Abstract
The luminescent binary and ternary europium(III) complexes were prepared by employing ethyl-(3-fluorobenzoyl) acetate (m-EFBA) as primary ligand and neocuproine (neo), bathophenanthroline (batho), 1,10-phenanthroline (phen) and 2,2-bipyridyl (bipy) as secondary ligands. The synthesized complexes Eu(m-EFBA)3·(H2O)2 (C1), Eu(m-EFBA)3·neo (C2), Eu(m-EFBA)3·batho (C3), Eu(m-EFBA)3·phen (C4), Eu(m-EFBA)3·bipy (C5) were characterized by the means of elemental analysis (C, H and N), nuclear magnetic resonance spectroscopy (1H-NMR), infrared spectroscopy (IR), thermogravimetric analysis (TG/DTG), UV–visible and photoluminescence (PL) spectroscopy. The photoluminescence spectra of complexes exhibit the characteristic emission band at 613 nm assigned to hypersensitive 5D0 → 7F2 transition, responsible for the red color emission of complexes. The higher photoluminescence intensity of ternary europium(III) complexes C2–C5 as compared to binary complex C1, suggest that ancillary ligands neo, batho, phen and bipy enhance the process of sensitization from ligand (m-EFBA) to europium(III) ion. The luminescence decay time and quantum efficiencies of the complexes were determined to estimate the efficiency of energy transfer from ligand to metal ion. In addition, the Judd–Ofelt intensity parameters (Ω2, Ω4) were calculated from the emission intensities of 5D0 → 7F2 and 5D0 → 7F4 transitions of europium(III) ion respectively. The intramolecular energy transfer mechanism of the complexes were also investigated and results indicate that the ligand (m-EFBA) and ancillary ligands effectively transfer the energy to that of Eu(III) ion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 1942, Weissman investigated about the excitation process in lanthanide complexes; since than a lot of research interest has been aroused due to high luminescent properties of the complexes based on the absorption of light by the organic ligand and then transfer of energy to the lanthanide ion by the process of sensitization [1–3]. This intense luminescence property makes the lanthanide complexes potential applicant in optical materials [4–6], magnetism [7], sensors [8], catalyst, separation of gases [9, 10], organic [11] and bioorganic chemistry [12, 13]. The lanthanide complexes results from the complexation of organic ligand and metal ion which marks magnificent properties of complexes like intense emission band, long luminescence life time and hypersensitivity to ligand field [14, 15]. Among the lanthanide ions Eu(III) ion is used as an attractive lumophores due to sharp emission band in the solid state. But the direct excitation of ion is not possible due to limited amount of light absorption as a consequence of smaller value of molar absorption coefficient which results in weak luminescence intensity [16]. To circumvent the problem of this little absorption of light, an organic ligand was added which can apprehend greater amount of light as compared to metal ion. Further the energy is transferred to the resonating level of lanthanide ion by the process of intramolecular energy transfer. For this effective energy transfer, there should be compatibility between the triplet energy level of ligand and the emitting level of lanthanide ion which depends upon the choice of organic ligand [17]. Moreover the ligands containing high energy O–H and C-H oscillators, nonradiatively suppress the excited state of lanthanide ion, thereby results in low luminescence [18, 19]. Hence the use of a fluorine functionalised β-ketoester ligand having low frequency C-F oscillators reduces the energy losses due to vibrational energy of ligand [20, 21]. In binary complex, the organic chromophoric moiety and solvent molecules fulfills the coordination sphere around europium(III) ion while in ternary complexes the ancillary ligands substitute these solvent molecules and decreases the O–H vibrational quenching resulting higher photoluminescence intensity of complex C2–C5 [22].
