Abstract
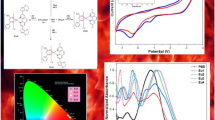
Two new europium (III) complexes have been synthesized with 1,3[bis(4methoxyphenyl)]propane1,3dionato (HBMPD) as main ligand and 2,2′-bipyridyl (bipy) or 1,10-phenanthroline (phen) as an auxiliary ligand. The main ligand HBMPD has been synthesized by ecofriendly microwave approach and complexes by solution precipitation method. The resulting materials are characterized by IR, 1H-NMR, elemental analysis, X-ray diffraction, UV-visible and TG-DTG techniques. The photoluminescence (PL) spectroscopy depicts the detail analysis of photophysical properties of the complexes, their results show that the ligand interact with Eu (III) ion which act as antenna and transfers the absorbed energy to the central europium(III) ion via sensitization process efficiently. As a consequence of this interaction, these materials exhibit excellent luminescent intensity, long decay time (τ), high quantum efficiency (η) and Judd-Ofelt intensity parameter (Ω2). The CIE coordinates fall under the deep red region, matching well with the NTSC (National Television Standard Committee) standard. Hence, these highly efficient optical materials can be used as a red component in organic light emitting diodes (OLEDs) and full color flat panel displays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The optical features of lanthanide ions sensitized with various ligands have attracted the attention of researchers owing to their fascinating properties in many research areas like luminescent materials for organic light emitting diodes (OLEDs), optical fibers, lasers [1–3], chemical and biological sensors [4, 5]. The major interest in exploring the europium complexes is their intriguing luminescent properties such as large stokes shifts, long lifetime and line like emission profile in visible region due to screening of 4fn by 5 s and 5p electrons [6–8]. The 4f-4f intra-configurational forbidden transitions of the europium (III) ion and NH, OH and CH oscillators of the solvent molecules diminish the emission intensity by depopulating the excited state of metal ion by non-radiative decay path, which quench luminescence. In this problem, organic ligands play significant role to sensitize the weak luminescence of europium metal, acting as antennae and also shield the central metal ion from fundamental interaction of NH, OH and CH high energy oscillators of solvents. An organic moiety with magnificent absorption coefficient act as sensitizer or an antenna, which absorbs energy, then reassign the energy from its lowest triplet energy level to the emitting resonance levels of europium(III) ion and hence facilitates the sensitization process [9]. This organic moiety enhances the luminescent intensity of electric dipole 5D0 → 7F2 transition by creating asymmetric coordination environment in the complex. The introduction of auxiliary ligand further enhance the luminescence as it restricts the 4f-4f forbidden transition, decrease the loss of energy by non-radiative decay, moreover, confers stability and rigidity to the complex. In aforementioned perspective of ligands, we introduced an electron donating –OCH3 group in the main organic ligand [10–12] and an auxiliary ligand like bipy or phen having synergistic effect with main ligand and noticed a dramatically enhanced luminescence. So, the quest of new organic ligand possessing extensive π-conjugation which affirm the complexation with trivalent europium(III) ion, meet with the synthesis of β-diketonato HBMPD ligand.
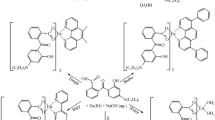
Here in the present communication, we have synthesized new β-diketonato ligand, namely HBMPD by microwave irradiation and employed this ligand for the synthesis of europium (III) complexes incorporating auxiliary ligands phen or bipy, ensuing to tris[1,3-bis(4-methoxyphenyl)propane-1,3-dionato](bipyridyl) europium(III) (Eu1) and tris[1,3-bis(4-methoxyphenyl)propane-1,3-dionato](1,10-phenanthroline) europium(III) (Eu2). The spectral, elemental analyses, X-ray, thermal behavior and photophysical studies of these novel Eu (III) complexes are reported. Moreover, the synergistic effect of auxiliary ligands on photophysical properties of these europium complexes is expatiated. On the basis of emission spectra, the CIE color coordinates (x and y), luminescence decay time (τ), radiative decay rate (Arad), nonradiative rate (Anrad), quantum efficiency (η) and Judd-Ofelt intensity parameter (Ω2) are determined and analyzed. The sensitization process and mechanistic investigation of intramolecular energy transfer in these europium(III) complexes are proposed.
Experimental
Materials
High purity europium nitrate [Eu (NO3)3.5H2O; 99.9] and gadolinium nitrate [Gd (NO3)3.5H2O; 99.9] were acquired from Sigma Aldrich. The solvents and other chemicals like 2,2′-bipyridyl, 1,10-phenanthroline, 4-methoxyacetophenone, 4-methoxybenzoyl chloride and potassium carbonate were purchased from commercial source and used as received. The ligand HBMPD was synthesized adopting ecofriendly microwave approach and then recrystalized three times with DMSO to obtain ligand of high purity.
