Abstract
Five new europium(III) complexes Eu(p-EFBA)3.(H2O)2 (C1), Eu(p-EFBA)3.neo (C2), Eu(p-EFBA)3.batho (C3), Eu(p-EFBA)3.phen (C4), Eu(p-EFBA)3.bipy (C5) have been synthesized by using ethyl-(4-fluorobenzoyl) acetate (p-EFBA) as β-ketoester ligand and neocuproine (neo), bathophenanthroline (batho), 1,10-phenanthroline (phen) and 2,2-bipyridyl (bipy) as ancillary ligands. The synthesized complexes C1-C5 were characterized by elemental analysis, nuclear magnetic resonance spectroscopy (1H-NMR), infrared (IR) spectroscopy, thermogravimetric analysis (TG/DTG), UV-visible and photoluminescence (PL) spectroscopy. The relative study of luminescence spectra of complexes with the previously reported complexes of isomeric ligand (ortho and meta substituted ligand) indicate the higher luminescence properties of complexes as an effect of fluorine position on β-ketoester ligand. The para substituted ligand shows a remarkable effect on quantum efficiencies and Judd-Ofelt intensity parameters (Ω2, Ω4) of the complexes. The higher value of intensity parameter Ω2 associated with hypersensitive 5D0 → 7F2 transition of europium(III) ion revealing highly polarizable ligand field. The purposed energy transfer mechanism of complexes indicates the efficient energy transfer in complexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interesting luminescent behaviour of organic rare earth complexes initiate the utilization of these complexes in magnetism [1], sensors [2], fluoroimmunoassays [3], chelates for bioanalysis and imaging [4, 5], catalyst [6], luminescent devices and displays [7]. The synthesis of these metal complexes particularly involves the coordination between organic ligand and trivalent lanthanide ion, owing to smaller molar absorption coefficient inherent with 4f-4f transition of lanthanide ion which is not allowed according to Laporte’s rule [8–10]. The organic ligand inserted into the coordination environment of lanthanide ion should be able to harvest energy and effectively transfer it to the resonating level of metal ion. Following this pioneer investigation by Weissman and co-worker [11]; a wide variety of complexes involving organic ligand and Eu(III) and Tb(III) ions as main luminescent centers have been synthesized. Based on the different choices of chromophoric organic ligands, functionalised β-ketoester ligands are found to be best candidate for the synthesis of the complexes. Particularly the fluorination of ligand with low energy C-F oscillators reduces the non-radiative energy losses occur due to vibrational energy of C-H oscillators, resulting higher luminescence of metal complexes [12, 13]. In addition, the fluorination of ligand possesses significant effect on the properties of materials mainly contributed by its small size and highest electronegativity. The thermal stability is also affected by the substitution of fluorine on the ligand resulting longer luminescence life time [14]. Thus the fluorinated ligand provide a fascinating prospect in the synthesis of organic metal complexes which prompt us to synthesize a series of complexes based on organic ligand having different position of substituent fluorine and to study the effect of substituent position on the luminescent properties of these complexes.
The present communication deals with the synthesis of five new europium(III) complexes (C1-C5) by using ethyl-(4-fluorobenzoyl) acetate (p-EFBA) as fluorinated β-ketoester ligand and neocuproine, bathophenanthroline, 1,10-phenanthroline and 2,2-bipyridyl as ancillary ligands. All the synthesized complexes were characterized by elemental analysis, 1H–NMR, IR, thermogravimetric analysis, UV-visible and photoluminescence spectroscopy. The investigation is made on the basis of fluorine substituent position (ortho, meta and para) on the ligand and its effect on the luminescent properties is studied. The luminescence properties of complexes are compared and effect of different position of fluorine is studied. The calculated quantum efficiencies and intensity parameters of complexes further confirms the obtained results.
Experimental
Materials and Methods
The chemicals and solvent used were of reagent grade and used without further purification. Lanthanide nitrates and ancillary ligands used were purchased from commercially available sources. Elemental analyses (C, H and N) of complexes were performed on Perkin Elmer 2400 CHN elemental analyser. The concentration of Eu(III) ion determined by complexometric titration with ethylenediaminetetraacetate (EDTA). Nuclear magnetic resonance spectra (1H–NMR) were obtained on a Bruker Avance II 400 NMR spectrometer in deuterated chloroform solution by using tetramethylsilane (TMS) as standard. The Infrared spectra (IR) were carried out with Perkin Elmer Spectrum 400 spectrometer in the range of 4000–400 cm−1 using dried KBr disks. Shimadzu-2450 UV-Visible Spectrophotometer was employed to measure the UV-visible absorption spectra of the complexes. Thermogravimetric analyses (TG/DTG) were performed at heating rate of 10 °C/min on SDT Q600. Luminescent (PL) measurements were recorded with Hitachi F-7000 fluorescence spectrophotometer equipped with a xenon lamp as excitation source. The decay time of complexes were calculated by using software (FL solution of F-7000) of the spectrophotometer.
