev 123:6197–6232
Li JR, Sculley J, Zhou HC (2012) Chem Rev 112:869–932
Zhang JP, Zhang YB, Lin JB, Chen XM (2012) Chem Rev 112:1001
Yoshioka S, Inokuma Y, Duplan V, Dubey R, Fujita M (2016) J Am Chem Soc 138:10140
Brunet G, Safin DA, Korobkov I, Cognigni A, Murugesu M (2016) Cryst Growth Des 16:4043
Schmidt BM, Osuga T, Sawada T, Hoshino M, Fujita M (2016) Angew Chem Int Ed 55:1561
Zheng SR, Yang QY, Liu YR, Zhang JY, Tong YX, Zhao CY, Su CY (2008). Chem Commun. https://doi.org/10.1039/B711457E
Fu HR, Xu ZX, Zhang (2015) J Chem Mater 27:205
Feng Y, Cai SL, Gao Y, Zheng SR (2018) J Solid State Chem 265:64–71
Deng SQ, Mo XJ, Zheng SR, Jin X, Gao Y, Cai SL, Fan J, Zhang WG (2019) Inorg Chem 58:2899–2909
Deng SQ, Miao YL, Tan YL, Fang HN, Li YT, Mo XJ, Cai SL, Fan J, Zhang WG, Zheng SR (2019) Inorg Chem 58:13979–13987
Feng Y, Zou MY, Hu HC, Li WH, Cai SL, Zhang WG, Zheng SR (2022) Chem Commun 58:5013–5016
Feng Y, Wu LH, Zhang CH, Zhou BX, Zheng SR, Zhang WG, Cai SL, Fan J (2023) Dalton Trans 52:12087–12097
Deng SQ, Mo XJ, Cai SL, Zhang WG, Zheng SR (2019) Inorg Chem 58:14660–14666
Wang GQ, Huang JF, Huang XF, Deng SQ, Zheng SR, Cai SL, Fan J, Zhang WG (2021) Inorg Chem Front 8:1083–1092
Xian JY, Yang KL, Wang HN, Feng ML, Cai SL, Song HY, Zheng SR, Zhang WG (2021) Inorg Chem Commun 130:108720
Xian JY, Huang ZY, Xie XX, Lin CJ, Zhang XJ, Song HY, Zheng SR (2022) Chinese J. Struct Chem 42:100005
CrysAlisPro Software System (2021) Rigaku Oxford Diffraction
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339–341
Sheldrick GM (2015) Acta Crystallogr Sect A Found Adv 71:3–8
Sheldrick GM (2015) Acta Crystallogr Sect C Struct Chem 71:3–8
Lin H, Liu Y, Ji M, Zhao J, Liu G (2020) Inorg Chem Commun 112:107734
Yu H, Sun J (2021) CrystEngComm 23:1744–1755
Li K, Blatov VA, Fan T, Zheng TR, Zhang YQ, Li BL, Wu B (2017) CrystEngComm 19:5797–5808
Li TT, Liu YM, Wang T, Zheng SR (2017) Inorg Chem Commun 84:5–9
Han YH, Zeng DR, Li JM, Wang XL, He KH, Fang ZJ, Shi ZF (2018) J Coord Chem 71:2674–2690
Spek AL (2005) PLATON, a multipurpose crystallographic tool. Utrecht University, Utrecht
Naresh K, Roopa BV, Kumar M (2021) Coord Chem Rev 427:213550
Xie W, Wu J, Hang X, Zhang H, Shen K, Wang Z (2021) Front Chem 9:708314
She M, Wang Z, Chen J, Li Q, Liu P, Chen F, Zhang S, Li J (2021) Coord Chem Rev 432:213712
Mukherjee D, Pal A, Pal SC, Saha A, Das MC (2022) Inorg Chem 61:16952–16962
Han LJ, Yan W, Chen SG, Shi ZZ, Zheng HG (2017) Inorg Chem 56:2936–2940
Wang H, Qin J, Huang C, Han Y, Xu W, Hou H (2016) Dalton Trans 45:12710–12716
Han LJ, Kong YJ, Hou GZ, Chen HC, Zhang XM, Zheng HG (2020) Inorg Chem 59:7181–7187
Liu HF, Tao Y, Wu TX, Li HY, Zhang XQ, Huang FP, Bian HD (2022) Appl Organomet Chem 36:e6456
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of P. R. China (Grant Nos. 22171092, 92056113 and 22073032) and the Science and Technology Project of Qingyuan (2023KJJ013).
Author information
Authors and Affiliations
Contributions
Bing-Xun Zhou perform the syntheisis and crystal structure solution of compound 1, and wrote the original draft. Xian Lin, and Hui-Qi Xie perform the characterization of compound 1. Liang-Hua Wu and Chu-Hong Zhang help to prepare figures 1–7. Hai-Yan Song and Sheng-Run Zheng provide the conceptualization, review and editing the manuscript. Jun Fan help to analysis the data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, BX., Lin, X., Xie, HQ. et al. Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer from a New Semirigid Tripodal Nitrogen-Containing Heterotopic Ligand. J Chem Crystallogr (2024). https://doi.org/10.1007/s10870-024-01023-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10870-024-01023-4