In this communication, five new europium(III) complexes have been synthesized by using ethyl-(3-fluorobenzoyl) acetate (m-EFBA) as fluorine functionalised β-ketoester ligand and neocuproine, bathophenanthroline, 1,10-phenanthroline and 2,2-bipyridyl as ancillary ligands. All the synthesized complexes (C1–C5) were characterized by elemental analysis, 1H-NMR, IR, thermogravimetric analysis, UV–visible and photoluminescence spectroscopy. Luminescence quantum efficiency and Judd–Ofelt intensity parameters were also calculated to investigate the influence of organic ligand as well as ancillary ligand on the photoluminescence properties of europium(III) ion. The investigation of energy transfer mechanism forms the basis of utilisation of these complexes in the development of highly luminescent materials.
2 Experimental
2.1 Materials and methods
All the chemicals and solvents employed were of analytical reagent grade and used as such without further purification. Lanthanide nitrates (Eu(NO3)3·5H2O and Gd(NO3)3·5H2O), neocuproine, bathophenanthroline, 1,10-phenanthroline and 2,2-bipyridyl were purchased from commercially available sources.
The elemental analyses of complexes were carried out on Perkin Elmer 2400 CHN elemental analyser. The europium(III) ion content of complexes estimated by the complexometric titration with ethylenediaminetetraacetate (EDTA). The Infrared spectra (IR) were performed in the range of 4000–400 cm−1 with Perkin Elmer Spectrum 400 spectrometer using dried KBr disks. Nuclear magnetic resonance spectra (1H-NMR) were analysed on a Bruker Avance II 400 NMR spectrometer in CDCl3 solution with tetramethylsilane (TMS) as internal standard. Thermogravimetric analyses (TG/DTG) were performed on SDT Q600 with a heating rate 10 °C/min to assess the thermal stability of complexes. UV–visible absorption spectra of the complexes were measured with Shimadzu-2450 UV–visible Spectrophotometer. Photoluminescent (PL) measurements were made on a Hitachi F-7000 fluorescence spectrophotometer equipped with a xenon lamp as the source of excitation. The luminescent lifetimes were recorded by software (FL solution of F-7000) of the spectrophotometer.
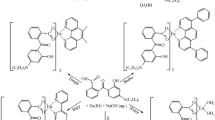
2.2 Synthesis of β-ketoester ligand (Scheme 1)
A mixture was prepared by dissolving 3-fluorobenzoyl chloride and 2, 2-dimethyl-1,3-dioxane-4,6-dione in dry methylene chloride (CH2Cl2). 4-(dimethylamino) pyridine was added into the reaction mixture at about 0 °C and stirred for 1 h at room temperature. The resulting mixture was washed with water and then with 1 N HCl and further dried by using anhydrous Na2SO4. The product formed was mixed with ethanol in toluene and heated at 90 °C by using reflux condenser for approximately 24 h. The excess ethanol and solvents were removed and progress of reaction was checked by TLC [23]. The final product m-EFBA was obtained with 57 % yield. IR(KBr): cm−1 3639 (b), 2983 (s), 1741 (s), 1691 (s), 1649 (s), 1587 (s), 1442 (s), 1325 (w), 1274 (s), 1215 (s), 1165 (m), 1029 (s), 879 (s), 790 (m), 680 (s), 590 (m); 1H NMR (CDCl3, 400 MHz): δ 12.56 (s, 1H, enolic OH), 7.48 (m, 4H, Ar–H), 5.66 (s, 1H, C=CH), 3.98 (s, 2H keto CH2), 3.69 (q, 2H, OCH2CH3), 1.33 (t, 3H, OCH2CH3) ppm.
2.3 Synthesis of europium complexes (Scheme 1)
An aqueous solution of europium nitrate (1 mmol) was prepared and then ethanolic solution of ligand m-EFBA (3 mmol) was added to the solution with constant stirring on magnetic stirrer drop by drop. The pH value of the solution was adjusted to 6.5–7 with 0.05 M sodium hydroxide solution (NaOH), which leads to the formation of white color precipitates. The resulting precipitates were collected by the filtration, washed with water and ethanol for the eviction of free ligand. The residue was dried in vacuum desiccator to procure the white powder of binary complex (C1).