Instrumental Measurements
The europium content was enumerated by complexometric titration with disodium ethylenediaminetetraacetate (EDTA) salt. The elemental CHN analyses were performed with Perkin Elmer 2400 CHN Elemental Analyzer. The 1H-NMR spectra of complexes were executed by Bruker Avance II 400 NMR spectrometer in chloroform solvent using tetramethylsilane (TMS) as an internal standard (δ = 0.00 ppm). The infrared spectra were obtained by Shimadzu FT-IR Affinity-1 with KBr pellets in the range of 4000–400 cm−1. The UV-visible absorption spectra were measured on Shimadzu-2450 UV-visible spectrophotometer. The thermal behavior in form of TG-DTG curves were determined by Hitachi STA-7300 thermal system under N2 atmosphere with a heating rate of 20°C/min. Powder X-ray diffraction patterns were recorded on Rikagu Ultima IV diffractometer of 40 kV tube voltage and 40 mA tube current with CuKα as a radiation source at a scanning rate of 3° min−1 in the 2θ angular range of 10–80°. The excitation, emission spectra and decay curves of the complexes were carried out by Hitachi F-7000 fluorescence spectrophotometer equipped with xenon lamp as radiation source in UV-visible range. These measurements were made with the scanning rate of 240 nm/min at 400 PMT and 2.5 nm slit width for excitation and emission process. The decay time values of the complexes were obtained by FL solution software of F-7000 spectrometer under time scan mode.
Synthesis of Ligand
The ligand HBMPD was synthesized adopting microwave irradiation method as illustrated in Scheme 1. To a mixture of 4-methoxybenzoyl chloride (0.68 ml, 5 mmol), 4-methoxyacetophenone (0.75 g, 5 mmol) and potassium carbonate (1.38 g, 10 mmol), a few drops of water were added to make a homogenous mixture. Then resulting mixture was exposed to microwave radiation (300 W) for the 40 s (8 × 5 s). The completion of the reaction was assured by thin layer chromatography (TLC). The reaction mixture was diluted with crushed ice followed by acidification with hydrochloric acid. The solid was separated out and purified by recrystalization process, repeating the process three times in DMSO. The yellow crystalline solid of HBMPD was obtained in 90 % yield with 112°C melting point. IR (KBr): cm−1 3480 (b), 3045 (w), 2949 (w), 1680 (s), 1597 (s), 1490 (s), 1446 (s), 1307 (s), 1251 (s), 1024 (s), 837 (s), 783 (s), 509 (m); 1H-NMR (400 MHz, CDCl3): δ 12.07 (s, 1H, enolic OH), 7.93–6.91 (m, 8H, Ar-H), 6.83 (s, 1H, enol CH), 4.80 (s, 2H, keto-CH2), 4.62 (s, 6H, OCH3) ppm. Anal. Calcd for C17H16O4 formula of HBMPD ligand: C, 71.83, H, 5.63; found: C, 71.12; H, 5.21.
Synthesis of Complexes
An ethanolic solution of ligand HBMPD (0.909 g, 3.2 mmol) and bipy (0.15 g, 1.0 mmol) for complex Eu1 or phen (0.18 g, 1.0 mmol) for complex Eu2 was slowly added to the aqueous solution of europium nitrate (0.42 g, 1.0 mmol) and the reaction mixture was allowed to stir for 3 h at about 50–55°C temperature on magnetic stirrer. The pH of the resulting mixture was adjusted to 6.5–7.0 by carefully addition of aqueous NaOH solution (0.05 M). The yellow precipitates were collected after filtration and washed with distilled water followed by ethanol to take away the unreacted ligand. The solid was dried at 60° C in hot air oven.
A gadolinium (III) binary complex (Gd3) i.e. [Gd(BMPD)3(H2O)2] was also prepared to calculate the lowest triplet level of ligand HBMPD, a similar method was adopted as in the synthesis of europium complexes using the mixture of HBMPD (0.90 g, 3.2 mmol) in ethanol and aqueous solution of gadolinium nitrate (0.45 g, 1.0 mmol).
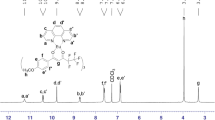
[Eu(BMPD)3bipy] (Eu1): yellow solid, yield 72 %; IR (KBr): cm−1 3061 (w), 3008 (w), 2948 (w), 1595 (s), 1539 (s), 1481 (s), 1444 (s), 1390 (s), 1247 (s), 1174 (s), 1114 (s), 1026 (s), 837 (s), 516 (w), 460 (w); 1H-NMR (400 MHz, CDCl3): δ 7.93–7.01 (m, 32H, Ar-H), 4.62 (s, 18H, −OCH3), 3.84 (s, 3H, enol CH). Anal. Calcd for EuC61H53N2O12: C, 63.27; H, 4.58; N, 2.42; Eu, 13.14; found: C, 62.82; H, 4.25; N, 2.16; Eu, 12.96.