Synthesis
The synthetic route of ligand (p-EFBA) and corresponding europium(III) complexes C1-C5 shown in Scheme 1.
Synthesis of β-ketoester Ligand
To a solution of 4-fluorobenzoyl chloride and 2, 2-dimethyl-1,3-dioxane-4,6-dione in dry methylene chloride, addition of 4-(dimethylamino) pyridine was done at about 0 °C and the resulting mixture was stirred at room temperature for one hour. The mixture was washed with water and 1 N HCl and then dried by employing anhydrous sodium sulphate. The resulting product was dissolved with ethanol in toluene and heated on reflux condenser at 90 °C for 24 h. Subsequently solvents were allowed to evaporate at room temperature and product formed was recrystallized with ethanol [15]. Ligand ethyl-(4-fluorobenzoyl) acetate (p-EFBA) was obtained with 56% yield. IR(KBr): cm−1 3670 (b), 2984 (s), 1740 (s), 1692 (s), 1648 (s), 1587 (s), 1443 (s), 1412 (s), 1326 (w), 1275 (s), 1217 (s), 1148 (m), 1030 (s), 876 (s), 591 (m); 1H NMR (CDCl3, 400 MHz): δ 12.54 (s, 1H, enolic OH), 7.46 (m, 4H, Ar-H), 5.64 (s, 1H, C = CH), 3.96 (s, 2H keto CH2), 3.67 (q, 2H, OCH2CH3), 1.32 (t, 3H, OCH2CH3) ppm.
Synthesis of europium(III) Complexes C1-C5
The synthesis of ternary complexes C2-C5 follow the similar procedure as used for the synthesis of binary complex C1 but differed only in addition of 1 mmol ethanolic solution of ancillary ligand (neo batho, phen and bipy) respectively. For the synthesis of complex C1, an ethanolic solution of ligand p-EFBA (3 mmol) was added dropwise to the aqueous solution of europium nitrate (1 mmol) and stirred the mixture on magnetic stirrer. The pH of solution was adjusted (6.5–7) with 0.05 M sodium hydroxide solution, leading to white precipitates in mixture which were collected by filtration. The washing of precipitates with water and ethanol makes complete removal of free ligand from mixture. The white powder of complex C1 was obtained by drying solid residue in vacuum desiccator.
Eu(p-EFBA)3.(H2O)2 (C1): White powder, yield 79%; IR(KBr): cm−1 3612 (b), 2984 (s), 1733 (s), 1630 (s) 1517 (s), 1480 (s), 1385 (s), 1283 (s), 1215 (s), 1094 (m), 1036 (s), 943 (s), 875 (s), 732 (m), 705 (m), 423 (m); 1H NMR (CDCl3, 400 MHz): δ 7.24 (m, 12H, Ar-H), 4.34 (q, 6H, OCH2CH3), 2.69 (s, 3H, C = CH), 1.36 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC33H34O11F3: C, 48.82; H, 4.19; Eu, 18.24; Found: C, 48.54; H, 4.13; Eu, 18.19.
Eu(p-EFBA)3.neo (C2): White powder, yield 77%; IR(KBr): cm−1 2984 (s), 2360 (m), 1741 (s), 1628 (s), 1582 (s), 1520 (s), 1478 (s), 1283 (s), 1211 (s), 1171 (s), 1096 (s), 1036 (s), 946 (s), 876 (m), 737 (m), 709 (m), 530 (s), 420 (m); 1H NMR (CDCl3,400 MHz): δ 8.31 (d, 2H, Ar-H), 7.87 (m, 4H, Ar-H), 7.49 (m, 12H, Ar-H), 4.51 (q, 6H, OCH2CH3), 3.49 (s, 6H, Ar-H), 2.61 (s, 3H, C = CH), 1.34 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC47H42N2O9F3: C, 57.36; H, 4.27; N, 2.84; Eu, 15.05; Found: C, 57.21; H, 4.24; N, 2.71; Eu, 14.96.