Eu(m-EFBA)3·(H2O)2 (C1): White powder, yield 81 %; IR(KBr): cm−1 3608 (b), 2983 (s), 1732 (s), 1629 (w) 1516 (s), 1479 (s), 1384 (s), 1282 (s), 1217 (s), 1093 (m), 1035 (s), 945 (s), 877 (s), 786 (s), 729 (s), 671 (m), 422 (m); 1H NMR (CDCl3, 400 MHz): δ 7.27 (m, 12H, Ar–H), 4.36 (q, 6H, OCH2CH3), 2.70 (s, 3H, C=CH), 1.38 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC33H34O11F3: C, 48.82; H, 4.19; Eu, 18.24; Found: C, 48.39; H, 3.95; Eu, 18.20.
Ternary europium(III) complexes C2–C5 were accomplished by adopting the similar method as used for the synthesis of binary complex C1 but the reaction mixture of complex C2 also contain the ethanolic solution of neo (1 mmol) alongwith the aqueous solution of europium nitrate (1 mmol) and ethanolic solution of ligand m-EFBA (3 mmol). In similar way, ethanolic solution of batho (1 mmol) for complex C3, phen (1 mmol) for complex C4 and bipy (1 mmol) for complex C5 were used to synthesize the ternary europium(III) complexes.
Eu(m-EFBA)3.neo (C2): White powder, yield 80 %; IR(KBr): cm−1 2983 (s), 1743 (s), 1627 (s), 1583 (s), 1519 (s), 1479 (s), 1282 (s), 1212 (s), 1170 (s), 1095 (s), 1037 (s), 947 (s), 879 (m), 785 (m), 731 (m), 671 (m), 637 (m), 532 (s), 421 (m); 1H NMR (CDCl3,400 MHz): δ 8.32 (d, 2H, Ar–H), 7.89 (m, 4H, Ar–H), 7.49 (m, 12H, Ar–H), 4.52 (q, 6H, OCH2CH3), 3.50 (s, 6H, Ar–H), 2.63 (s, 3H, C=CH), 1.36 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC47H42N2O9F3: C, 57.36; H, 4.27; N, 2.84; Eu, 15.05; Found: C, 57.27; H, 4.24; N, 2.73; Eu, 15.29.
Eu(m-EFBA)3·batho (C3): White powder, yield 79 %; IR(KBr): cm−1 2980 (s), 1735 (s), 1618 (s), 1581 (s), 1517 (s), 1479 (s), 1370 (s), 1278 (s), 1211 (s), 1165 (s), 1039 (s), 877 (s), 783 (m), 734 (m), 704 (m), 543 (s), 420 (m); 1H NMR (CDCl3, 400 MHz): δ 10.32 (d, 2H, Ar–H), 9.12 (d, 2H, Ar–H), 8.31 (m, 12H, Ar–H), 7.42 (d, 2H, Ar–H)), 6.78 (s, 10H, Ar–H), 4.21 (q, 6H, OCH2CH3), 2.54 (s, 3H, C = CH), 1.41 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC57H46N2O9F3: C, 61.76; H, 4.15; N, 2.52; Eu, 13.36; Found: C, 61.42; H, 4.01; N, 2.35; Eu, 13.24.
Eu(m-EFBA)3.phen (C4): White powder, yield 81 %; IR(KBr): cm−1 2935 (s), 1735 (s), 1626 (s), 1577 (s), 1508 (s), 1482 (s), 1424 (s), 1384 (s), 1307 (s), 1222 (s), 1186 (s), 1093 (s), 1031 (s), 942 (m), 843 (s), 765 (m), 725 (m), 639 (m), 512 (s), 418 (m); 1H NMR (CDCl3, 400 MHz): δ 9.43 (d, 2H, Ar–H), 8.43 (m, 4H, Ar–H), 7.67 (d, 2H, Ar–H), 7.36 (m, 12H, Ar–H), 6.98 (d, 2H, Ar–H) 4.43 (q, 6H, OCH2CH3), 2.63 (s, 3H, C=CH), 1.39 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC45H38N2O9F3: C, 56.32; H, 3.97; N, 2.93; Eu, 15.49; Found: C, 56.04; H, 2.98; N, 2.91; Eu, 15.43.