[Eu(BMPD)3phen] (Eu2): yellow solid, yield 74 %; IR (KBr): cm−1 3049 (w), 3004 (w), 2951 (w), 1595 (s), 1535 (s), 1483 (s), 1442 (s), 1388 (s), 1249 (s), 1174 (s), 1028 (s), 839 (s), 516 (w), 462 (w); 1H-NMR (400 MHz, CDCl3): δ 7.93–7.0 (m, 32H, Ar-H), 4.62 (s, 18H, −OCH3), 3.84 (s, 3H, enol CH). Anal. Calcd for EuC63H53N2O12: C, 64.01; H, 4.50; N, 2.40; Eu, 12.90; found: C, 63.82; H, 4.15; N, 2.10; Eu, 12.48.
Gd(HBMPD)3(H2O)2 (Gd3): yellow solid, yield 64 %; IR (KBr): cm−1 3401 (b), 3068 (w), 3003 (w), 2947 (w), 2837 (w), 1597 (s), 1479 (s), 1375 (s), 1305 (s), 1234 (s), 1180 (s), 1116 (s), 1022 (s), 835 (s), 796 (s), 466 (m); 1H-NMR (400 MHz, CDCl3): δ 7.93–7.01 (m, 24H, Ar-H), 4.62 (s, 18H, −OCH3), 3.64 (s, 3H, enol CH). Anal. Calcd for GdC51H49O14: C, 58.73; H, 4.70; Gd, 15.06; found: C, 58.10; H, 3.98; Gd, 14.62.
Results and Discussion
Elemental and Spectral Analysis
The elements (C, H and N) of ligand HBMPD ligand and their europium complexes are analyzed, results are listed in Table 1. These are in good concordance with values calculated on the basis of proposed molecular formulas. The molecular composition of the complexes conforms to Eu (HBMPD)3bipy (Eu1) and Eu(HBMPD)3phen (Eu2). These complexes are found to be highly soluble in DMSO, chloroform and stable under atmospheric condition.
The significant data of FT-IR spectra of HBMPD ligand, Eu1 and Eu2 complexes are arranged in Table 2. The binding nature of ligands to the europium (III) ion is assessed by a comparative study of ligand and spectra of complexes. A broad absorption band at 3480 cm−1 attributed to the stretching vibration of enolic –OH is absent in the spectra of complexes, proposing the involvement of enolic –OH of ligand in coordination to the Eu (III) ion. The strong absorption bands of ›CO stretch at 1595 cm−1 in complexes spectra exhibit a noticeable decrease in the ›CO frequency compared to the spectrum of ligand, suggesting the coordination of ligand to the metal through oxygen atom of ›CO group of ligand, which is further affirmed by visualizing a medium intense band of Eu-O vibration in 460–462 cm−1 range. Similarly, the enolic C = C stretching vibration of complexes spectra are red shifted relative to spectrum of ligand, indicating the extension of π-conjugated system in complexes. The IR spectra of complexes only show the intense absorption band in the frequency range 1539–1535 cm−1 ascribed to the ›C = N stretch vibration of auxiliary ligands (bipy or phen), ensuring the coordination of bipy or phen ligand to the central metal ion through nitrogen atom. This data is further confirmed by a medium intense band of Eu-N stretching vibration in 516–517 cm−1 range. In 1H-NMR spectra, the singlet peaks noticed in spectrum of ligand only at 12.07 ppm and 4.80 ppm are ascertained to enolic –OH and ketonic –CH2 protons, respectively. These signals disappeared in the spectra of complexes, suggesting that the ligand in enolic form coordinates with the Eu(III) ion. The proton signal of enolic –CH at 6.83 ppm in the spectrum of ligand shifts to upfield at 3.84 ppm in the spectra of complexes, intends the paramagnetism of lanthanide ion [13].
Figure 1 displays the UV-visible absorption spectra of free ligand and their Eu1 and Eu2 complexes in DMSO solution (1 × 10−5 mol/L) at room temperature [14, 15]. The UV-absorption profile of europium complexes is similar to the ligand HBMPD absorption profile, proposing that the coordination of europium metal ion does not affect the singlet excited level of ligand in significant way. The maximum absorption at 278 nm and 360 nm is associated with the π-π* transition of organic ligands as the europium ion shows negligible absorption in this range. According to the Beer’s Lambert law, the observed values of molar absorption coefficient in Eu1 and Eu2 complexes are 1.5 × 103 Lmol−1 cm−1 and 1.3 × 103 Lmol−1 cm−1 respectively.
Thermal Analysis and X-Ray Measurements
In order to examine the thermal behavior of Eu1 and Eu2 complex, TG-DTG analyses have been carried out in N2 atmosphere with the range from ambient temperature to 800°C at a heating rate of 20°C/min, resulting traces are depicted in Fig. 2a-2b respectively. The Eu1 and Eu2 complexes show initial little mass loss of about 1–3 % which represents the loss of moisture present in complexes and stable about 250°C temperature. Further mass loss upto 700°C temperature corresponds to the removal of three molecule of BMPD ligand and bipy or phen molecules, leaving residue of europium oxides. The TG-DTG curves demonstrate the high thermal stability of complexes, which is quite favourable for the fabrication of OLEDs and other display devices.