Eu(p-EFBA)3.batho (C3): White powder, yield 82%; IR(KBr): cm−1 2981 (s), 1738 (s), 1617 (s), 1582 (s), 1518 (s), 1479 (s), 1370 (s), 1279 (s), 1212 (s), 1165 (s), 1096 (s), 1040 (s), 875 (s), 735 (m), 702 (m), 541 (s), 422 (m); 1H NMR (CDCl3, 400 MHz): δ 10.31 (d, 2H, Ar-H), 9.11 (d, 2H, Ar-H), 8.31 (m, 12H, Ar-H), 7.40 (d, 2H, Ar-H)), 6.76 (s, 10H, Ar-H), 4.20 (q, 6H, OCH2CH3), 2.52 (s, 3H, C = CH), 1.39 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC57H46N2O9F3: C, 61.76; H, 4.15; N, 2.52; Eu, 13.36; Found: C, 61.57; H, 4.04; N, 2.38; Eu, 13.22.
Eu(p-EFBA)3.phen (C4): White powder, yield 81%; IR(KBr): cm−1 2982 (s), 1733 (s), 1624 (s), 1576 (s), 1511 (s), 1481 (s), 1423 (s), 1382 (s), 1311 (s), 1223 (s), 1182 (s), 1092 (s), 1033 (s), 941 (m), 871 (s), 743 (m), 704 (m), 538 (s), 419 (m); 1H NMR (CDCl3, 400 MHz): δ 9.41 (d, 2H, Ar-H), 8.42 (m, 4H, Ar-H), 7.65 (d, 2H, Ar-H), 7.36 (m, 12H, Ar-H), 6.94 (d, 2H, Ar-H) 4.42 (q, 6H, OCH2CH3), 2.65 (s, 3H, C = CH), 1.38 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC45H38N2O9F3: C, 56.32; H, 3.97; N, 2.93; Eu, 15.49; Found: C, 56.13; H, 3.76; N, 2.82; Eu, 15.38.
Eu(p-EFBA)3.bipy (C5): White powder, yield 79%; IR(KBr): cm−1 2979 (s), 1742 (s), 1622 (s), 1583 (s), 1514 (s), 1483 (s), 1369 (s), 1272 (s), 1215 (s), 1161 (s), 1094 (s), 1036 (s), 942 (s), 876 (s), 732 (m), 708 (m), 529 (s), 428 (m); 1H NMR (CDCl3, 400 MHz): δ 8.55 (d,2H, Ar-H), 7.98 (d, 2H, Ar-H), 7.56 (m, 4H, Ar-H),7.30 (m, 12H, Ar-H), 4.31 (q, 6H, OCH2CH3), 2.66 (s,1H, C = CH), 1.29 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC43H38N2O9F3: C, 55.41; H, 4.08; N, 3.01; Eu, 15.89; Found: C, 55.29; H, 3.94; N, 2.91; Eu, 15.71.
Results and Discussion
Composition and Spectroscopic Characterization of Complexes
The elemental analysis data of C, H, N and europium(III) ion of complexes are represented in Table 1. The obtained results are compared with the theoretically calculated values which confirms the proposed formulae Eu(p-EFBA)3.(H2O)2, Eu(p-EFBA)3.neo, Eu(p-EFBA)3.batho, Eu(p-EFBA)3.phen, Eu(p-EFBA)3.bipy for complexes C1-C5 respectively.
The infrared spectral data of ligand p-EFBA and corresponding europium(III) complexes C1-C5 are listed in Table 2. In comparing with complexes C2-C5, ligand and complex C1 shows a broad band at 3670 cm−1 and 3612 cm−1 accredited with vibrational frequency of enolic O-H and absorbed or coordinated water molecule respectively. The absence of band in C2-C5 hinting the coordination of enolic O-H with the europium(III) ion. A shift in vibrational frequencies of > C = O and >C = C bands in complexes occurs due to extension of π-conjugation in complexes [16]. A strong band at 1576–1583 cm−1 in complexes C2-C5 assigned to C = N bands of coordinating ancillary ligands which are supported by the presence of Eu-N bands in complexes. The binding mode of ligand with europium ion further approved by the appearance of new absorption bands Eu-O at 419–428 cm−1 in the spectra of complexes C1-C5 [17, 18]. The observation indicates that europium ion coordinated through enolic O-H and > C = O group of ligand in complexes.
1H–NMR spectral analysis of ligand and complexes in the deuterated chloroform solution confirms the bonding mode of ligand with europium(III) ion. In the spectrum of ligand, there is a singlet at 12.54 ppm and 3.96 ppm attributed to enolic O-H and ketonic -CH2 peak respectively [19]. The coordination of ligand with europium ion is confirmed by the absence of specific proton peak (enolic and ketonic proton) in complexes. Due to paramagnetic behavior of europium ion, a singlet at 5.64 ppm in spectrum of ligand ascribed to methine proton move toward higher field value in spectra of complexes [20].