Eu(m-EFBA)3.bipy (C5): White powder, yield 83 %; IR(KBr): cm−1 2979 (s), 1740 (s), 1620 (s), 1584 (s), 1516 (s), 1481 (s), 1363 (s), 1279 (s), 1211 (s), 1159 (s), 1095 (s), 1038 (s), 946 (s), 876 (s), 783 (m), 732 (m), 671 (m), 558 (s), 525 (s), 430 (m); 1H NMR (CDCl3, 400 MHz): δ 8.57 (d,2H, Ar–H), 8.01 (d, 2H, Ar–H), 7.60 (m, 4H, Ar–H),7.31 (m, 12H, Ar–H), 4.32 (q, 6H, OCH2CH3), 2.67 (s,1H, C = CH), 1.31 (t, 9H, OCH2CH3) ppm; Anal. Cal. For EuC43H38N2O9F3: C, 55.41; H, 4.08; N, 3.01; Eu, 15.89; Found: C, 55.26; H, 3.96; N, 2.96; Eu, 15.78.
Gadolinium(III) binary complex C6 [Gd(m-EFBA)3.(H2O)2] was synthesized to calculate the triplet energy state of ligand m-EFBA by adopting the similar method as used for the synthesis of europium(III) complex C1.
Gd(m-EFBA)3.(H2O)2 (C6): White powder, yield 54 %; IR(KBr): cm−1 3611 (b), 2981 (s), 1733 (s), 1625 (w) 1511 (s), 1476 (s), 1381 (s), 1213 (s), 1092 (m), 1027 (s), 874 (s), 781 (s), 724 (s), 673 (m), 423 (m);1H NMR (CDCl3, 400 MHz): δ 7.31 (m, 12H, Ar–H), 4.33 (q, 6H, OCH2CH3), 2.69 (s, 3H, C=CH), 1.40 (t, 9H, OCH2CH3) ppm; Anal. Cal. For GdC33H34O11F3: C, 48.70; H, 4.18; Gd, 18.45; Found: C, 47.88; H, 4.15; Gd, 18.40.
3 Results and discussion
3.1 Composition and spectroscopic characterization of complexes
Elemental analysis data of europium(III) ion, carbon, hydrogen and nitrogen of the complexes C1–C5 are listed in Table 1. The europium(III) ion content of complexes is determined by the complexometric titration with EDTA. The results obtained are in good consistency with the theoretically calculated values and clearly affirms the composition of complexes as formulae Eu(m-EFBA)3·(H2O)2, Eu(m-EFBA)3·neo, Eu(m-EFBA)3·batho, Eu(m-EFBA)3·phen, Eu(m-EFBA)3·bipy for complexes C1–C5 respectively.
The characteristic IR bands of ligand m-EFBA and its europium complexes C1–C5 are displayed in Table 2. The spectra of europium(III) complexes shows the ligand specific bands with relevant shifts due to the formation of complex with Eu(III) ion. The IR spectra of ligand and complex C1 display a broad band at 3639 and 3608 cm−1 assigned to enolic O–H stretching vibration and vibrational frequency of coordinated water molecule, respectively. The coordination of enolic O–H with the europium(III) ion is suggested by the absence of this broad band in the IR spectra of complexes C2–C5. The absorption bands at 1691 and 1587 cm−1 in the spectra of ligand ascribed to >C=O and >C=C shifted to 1618–1629 and 1508–1519 cm−1 in the complexes due to the extended π-conjugation of europium complexes [24]. The spectra of complex C2–C5 shows a strong band at 1577–1584 cm−1 attributed to C=N bonds of ancillary ligands. The bonding of ancillary ligands to europium ion is confirmed by the appearance of new bands in the range of 512–543 cm−1 indicating the Eu–N coordination in the complex. The coordination of ligand to europium ion is further supported by the appearance of new peaks in the range 418–430 cm−1 assigned to Eu–O bands in the spectra of complexes C1–C5 [25, 26].