The powder X-ray diffraction patterns of Eu3+ complexes Eu1–2 are displayed in Fig. 3. The sharp prominent peaks at 2θ of 12.83, 14.10, 16.39, 20.22, 25.57, 28.11 of Eu1 and 11.30, 14.10, 16.66, 18.43, 20.22, 28.11 of Eu2 manifest the crystalline nature of the complexes. The size of particles is estimated with the help of Scherrer’s equation, D = 0.941λ/β cos θ, where D is the average particle size, λ the X-ray wavelength (0.15418 nm) and θ and β are the diffraction angle and full width at half maximum, respectively [16, 17]. The calculated particle size of Eu1 and Eu2 complexes from Scherrer’s eq. is 36.78 nm and 27.80 nm respectively. The PXRD study designates the crystalline nature and nano range particle size of the complexes.
Optical Features
The emission spectra of Eu1 and Eu2 complexes in solid state at 298 K (Fig. 4), being excited at maxima 397 nm of excitation spectra, afford the greatest emission intensity of europium ion. The narrow characteristics emission peaks at 534, 540, 580, 590, 613 and 652 nm associated with the 5D0,1 → 7Fj (where j = 0, 1, 2, 3) transition of europium (III) ion. No ligand based emission peaks are noticed, demonstrating the effective transfer of absorbed energy from ligand to the emitting levels of europium(III) ion. It is well known that the emission peaks of europium ion are highly sensitive to change in symmetry and local chemical environment of the coordination sphere [18–20]. This can be reflected in the intense 5D0 → 7F2 electric dipole transition at 613 nm, which is hypersensitive to even slight variation in the coordination environment being amenable for pure red color of the Eu3+ ion [21], while the parity-allowed magnetic dipole transition 5D0 → 7F1 at 592 nm is insensitive to the coordination environment [22]. Hence, the monochromaticity of complexes can be calculated from the intensity ratio of electric dipole transition to magnetic dipole transition (I2/ I1 or red/orange) which manifest the low symmetry around Eu (III) ion in coordination sphere [23]. On the basis of data the intensity rations (I2/I1) computed are 13.4 (Eu1) and 9.3 (Eu2), pointing to the nonexistence of an inversion center around central europium ion. The emission peaks at 580 nm and 652 nm are weak as their associated transition 5D0 → 7F0, 3 are forbidden both in electric as well as in magnetic dipole scheme. We observed one line for 5D0 → 7F0 transition and stark splitting of 5D0 → 7F1 and 5D0 → 7F2 transition in emission spectra due to ligand field around europium(III) ion, suggesting the single chemical environment around europium(III) ion with low symmetry [24, 25]. In addition, rigid auxiliary ligand (bipy or phen) have strong affinity towards lanthanide ions which induced an asymmetry in the complex coordination environment, restricting the thermal vibration as well as f-f forbidden transitions and reducing the non-radiative loss of energy that would enhance the intensity of the complexes [26, 27].
Figure 5 depicts the normalized excitation spectra of europium (III) complexes Eu1 and Eu2 measured by monitoring the intense 5D0 → 7F2 peak of europium ion at 613 nm in solid state at 298 K. The figure displays a broad band from 225 nm to 500 nm, with an excitation maxima 397 nm (7F0 → 5 L6) ascribed to the π-π* electronic transition of β-diketonato ligand HBMPD. The excitation spectra also exhibit low intensity peaks in the longer wavelength region at 419 nm and 465 nm corresponding to 7F0 → 5D2,3 transitions of Eu3+ ion respectively.
Figure 6 shows the CIE (Commission Internationale de Eclairage) chromaticity coordinates (x and y) of Eu1 and Eu2 complexes estimated from the emission spectra excited at 397 nm and data is tabulated in Table 3. The CIE coordinates for Eu2 (x = 0.6174 and y = 0.3294) fall in red region which moves to deeper red region in case of Eu1 (x = 0.6428, y = 0.3454). Also, these values are close to the standard red color values of National Television Standard Committee (NTSC, x = 0.67, y = 0.33), Society of Motion Picture and Television (SMPTE, x = 0.63, y = 0.34) and European Broadcasting Union (EBU, x = 0.64, y = 0.33), which can be explicated on the basis of high intensity rations (I2/I1 or red/orange) in complexes.
Luminescence Decay Time (τ), Quantum Efficiency (η) and Judd-Ofelt Analysis
In order to explore the coordination environment around central europium (III) ion in complexes, the luminescence decay time of 5D0 excited level of Eu3+ ion in complexes is recorded by monitoring the most intense emission peak (5D0 → 7F2) at 613 nm in solid state (Fig. 7), results are summarised in Table 3. The decay profile of 5D0 → 7F2 transition obey single exponential function as I = I 0 exp. (−t/τ) where τ is the radiative decay time, I and I0 are the luminescence intensities at time t and 0, respectively. The results of the decay curves proposed that the europium(III) ion is present in single chemical environment in complexes [28].