Thermal Behaviour (TG/DTG)
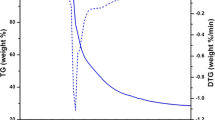
The thermal analysis (TG/DTG) of europium(III) complexes are carried out from ambient temperature to 1070 °C under the environment of nitrogen gas alongwith a heating rate of 10 °C min−1. Figure 1 depict the decomposition processes of complex Eu(p-EFBA)3.bipy, chosen as representative for all the complexes as an effect of similar pattern followed by them. The TG curve demonstrate the start of decomposition around 235 °C and continue upto 507 °C with a loss of weight 66.94% (theoretical weight loss 67.34%) indicating the loss of three organic ligand coordinating to europium ion in the complex. The second stage of decomposition occurs in the temperature range of 507 °C–959 °C with a loss of 16.65% weight (theoretical weight loss 16.75%) estimated with the removal of 2,2-bipyridine ligand from complex. The DTG curve of complex also support the decomposition pattern by the presence of peaks at 271 °C and 951 °C corresponds with the weight losses. Finally remaining product of approximately 14.58% associated with the oxides of europium. The analysis indicate the higher thermal stability of europium complexes.
UV-Visible Absorption Spectra
The UV-visible absorption spectra of ligand (p-EFBA) and its corresponding binary and ternary Eu(III) complexes are shown in Fig. 2. The measurements were made in chloroform solution at room temperature in the range 200–400 nm. The free ligand shows strong absorption at 318 nm. Although the spectral profile of europium(III) complexes were similar to that of ligand but chelating nature of ligand around central metal ion extends the conjugated system as well as electron density around ion which causing the absorption bands of complexes to shift toward higher wavelength [21].
Photoluminescence Spectra
Figures 3 and 4 represents the excitation and emission spectra of complexes obtained at room temperature by indexing the intense emission band 5D0 → 7F2 and broad excitation band 7F0 → 5D4 respectively. The excitation spectra of complexes consist of a broad band at 366–369 nm assigned to π → π* transition of ligand, alongwith a narrow peak at 463 nm (C1-C3, C5) and 466 nm (C4) associated with intraconfigurational 4f-4f transition 7F0 → 5D2 of europium ion [22]. The higher intensity of broad band clearly suggests the role of ligand in energy transfer processes to metal ion.
The emission spectra of europium(III) complexes C1-C5 shows magnificent luminescence behaviour on excitation with UV light. The spectra were measured in 500–700 nm range with slit width of 2.5 nm and voltage of 400PMT in the solid state. Figure 4 displays the characteristic emission peaks 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 of europium ion at 580 nm, 590 nm, 613 nm, 652 nm and 686 nm arise as a result of intraconfigurational transitions from the emitting state (5D0) to the low lying multiplet 7FJ (J = 0,1,2,3,4) of central metal ion [23]. The spectra of complexes are devoid of peaks related to free ligand pointing out the efficient sensitization mechanism in complexes. In accordance with the selection rule followed by the forced electric and magnetic dipole transition, the intense emission peak at 612 nm associated with the electric dipole transition and weaker intensity band at 590 nm belongs to magnetic dipole transition [24]. The intensity ratio of these characteristic bands imparts useful information about the coordination environment of central metal ion. The complexes shows a centrosymmetric behaviour around the europium ion with an intensity ratio lower than 0.7 while the value higher than 8 reflects the asymmetric environment possessed by luminescent centre [25]. Thus the higher intensity ratio of studied complexes directs the asymmetric environment around Eu(III) ion which is also pointed by the presence of stark splitting in emissions bands of complexes. The emission spectra also indicate a weak emission band at 535 nm attributed to 5D1 → 7F0 transition of europium ion. As a result of weak interaction between the ligand field Hemiltonian and central europium ion there is appearance of electric and magnetic forbidden transition 5D0 → 7F0 in the spectra [26]. Additionally, the spectra also shows the presence of primarily forbidden 5D0 → 7F3 transition of Eu(III) ion. The complexes shows similar behaviour of emission spectra with an increasing intensity parameter from C1-C5 due to the presence of ancillary ligands in complexes (C2-C5) which restrict the O-H vibrations and thermal quenching resulting enhanced luminescence of complexes. The substitution of ancillary ligands also diminished the non radiative losses of energy and effectively increases the luminescence [27]. Figure 5 clearly displays the higher luminescence properties of complexes from the previously reported complexes of isomeric ligands on the basis of combined effects of electron withdrawing and resonance phenomenon attributed to position of substituent fluorine in the ligand [28]. The photoluminescence data of complexes C1-C5 tabulated in Table 3.