The binding characteristic of ligand in europium(III) complexes were also studied by the analysis of 1H-NMR spectral data of free ligand and its corresponding complexes in CDCl3 solvent. The spectra of ligand displays enolic proton peak at 12.56 ppm and ketonic proton peak at 3.98 ppm which gets disappeared in the spectra of complexes indicating that ligand coordinates to europium ion in enolic form. The singlet at 5.66 ppm assigned to methine proton of ligand shifted upfield in the spectra of europium(III) complexes indicating the paramagnetism of europium(III) ion [27, 28].
3.2 Thermal analysis (TG/DTG)
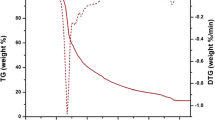
The thermal behavior of europium(III) complexes were investigated with a heating rate of 10 °C min−1 from ambient temperature to 800 °C under the atmospheric condition of nitrogen gas. All the complexes follow similar decomposition pattern, hence the complex Eu(m-EFBA)3·bipy is used as analogue to study the thermal behavior of complexes and thermogravimetric (TG) and differential thermogravimetric (DTG) curve of complex are shown in Fig. 1. The TGA curve indicates that decomposition of europium(III) complex occur in two steps. The first step involves the mass loss of 1.93 % up to 230 °C temperature attributed to the absorbed water and solvent molecule. The second decomposition step from 230 to 620 °C leads to mass loss of 66.98 % which is consistent with the decomposition of three organic ligands from the complex. The main decomposition step also confirmed by the appearance of peak at 269 °C in the DTG curve. The complex maintains 31.09 % mass up to 800 °C corresponds to the residual ancillary ligand and europium oxides. The high thermal stability of europium complexes makes these complexes suitable for the fabrication of luminescent devices.
3.3 UV–visible absorption spectra
Figure 2 displays the UV–vis absorption spectra of the organic ligand (m-EFBA) and its corresponding Eu(III) complexes in the chloroform solution (c = 1 × 10−5 M). The spectra of free ligand exhibit the absorption maxima at 301 nm while europium(III) complexes C1–C5 undergoes red shift (304–315 nm) as compared to that of ligand. The absorption band in complexes shifted as a result of extended π → π* conjugation of the chromophoric ligand indicating the coordination of ligand with europium(III) ion [29].
3.4 Photoluminescence spectra
The solid state excitation spectra of Eu(III) complexes C1–C5 are recorded by monitoring the emission band of Eu3+ ion at 613 nm as shown in Fig. 3. The excitation spectra of complexes exhibit broad band at 365–379 nm in the range 200–500 nm attributed to excitation of ligand. The spectra also display sharp bands at 465 nm, 535 nm assigned to intraconfigurational transitions 7F0 → 5D2 and 7F1 → 5D1 of Eu3+ ion [30]. The stronger intensity of broad band as compared to intraconfigurational transitions of Eu3+ ion clearly indicates that europium(III) ion is effectively sensitized by the ligand. The photoluminescence data of complexes C1–C5 tabulated in Table 3.