The luminescence quantum efficiency of 5D0 excited level of Eu3+ ion in Eu1 and Eu2 complexes are calculated from the decay time and emission spectra of complexes using following equations [29].
Here Arad and Anrad represent radiative and non-radiative transitions rates respectively and considering that only these transition are responsible for the depopulation of 5D0 excited level of europium ion. The luminescence decay time is related to radiative and non-radiative transitions according to equation (2).
The radiative transition rate (Arad) is determined by the summation of radiative rates A0J for the 5D0 → 7F0–3 transitions of europium ion. A0J is calculated by applying equation (3) [30–33]
Here, I01 and I0J are the integrated intensities; ν01 and ν0J are the energy barycenters of 5D0 → 7F1 and 5D0 → 7F0–3 transitions respectively. From the emission spectra, it is noticed that 5D0 → 7F1 transition does not depend on local chemical environment of Eu3+ ion, therefore it may be used as a reference and their value can be calculated to be 50 s−1 approximately [34]. The calculated values of Arad, Anrad and η from the above equations are listed in Table 3.
The Judd-Ofelt intensity parameters i.e. Ω2 and Ω4 give the information about structural changes around the europium ion in the complexes. Out of these parameters, the Ω2 is more sensitive to the symmetry and ligand field of the complex which is estimated from the 5D0 → 7F2 electronic transition of emission spectra of complexes using equation 3 while Ω4 is not calculated because 5D0 → 7F4 transition is not observed in emission spectra.
Here, ћ is taken as Planck’s constant over 2π, c is velocity of light, e is the electronic charge, ω is angular frequency of electronic transition, χ is Lorenz local field correction [\( {n}_0^2 \)(\( {n}_0^2 \) + 2)2/9] in which 1.5 average refractive index is used and \( {\langle {5}_{D_0}\vert {U}^{\left(\lambda \right)}\vert {7}_{F_j}\rangle}^2 \) is taken as 0.0032, representing the square reduced matrix elements [35]. The higher value of Ω2 intensity parameter in Eu1 as compared to Eu2 as summarized in Table 3, indicating the highly sensitive nature of 5D0 → 7F2 transition and suggesting that the europium ion is presented in highly polarizable chemical environment in these complexs. As a consequence of this, Eu1 complex possesses higher luminescence intensity than Eu2 complex which corresponds to the results of emission spectra.
Antenna Effect and Energy Transfer Process
In general, the sensitization process in europium (III) complexes consists of excitation of the organic ligand upto singlet level because the europium(III) ion exhibits very low absorption due to f-f forbidden transition which makes direct excitation of Eu3+ ion impossible, therefore the absorption maxima of organic ligand falls in the excitation spectra of complex. First, the energy of excited ligand is transferred from singlet level to triplet level via intersystem crossing (ISC) and then nonradiatively to the 5Dj manifold, finally to the most emitting 5D0 level of europium(III) ion by internal conversions. Therefore, the effective energy transfer from ligand to Eu3+ ion i.e. antenna effect is responsible for intense red emission in the complexes under UV irradiation. The antenna effect of both the complexes (Eu1 and Eu2) depicted in Fig. 8 and Fig. 9 respectively, Eu1 complex is taken as to describe the antenna effect in detail.
a The absorption spectra of bipy (1) and HBMPD (2); excitation spectrum of Eu1 monitored at 613 nm (3). b The emission spectra of bipy excited at 322 nm (1) and HBMPD excited at 360 nm (3); absorption spectrum of europium(III) nitrate (2). The inset shows the overlap between absorption of HBMPD (4) and emission of bipy spectra (5). All absorption spectra are measured at (1 × 10−5 mol/L) in DMSO. All the spectra are normalized to a constant intensity set at the maximum of the spectra
a The absorption spectra of phen (1) and HBMPD (2); excitation spectrum of Eu2 monitored at 613 nm (3). b The emission spectra of phen excited at 322 nm (1) and HBMPD excited at 360 nm (3); absorption spectrum of europium(III) nitrate (2). The inset shows the overlap between absorption of HBMPD (4) and emission of phen spectra (5). All absorption spectra are measured at (1 × 10−3 mol/L) in DMSO. All the spectra are normalized to a constant intensity set at the maximum of the spectra
In order to understand the antenna effect, the overlap between the excitation spectrum of Eu1 complex and absorption spectra of HBMPD and bipy ligands (Fig. 8a) should be considered which suggests that the central europium (III) ion is sensitized by the ligands (HBMPD and bipy) [26, 36–39]. It is also observed that the overlap between Eu1 complex excitation spectrum and HBMPD absorption spectrum is more than that between Eu1 complex excitation spectrum and bipy absorption spectrum indicating that the HBMPD ligand is more effective sensitizer than bipy for the europium ion, hence the transfer of intramolecular energy primarily exist between the triplet level of HBMPD ligand and europium ion. Fig. 8b exhibits the overlap between absorption spectrum of europium(III) nitrate and emission spectra of HBMPD as well as bipy ligands, indicating that the both HBMPD and bipy ligands can sensitize the central europium(III) ion [40, 41]. The inset of Fig. 8b depicts the overlap between absorption spectrum of HBMPD and emission of spectrum of bipy, suggesting that the bipy ligand absorbed the energy firstly which is transferred to the HBMPD ligand as shown in Fig. 10.