The CIE (Commission Internationale de I′ Eclairage) color coordinate diagram are applied to observe the luminescence intensity of organic metal complexes in terms of x and y values. The coordinates (x, y) recorded by employing the emission spectra of complexes and given in Table 3. The coordinates of complexes fall in red region of diagram but due to close proximity between values only C1 and C5 values shown in Fig. 6. The calculated values are comparable with the Society of Motion Picture and Television (SMPTE, x = 0.63, y = 0.34), National Television System committee (NTSC 1987, illuminant red 0.63, 0.34) and Europian Broadcasting Union (EBU illuminant red 0.64, 0.33) reference color systems. The observed values mark the complexes as worthy candidates in regime of luminescent materials.
Luminescence Quantum Efficiency (η) and Judd-Ofelt Parameters
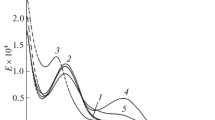
The luminescence quantum efficiency (η) is an important parameter to study the sensitization effect of ligand field on the central metal ion. Hence to calculate quantum efficiencies, an investigation based on luminescence decay time and emission intensity of complexes has been made [29]. The luminescence decay curve of europium(III) complexes C1-C5 are recorded by monitoring the intense emission band 5D0 → 7F2 at 613 nm and displayed in Fig. 7. Decay curve of all complexes show monoexponential behaviour which is directed by the equation I = I 0 exp ( − t/ τ) where τ is the life time for radiative emission, I 0 and I are the luminescence intensities at time 0 and t respectively, suggesting presence of similar ligand field around europium(III) ion [30]. The obtained results are listed in Table 3. By inversing this luminescence lifetime, total transition rates of radiative (Arad) and nonradiative (Anrad) processes can be calculated according to equation [31]
The radiative rate Arad can be calculated by carrying out the sum of individual radiative rate A0J for each emission transition (5D0 → 7FJ) of europium ion. The individual transition rates can be estimated by the given equation [32]
where A01 is the spontaneous emission coefficient for 5D0 → 7F1 transition having a value found to be 50 s−1 [33]. Depending on the independent coordination environment around europium(III) ion for magnetic dipole transition, A01 is used as standard to calculate the A0J value for each transition (J = 0,2,3,4) of europium ion. Ѵ0J is the energy barycentre and I0J is the integrated emission intensity for different transitions (5D0 → 7FJ) respectively.
The radiative and nonradiative processes affect the quantum efficiency of complexes as expressed by following equation [34]
The results for efficiency parameters are tabulated in Table 3. The higher quantum efficiency of complexes indicates that ligand acts as good sensitizer for europium ion.
The Judd-Ofelt intensity parameters Ωλ (λ = 2, 4) of europium(III) complexes C1-C5 were estimated by making utilisation of calculated radiative transition rate (Arad) and Judd-Ofelt theory which was developed by Judd and Ofelt for the analysis of laporte forbidden 4f-4f electronic transitions [35]. The intensity parameters Ωλ for the emissions 5D0 to 7FJ (J = 2, 4) can be calculated by following equation [36]:
Where χ = n2(n2 + 2)2/9 is Lorentz local field correction, n is the refractive index (1.5) and e is the electronic charge (4.80 × 10−10 esu) used. \( {\langle {5}_{D_0}\vert {U}^{\left(\lambda \right)}\vert {7}_{F_J}\rangle}^2 \) are the square reduced matrix elements 0.0032 (J = 2) and 0.0023 (J = 4) respectively. The Judd-Ofelt intensity parameters for complxes C1-C5 are represented in Table 3.
The intensity parameter for transition 5D0 → 7F2 (Ω2) related with the hypersensitive and covalence character of chemical environment around europium(III) ion while Ω4 associated with the interionic bond distances [37]. The intense emission of transition 5D0 → 7F2 leads to stronger radiative rates and resulting much higher value of Ω2 as compared to Ω4 in all the complexes [38]. A comparative analysis of efficiency and intensity parameters of highly intense complex Eu(p-EFBA)3.bipy (C5) with the previously reported complexes Eu(o-EFBA)3.bipy and Eu(m-EFBA)3.bipy shown in Table 3 which clearly reveals the effect of fluorine position on the luminescent properties and confirms that para position of fluorine is most significant for the development of highly luminescent complexes [39, 40].