The emission spectra of Eu(III) complexes C1–C5 in solid state are displayed in Fig. 4 and the characteristic emission peaks of Eu3+ ion are recorded in the range of 500–700 nm with 2.5 nm slit width and 400 PMT voltage resulting due to 4f–4f transition from the resonating level 5D0 to ground state multiplet 7FJ (J = 0,1,2,3,4) of europium(III) ion. The emission transitions of Eu(III) ion employed to study the chemical environment of central europium(III) ion. The complexes shows emission peaks at 580, 590, 613, 652 and 703 nm attributed to 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 transitions of Eu(III) ion respectively [31]. Furthermore, a weak intensity emission peak is also observed at 535 nm associated with the high frequency transition of 5D1 → 7F0. The 5D0 → 7F1 is parity allowed magnetic dipole transition and independent to the local environment around Eu3+ ion. The 5D0 → 7F2 transition is an electric dipole transition whose intensity strongly depend upon the chemical environment around Eu(III) ion. This hypersensitive transition is bounded to red emission of the europium(III) complexes [32]. The intensity ratio (I1/I2 = 5D0 → 7F2/5D0 → 7F1) of complexes less than 0.7 indicates the centre of symmetry while more than 8 reveals the asymmetric environment around Eu(III) ion [33]. The higher intensity of electric dipole transition compared to magnetic dipole transition in the studied complexes indicates the low symmetry environment around Eu(III) ion. The 5D0 → 7F0 transition is principally forbidden transition but the J-mixing phenomenon is responsible for this transition to occur in the emission spectra [34]. This phenomenon is the mixing between the 7F0 and 7F2 components of the ligand field Hamiltonian and arises due to weak interaction between the Eu(III) ion and the ligand field. The maximum number of stark splitting level shown in the electronic transitions of europium(III) complexes indicates the low symmetry around the central europium ion [35]. It is further important to note the absence of residual ligand emission in the spectra of complexes which marked the role of triplet excited state of ligand in the sensitization process. The O–H vibration in the complex C1 effectively quench the luminescence intensity of Eu(III) ion (Fig. 4), hence the introduction of ancillary ligand in place of water molecule increases the intensity as a result of effective transfer of energy to Eu(III) ion [36].
3.5 CIE color coordinates, decay time (τ), quantum efficiency (η) and Judd–Ofelt parameters
The CIE (Commission Internationale de I’ Eclairage) chromaticity coordinates (x, y) of europium(III) complexes calculated from respective emission spectra are listed in Table 3. The color coordinates of complexes fall in the red region of color gamut space and lie very close to each other hence the coordinates of complexes, only C1 and C5 are displayed in Fig. 5 and are found to be well comparable with Europian Broadcasting Union (EBU illuminant red 0.64, 0.33) and National Television System committee (NTSC 1987, illuminant red 0.63, 0.34) primary color systems. The emission color in the red region proclaims the accessibility of the complexes as red component of tricolor based organic light emitting diodes (OLEDs).
The luminescence lifetimes of europium(III) complexes (C1–C5) are analysed for 5D0 → 7F2 transition monitored at emission wavelength 613 nm by assessing FL solution software of F-7000 spectrometer. The decay times derived by the equation \(I = I_{0} { \exp }( - t/\tau )\) where τ is the decay time for radiative emission, I 0 and I are the luminescence intensities at time 0 and t respectively [37] and tabulated in Table 3. The monoexponential behaviour of decay curve represented by Fig. 6 clearly reveals the presence of homogenous arrangement around the central europium ion [38]. The decay time value of complex C1 is smaller as compared to the life time values observed for the complexes C2–C5 due to the prominent nonradiative decay supported with vibronic coupling of the solvent molecules [39]. The longer lifetime values of the complexes C2–C5 indicates the presence of respective ancillary ligands which suppresses the nonradiative decay channel among various transition levels and effectively transfer the energy to the metal ion.