The lowest excited singlet (S1; 23,255 cm−1) and triplet (T1; 22,538 cm−1) levels of HBMPD ligand are estimated from the UV-visible absorption edge wavelength of spectra and shortest phosphorescence emission wavelength of Gd3 complex (Fig. S1). Similarly, the lowest singlet and triplet levels of bipy (S1; 29,900 cm−1, T1; 22,900 cm−1) and phen (S1; 31,000 cm−1, T1; 22,100 cm−1) are calculated which match with the literature [42, 43].
To make energy transfer process efficient, the energy gap (∆E) between emitting level of lanthanides ion (M3+) and ligand triplet level (T1) should be appropriate i.e. neither too large nor too small according to the Dexter’s theory [44]. A large energy gap diminishes the overlapping between the donor and acceptor that decreases the rate of energy transfer while a small energy gap encourages the back energy transfer from metal emitting level to triplet level of ligand. An empirical rule of Latva et al’s also suggests that the energy gap ∆E (T1-M3+) should be in 2000–5000 cm−1 range for efficient energy transfer process [45]. The lowest triplet level of HBMPD, bipy and phen are 3812, 4174 and 3374 cm−1 respectively above than the emitting level of europium (III) ion (18,726 cm−1) which are well suited for the efficient energy transfer process. From the above discussion the proposed energy transfer process in Eu1 and Eu2 complexes is depicted in Fig. 10 and Fig. S2 respectively.
Conclusion
In summary, we have successfully synthesized the HBMPD ligand by adopting ecofriendly microwave method and its two new europium (III) complexes. These complexes are characterized by IR, 1H-NMR, elemental analysis and their results clearly demonstrate that the europium(III) ion can be anchored effectively to the ligands molecules through the oxygen atoms of the ›CO group and nitrogen atoms of auxiliary ligands. The TG-DTG analyses indicate the high thermal stability of these materials. The optical properties show that the Eu (III) ion exhibit characteristics intense emission peak at 613 nm in red region and it is present in polarizable chemical environment, acting as only one luminescent center. Furthermore investigation of photophysical properties of these materials clearly reveals that HBMPD ligand acts as sensitizer or antenna and transfers the absorbed energy to the central Eu(III) ion efficiently. It is noteworthy that the N,N donor bidentate auxiliary ligand bipy and phen have synergistic effect with HBMPD ligand which enhance the luminescent features of the complexes. These highly efficient luminescent europium(III) complexes can be employed as excellent emitting material in OLEDs and display devices.
References
Koen B (2009) Lanthanide-based luminescent hybrid materials. Chem Rev 109:4283–4374
Ken K, Yasuhiro K, Yoshi O (2002) Plastic Optical Fiber Lasers and Amplifiers Containing Lanthanide Complexes. Chem Rev 102:2347–2356
Sebastiaan A, Jos JMH, Van den E, Ilpo M, Maxime AS, Wen TF (2013) Substituted phenanthroline as antennae in luminescent Eu (III) complexes. J Inorg Chem 36:6137–6147
Matthew ST, Marlin H, Dalibor S (2007) Cocktails of Tb3+ and Eu3+ Complexes: A General Platform for the Design of Ratiometric Optical Probes. J Am Chem Soc 129: 7570–7577.