Energy Transfer Mechanism
The purposed intramolecular energy transfer mechanism for highly intense complex C5 is shown in Fig. 8 to describe the sensitization process in complexes. In the sensitization process, first of all ligand gets excited to its singlet state by absorbing energy and then from singlet excited state to triplet excited state by the process of intersystem crossing (ISC). The triplet excited state of ligand transfers energy to the emitting level of europium ion [41]. Hence singlet and triplet excited states (S1; 27,472 cm−1, T1; 22,271 cm−1) of ligand play a significant role in energy transfer and calculated from UV-visible absorbance edge and phosphorescence spectra of corresponding gadolinium complex respectively [42]. The phosphorescence spectrum of complex is displayed in Fig. 9. The singlet and triplet excited states of ancillary ligand bipy (29,900 cm−1, 22,900 cm−1), phen (31,000 cm−1, 22,100 cm−1) and batho (29,000 cm−1, 21,000 cm−1) are calculated and found to be well matched with the literature [12].
The effective energy transfer in complexes attributed as a result of suitable energy difference between triplet state of ligand to the resonating level of europium(III) ion according to Latva’s empirical rule suggesting an optimal energy gap ∆E (T1-M3+) of the range 2000–5000 cm−1 [43]. The triplet level of ligand tailored to maintain the upper emitting level 5D1 (18,691 cm−1) and then vibrationally relaxes to the lower emitting level 5D0 (17,241 cm−1). From this emitting level 5D0 releases energy to ground 7Fj levels of europium ion and gives the luminescence [44]. Figure 8 clearly depicts that T1 state of ancillary ligand first absorb energy and then transfer this energy to the triplet level of p-EFBA, from this level the energy transmitted to the 5D1 level of europium ion. This also suggests the role of ancillary ligand in the enhancement of luminescence for complexes. The T1 levels of phen and batho ligand are lower and may be associated with back energy transfer processes resulting weaker luminescence complexes (C3, C4) compared to complex C5. The Dexter theory [45] and thermal de-excitation theory [46] helps to conclude the energy transfer processes in complexes.
Conclusion
In this study, a series of europium(III) complexes C1-C5 have been synthesized and characterized by elemental analysis, IR, 1H–NMR, UV-visible, TG/DTG and PL spectroscopy. The effect of position of substituent fluorine on the luminescence properties of complexes Eu(p-EFBA)3.(H2O)2 (C1), Eu(p-EFBA)3.neo (C2), Eu(p-EFBA)3.batho (C3), Eu(p-EFBA)3.phen (C4), Eu(p-EFBA)3.bipy (C5) was investigated successfully. The enhanced luminescence of complexes from previous reported complexes shows the remarkable effect of substituent position on luminescence properties. The effect of position studied as a combined effect of resonance phenomenon and electron withdrawing nature of substituent attached. The studied complexes show higher intensity and efficiency parameters which pointing the utilisation of complexes in development of excellent luminescent materials.
References
Liu JQ, Wang YY, Batten SR, Sakiyama H, Ma DY (2012) An unusual self-penetrated metal–organic framework from mixed ligands: synthesis, structure and magnetism. Inorg Chem Commun 19:27–30
Zhu X, Zheng HY, Wei XF, Lin ZY, Guo LH, Qiu B, Chen GN (2013) Metal–organic framework (MOF): a novel sensing platform for biomolecules. Chem Commun 13:1276–1278
Mukkala VM, Helenius M, Hemmila I, Kankare J, Takalo H (1993) Development of luminescent europium(III) and terbium(III) chelates of 2,2′:6′,2″- terpyridine derivatives for protein labelling. Helv Chim Acta 76:1361–1378
Pandya S, Yu JH, Parker D (2006) Engineering emissive europium and terbium complexes for molecular imaging and sensing. Dalton Trans 23:2757–2766
Bünzli JC, Piguet C (2005) Taking advantage of luminescent lanthanide ions. Chem Soc Rev 34:1048–1077
Shi LX, Wu CD (2011) A nanoporous metal–organic framework with accessible Cu2+ sites for the catalytic Henry reaction. Chem Commun 10:2928–2930
Justel T, Nikol H, Ronda C (1998) New developments in the field of luminescent materials for lighting and displays. Angew Chem Int Ed 37:3084–3103
Bekiari V, Lianos P (1998) Strongly luminescent poly(ethyleneglycol)-2,2′-bipyridine lanthanide ion complexes. Adv Mater 10:1455–1458
Gao B, Qiao Z, Chen T (2014) Structure and photoluminescence properties of aromatic carboxylic acid-functionalised polysulfone with Eu(III) and Tb(III). Mater Chem Phys 143:1119–1130
Koen B (2009) Lanthanide-based luminescent hybrid materials. Chem Rev 109:4283–4374
Weissman SI (1942) Intramolecular energy transfer the fluorescence of complexes of europium. J Chem Phys 10:214–217
Raj DBA, Biju S, Reddy MLP (2008) One-, two-, and three-dimensional arrays of Eu3+-4,4,5,5,5-pentafluoro-1-(naphthalen-2-yl)pentane-1,3-dione complexes: synthesis, crystal structure and photophysical properties. Inorg Chem 47:8091–8100
Yang C, Fu LM, Wang Y, Zhang JP, Wong WT, Ai XC, Qiao YF, Zou BS, Gui LL (2004) A highly luminescent europium complex showing visible-light-sensitized red emission: direct observation of the singlet pathway. Angew Chem Int Ed 43:5010–5013
Martinelli C, Farinola GM, Pinto V, Cardone A (2013) Synthetic aspects and electro-optical properties of fluorinated arylenesvinylenes for luminescence and photovoltaics. Materials 6:1205–1236
Roh EJ, Keller JM, Olah Z, Ladarola MJ, Jacobson KA (2008) Structure–activity relationships of 1,4-dihydropyridines that act as enhancers of the vanilloid receptor 1 (TRPV1). Bioorg Med Chem 16:9349–9358
Sareen V, Gupta R (1996) Studies in fluorinated 1,3-diketones and related compounds. Part XVIII. Synthesis of some new tris(1,3-diketonato)lanthanide complexes. J Fluor Chem 76:149–150
Zhang W, Liu CH, Tang RR, Tang CQ (2009a) Preparation and luminescent properties of a novel carbazole functionalized bis-β-diketone ligand and corresponding Eu(III) and Tb(III) complexes. Bull Kor Chem Soc 30:2213–2216
Arvind M, Sageed K (1986) Studies on bis(p-dimethylaminobezylidene)benzidine complexes of trivalent lanthanides. Indian J Chem A 25:589–594
Bala M, Kumar S, Taxak VB, Boora P, Khatkar SP (2016) Optical features of efficient europium(III) complexes with β-diketonato and auxiliary ligands and mechanistic investigation of energy transfer process. J Fluoresc 26:1813–1823
Wang D, Zheng C, Fan L, Zheng J, Wei X (2012) Preparation and fluorescent properties of europium (III) complexes with β-diketone ligand and 2,2-dipyridine or 1,10-phenanthroline. Synth Met 162:2063–2068
An BL, Gong ML, Li MX, Zhang JM (2004) Synthesis, structure and luminescence properties of samarium (III) and dysprosium (III) complexes with a new tridentate organic ligand. J Mol Struct 687:1–6
Meijuan L, Xiaoping W, Qiang T, Qidan L (2013) Luminescence properties of polymers containing europium complexes with 4-tert-butylbenzoic acid. J Rare Earths 31:950–956
Shao G, Li Y, Feng K, Gan F, Gong M (2012) Diphenyl based β-diketonate europium(III) complexes as red phosphors applied in LED. Sensors Actuators B Chem 173:692–697
Kai J, Parra DF, Brito HF (2008) Polymer matrix sensitizing effect on photoluminescence properties of Eu3+-β-diketonate complex doped into poly-β-hydroxybutyrate (PHB) in film form. J Mater Chem 18:4549–4554
Kirby AF, Foster D, Richardson FS (1983) Comparison of 7FJ←5DO emission spectra for Eu(III) in crystalline environments of octahedral, near-octahedral, and trigonal symmetry. Chem Phys Lett 95:507–512
Richardson FS (1982) Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem Rev 82:541–552
Wang H, He P, Yan H, Gong M (2011) Synthesis, characteristics and luminescent properties of a new europium(III) organic complex applied in near UV LED. Sensors Actuators B Chem 156:6–11
Pu S, Yang T, Li G, Xu J, Chen B (2006) Substituent position effect on the optoelectronic properties of photochromic diarylethenes. Tetrahedron Lett 47:3167–3171