Daniel G, Stefan S, Hans GL, Niko H (2013) Six-Color Time-Resolved Förster Resonance Energy Transfer for Ultrasensitive Multiplexed Biosensing. J Am Chem Soc 135:1102–1109
Jiang X, Jen AK-Y, Huang D, Phelan GD, Londergan TM, Dalton LR (2002) The effect of ligand conjugation length on europium complex performance in light-emitting diodes. Synth Met 125: 331–336
Liang F, Zhou Q, Cheng Y, Wang L, Ma D, Jing X, Wang F (2003) Oxadiazole-Functionalized Europium (III) β-Diketonate Complex for Efficient Red Electroluminescence. Chem Mater 15:1935–1937
Fu L, Ferreira RAS, Silva NJO, Fernandes AJ, Ribeiro-Claro P, Goncalves IS, Bermudez VDZ, Carlos LD (2005) Structure–photoluminescence relationship in Eu (III) β-diketonate-based organic–inorganic hybrids Influence of the synthesis method: carboxylic acid solvolysis versus conventionalhydrolysis. J Mater Chem 15:3117–3125
de Sá GF, Malta OL, de Mello DC, Simas AM, Longo RL, Santa-Cruz PA, da Silva EF (2000) Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coor. Chem Rev 196:165–195
Yang L, Gong Z, Nie D, Lou B, Bian Z, Guan M, Huang C, Lee HJ, Baik WP (2006) Promoting near-infrared emission of neodymium complexes by tuning the singlet and triplet energy levels of β-diketonates. New J Chem 30:791–796
Hernández I. Y.-X Z, Motevalli M, Rendy HCT, Gillina WP, Wyatt PB (2013) Efficient sensitized emission in Yb (III) pentachlorotropolonate complexes. Chem Commun 49: 1933–1935
Wang D, Pi Y, Zheng C, Fan L, Hu Y, Wei X (2013) Preparation and photoluminescence of some europium (III) ternary complexes with β-diketone and nitrogen heterocyclic ligands. J. Alloy and Compound 574:54–58
Wang D, Zheng C, Fan L, Zheng J, Wei X (2012) Preparation and fluorescent properties of europium (III) complexes with β-diketone ligand and 2, 2-dipyridine or 1, 10-phenanthroline. J. Synt Met 162:2063–2068
Shao G, Li Y, Feng K, Gan F, Gong M (2012) Diphenylethyne based β-diketonate europium (III) complexes as red phosphors applied in LED. Sensors Actuat B 173:692–697
Taydakov I, Zaitsev B, Krasnoselskiy S, Starikova Z (2011) Synthesis, X-ray structure and luminescent properties of Sm3+ ternary complex with novel heterocyclic β-diketone and 1, 10-phenanthroline (Phen). J. Rare Earths 29:719–722
Davar F, Hassankhani A, Estarki MRL (2013) Controllable synthesis of metastable tetragonal zirconia nanocrystals using citric acid assisted sol–gel method. Ceram Int 39:2933–2941
Mohammadikish M, Davar F, Estarki MRL, Hamidi Z (2013) Synthesis and characterization of hierarchical ZnS architectures based nanoparticles in the presence of thioglycolic acid. Ceram Int 39:3173–3181
Francis B, Ambili Raj DB, Reddy MLP (2010) Highly efficient luminescent hybrid materials covalently linking with europium (III) complexes via a novel fluorinated β-diketonate ligand: synthesis, characterization and photophysical properties. Dalton Trans. 39:8084–8092
Hasegawa Y, Tsuruoka S-I, Yoshida T, Kawai H, Kawai T (2008) Enhanced Deep-Red Luminescence of Tris (hexafluoroacetylacetonato)samarium(III) Complex with Phenanthroline in Solution by Control of Ligand Coordination. J Phys Chem A 112: 803–807
Irfanullah M, Iftikhar K (2010) Hypersensitivity in the Luminescence and 4f –4f Absorption Properties of Mono- and Dinuclear EuIII and ErIII Complexes Based on Fluorinated β -Diketone and Diimine/Bis-Diimine Ligands. J Fluoresc 21:81–93
Zucchi G, Murugesan V, Tonfelier D, Aldakov D, Jeon T, Yang F, Thuery P, Ephritikhine M, Geffroy B (2011) Solution, Solid State, And film properties of a structurally characterized highly luminescent molecular europium plastic material excitable with visible light. Inorg Chem 50:4851–4856
Wang YB, Zheng XJ, Zhuang WJ, Jin LP (2003) Hydrothermal Synthesis and Characterization of Novel Lanthanide 2,2-Diphenyldicarboxylate Complexes. Eur J Inorg Chem 7:1355–1360
Xue FM, Ma Y, LM F, Hao R, Shao GS, Tang MX, Zhang JP, Wang Y (2010) A europium complex with enhanced long-wavelength sensitized luminescent properties. Phys Chem Chem Phys 12:3195–3202
Feng R, Jiang F-L, M-Y W, Chen L, Yan C-F, Hong M-C (2010) Structures and Photoluminescent Properties of the Lanthanide Coordination Complexes with Hydroxyquinoline Carboxylate Ligands. Cryst Growth Des 10:2306–2313
Li Y-J, Yan B (2010) Photoactive europium(III) centered mesoporous hybrids with 2- thenoyltrifluoroacetone functionalized SBA-16 and organic polymers. Dalton Trans 39:2554–2562
26. Bekiari V, Lianos P (1998) Strongly Luminescent Poly(ethylene glycol)-2,2′-bipyridine Lanthanide Ion Complexes. Adv Mater 10(17): 1455–1458.
Wang D, Zheng C, Fan L, Hu Y, Zheng J (2014) Photoluminescence behavior of europium(III) complexes containing 1-(4-tert-butylphenyl)-3-(2-naphthyl)-propane-1,3-dione ligand. Spectrochimica Acta Part A. 117: 245–249.
Gago S, Fernandes JA, Rainho JP, Sá Ferreira RA, Pillinger M, Valente AA, Santos TM, Carlos LD, Ribeiro-Carlo PJA, Goncüalves S (2005) Highly Luminescent Tris(β-diketonate)europium(III) Complexes Immobilized in a Functionalized Mesoporous Silica. Chem Mater 17:5077–5084
Soares-Santos PCR, Nogueira HIS, Félix V, Drew MGB, Ferreira RAS, Carlos LD, Trindade T (2003) Novel Lanthanide Luminescent Materials Based on Complexes of 3-Hydroxypicolinic Acid and Silica Nanoparticles. Chem Mater 15:100–108
Ferreira RAS, Carlos LD, Goncalves RR, Ribeiro SJL, Bermudez VD (2001) Energy-Transfer Mechanisms and Emission Quantum Yields In Eu3 + −Based Siloxane-Poly(oxyethylene) Nanohybrids. Chem Mater 13:2991–2998
Teotonio EES, Espínola JGP, Brito HF, Malta OL, Oliveira SF, Foria DLAD, Izumi CMS (2002) Influence of the N-[methylpyridyl]acetamide ligands on the photoluminescent properties of Eu(III)-perchlorate complexes. Polyhedron 21:1837–1844
Carlos LD, Messaddeq Y, Brito HF, Malta OL, Oliveira SF, Bermudez VD, Ribeiro SJL (2000) Full-Color Phosphors from Europium(III)-Based Organosilicates. Adv Mater 12:594–598
Hazenkamp MF, Blasse G (1990) Rare-earth ions adsorbed onto porous glass: luminescence as a characterizing tool. Chem Mater 2:105–110
Werts MHV, Jukes RTF, Verhoeven JW (2002) The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys Chem Chem Phys 4:1542–1548
Kodaira CA, Claudia A, Brito HF, Felinto MCFC (2003) Luminescence investigation of Eu3+ ion in the RE2(WO4)3 matrix (RE = La and Gd) produced using the Pechini method. J Solid State Chem 171:401–407
Kawa M, Frẻchet JMJ (1998) Self-Assembled Lanthanide-Cored Dendrimer Complexes: Enhancement of the Luminescence Properties of Lanthanide Ions through Site-Isolation and Antenna Effects. Chem Mater 10:286–296
Carlos LD, Sá Ferreira RA, Rainho JP, Bermudez VDZ (2002) Fine-Tuning of the Chromaticity of the Emission Color of Organic–Inorganic Hybrids Co-Doped with EuIII, TbIII, and TmIII. Adv Funct Mater 12:819–823
Okamoto Y, Ueba Y, Dzhanibekov NF, Banks E (1981) Rare earth metal containing polymers. 3. Characterization of ion-containing polymer structures using rare earth metal fluorescence probes. Macromolecules 14:17–22
Sabbatini N, Mecati A, Guardigli M, Balzani V, Lehn JM, Zeissel R, Ungaro R (1991) Lanthanide luminescence in supramolecular species. J Lumin 48–49: 463–468
Peng CY, Zhang HJ, JB Y, Meng QG, LS F, Li HR, Sun LN, Guo XM (2005) Synthesis, Characterization, and Luminescence Properties of the Ternary Europium Complex Covalently Bonded to Mesoporous SBA-15. J. Phys. Chem. B 109:15278–15287
Dang S, Sun LN, Zhang HJ, Guo XM, Li ZF, Feng J, Guo HD (2008) Near-Infrared Luminescence from Sol−Gel Materials Doped with Holmium(III) and Thulium(III) Complexes. J Phys Chem C 112:13240–13247
Ambili Raj DB, Francis B, Reddy MLP, Butorac RR, Lynch MV, Cowley H (2010) Highly Luminescent Poly(Methyl Methacrylate)-Incorporated Europium Complex Supported by a Carbazole-Based Fluorinated β-Diketonate Ligand and a 4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene Oxide Co-Ligand. Inorg Chem 49:9055–9063
Xyu H, Su Q (2003) Photoacoustic and luminescence properties study on energy transfer and relaxation processes of Tb(III) complexes with benzoic acid. J. Photochem. Photobiolog. A 155:73–78
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–850
Latva M, Takalo H, Mukkala VM, Matachescu C, RodriguezUbis JC, Kankare J (1997) Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin 75:149–169
Acknowledgments
The present work is financial supported by Council of Science and Industrial Research (CSIR) of India in the form of senior research fellowship (09/382(0155)/2012- EMR-I).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 121 kb)
Rights and permissions
About this article
Cite this article
Bala, M., Kumar, S., Taxak, V.B. et al. Optical Features of Efficient Europium(III) Complexes with β-Diketonato and Auxiliary Ligands and Mechanistic Investigation of Energy Transfer Process. J Fluoresc 26, 1813–1823 (2016). https://doi.org/10.1007/s10895-016-1873-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1873-5