Werts MHV, Jukes RTF, Verhoeven JW (2002) The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys Chem Chem Phys 4:1542–1548
Brito HF, Malta OL, Souza LR, Menezes JFS, Carvalho CAA (1999) Luminescence of the films of europium (III) with thenoyltrifluoroacetonate and macrocyclics. J Non-Cryst Solids 247:129–133
De Sà GF, Malta OL, de Mello DC, Simas AM, Longo RL, Santa-Cruz PA, da Silva Jr EF (2000) Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord Chem Rev 196:165–195
Carlos LD, Messaddeq Y, Brito HF, Ferreira RAS, Bermudez VD, Ribeiro SJL (2000) Full-color phosphors from europium(III)-based organosilicates. Adv Mater 12:594–598
Ferreira R, Pires P, Castro BD, Ferreira RAS, Carlos LD, Pischel U (2004) Zirconium organophosphonates as photoactive and hydrophobic host materials for sensitized luminescence of Eu(III), Tb(III), Sm(III) and Dy(III). New J Chem 28:1506–1513
Stanimirov S, Petkov I (2009) Photophysical properties of novel fluorescent poly(oxyethylene phosphate) tris(β-diketonate) europium (III) complexes. Spectrochim Acta A Mol Biomol Spectrosc 72:1127–1133
Zhang X, Zhou F, Shi J, Gong M (2009b) Sr3.5Mg0.5Si3O8Cl4: Eu2+ bluish–green-emitting phosphor for NUV-based LED. Mater Lett 63:852–854
Teotonio EES, Brito HF, Felinto MCFC, Kodaira CA, Malta OL (2003) Luminescence investigations on Eu(III) thenoyltrifluoroacetonate complexes with amide ligands. J Coord Chem 56:913–921
Ferreira RAS, Nobre SS, Granadeiro CM, Nogueira HIS, Carlos LD, Malta OL (2006) A theoretical interpretation of the abnormal 5D0→7F4 intensity based on the Eu3+ local coordination in the Na9[EuW10O36]·14H2O polyoxometalate. J Lumin 121:561–567
Li XP, Chen B, Shen R, Zhong H, Cheng L, Sun J, Zhang J, Zhong H, Tian Y, Du G (2011) Fluorescence quenching of 5DJ (J = 1, 2 and 3) levels and Judd–Ofelt analysis of Eu3+ in NaGdTiO4 phosphors. J Phys D Appl Phys 44:335403–335410
Devi R, Dalal M, Bala M, Khatkar SP, Taxak VB, Boora P (2016a) Investigations of luminescent behavior and intramolecular energy transfer mechanism of europium(III) complexes with fluorinated β-ketoester ligand. J Fluor Chem 181:36–44
Devi R, Dalal M, Bala M, Khatkar SP, Taxak VB, Boora P (2016b) Synthesis, photoluminescence features with intramolecular energy transfer and Judd–Ofelt analysis of highly efficient europium(III) complexes. J Mater Sci Mater Electron 27:12506–12516
Taha ZA, Ajlouni AM, Al-Hassan KA, Hijaki AK, Faiq AB (2011) Syntheses, characterization, biological activity and fluorescence properties of bis-(salicylaldehyde)-1,3-propylenediimine Schiff base ligand and its lanthanide complexes. Spectrochim Acta A Mol Biomol Spectrosc 81:317–323
Gawryszewska P, Sokolnicki J, Legendziewicz J (2005) Photophysics and structure of selected lanthanide compounds. Coord Chem Rev 249:2489–2509
Latva M, Takalo H, Mukkala VM, Matachescu C, Ubis JCR, Kankare J (1997) Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J Lumin 75:149–169
Lee JC, Leong YK, Kim JM, Kang JG (2014) Sensitized luminescence of Eu(III) complexes with Schiff-base and 1,10-phenanthroline: role of Schiff-base as a sensitizer. Spectrochim Acta A Mol Biomol Spectrosc 124:256–264
Dexter DL (1953) A theory of sensitized luminescence in solids. Chem Phys 21:836–850
Dean CRS, Shephred TM (1975) Evaluation of the intramolecular energy transfer rate constants in crystalline Eu(hfaa)4ButNH3. J Chem Soc Faraday Trans 2(71):146–155
Acknowledgements
This work was financially supported in the form of senior research fellowship (SRF) from UGC, New Delhi, India (Award No: 2121210101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors indicated no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Devi, R., Chahar, S., Khatkar, S.P. et al. Relative Study of Luminescent Properties with Judd-Ofelt Characterization in Trivalent Europium Complexes Comprising ethyl-(4-fluorobenzoyl) Acetate. J Fluoresc 27, 1349–1358 (2017). https://doi.org/10.1007/s10895-017-2069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2069-3