The luminescence quantum efficiency (η) of 5D0 emitting level of europium(III) complexes C1–C5 is determined on the basis of emission spectra and decay time to express how efficiently the radiative (Arad) and nonradiative (Anrad) processes compete with each other [40, 41]
where Arad and Anrad are the rate constants for radiative and nonradiative processes, respectively. The radiative rate constant can be estimated by summation of radiative rates (A0J) for each emission transition (5D0 → 7FJ) of Eu3+ ion. The radiative rate (A0J) can be calculated according to following equation [42]:
where I0J and \(\upnu_{0J}\) are integrated emission intensity and energy barrier for the 5D0 → 7FJ transition, respectively. A01 is the spontaneous emission coefficient for the magnetic dipole transition (5D0 → 7F1) which is independent of the chemical environment around central europium(III) ion, hence used as a reference to determine the A0J value for different transitions (for J = 0,2,3,4). The value of A01 is found to be 50 s−1 [43].
The radiative and nonradiative processes have found pronounced effect on the experimental luminescence lifetime as given by the equation [44]:
The obtained value for luminescence quantum efficiency, radiative and nonradiative rate constants are listed in Table 3. The luminescence quantum efficiency depends mainly on the decay time value and the intensity ratio of the electric dipole to magnetic dipole transition. The lower value of quantum efficiency in complex C1 due to smaller value of decay time and excessive non-radiative transitions produce as result of vibrations coordinated water molecule. Hence, the introduction of ancillary ligands in place of water molecules effectively enhance the transfer of energy to the europium(III) ion and the quantum efficiency. It is clearly represented in Table 3 that complex C5 possess higher quantum efficiency and longer decay time values which are in good concurrence with the luminescence intensity of complexes as shown in Fig. 4.
The Judd–Ofelt theory is an important tool to study the 4f–4f electronic transitions. The intensity parameters Ωλ of the ligand field comprehend the augmentation from forced electric dipole and dynamic coupling mechanism [45]. These intensity parameters Ωλ (λ = 2, 4) can be estimated from the emission intensities of the 5D0 to 7FJ (J = 2, 4) levels of europium(III) ion and given by following equation [46]:
where χ is the Lorentz local field correction given by n2(n2 + 2)2/9, n is the average index of refraction (1.5) used, \(\hbar\) is Planck’s constant over 2π, c is velocity of light and \(\left\langle {5_{{D_{0} }} \left| {U^{\left( \uplambda \right)} } \right|7_{{F_{J} }} } \right\rangle^{2}\) is the square matrix element 0.0032 (J = 2) and 0.0023 (J = 4) respectively. e is the electronic charge (4.80 × 10−10 esu) and ω is the angular frequency of the transition. The intensity parameters Ω2 and Ω4 for complxes C1–C5 are represented in Table 3.
The intensity parameter Ω2 associated with the hypersensitive behavior around the central Eu(III) ion and proportional to the covalency between the ligand field and europium(III) ion. The higher value of intensity parameter Ω2 for the complex C5 suggesting the highly asymmetric local environment around europium(III) ion and high covalency of ligand field [47]. The interionic bond distances and the ion to ligand bond distances affect the intensity parameter Ω4. 3.6 Energy transfer mechanism.
To investigate the energy transfer mechanism in europium(III) complexes, the phosphorescence spectra of complex C6 (Gd(m-EFBA)3·(H2O)2) and UV–visible absorbance edge of the ligand (m-EFBA) are employed to calculate the triplet (T1) and singlet (S1) energy states of ligand respectively [48]. The excited triplet state of ligand cannot transfer the energy to the higher energy emitting level of gadolinium(III) ion, hence the lowest phosphorescence band of complex gives the energy of triplet state of ligand as illustrated in Fig. 7. All the complexes C1–C5 follow the similar energy transfer mechanism, hence the highly luminescent complexes C3–C5 are employed to study the sensitization process in complexes and mechanism of energy transfer clearly illustrated in Figs. 8, 9 and 10 respectively. The singlet and triplet energy state of ligands [39] are given in Table 4.
It is clearly revealed from Table 4 that the energy difference between the singlet and triplet excited states [∆E (S1 − T1)] is optimal for the efficient intersystem crossing in complexes. The energy difference from the triplet state of ligand to the emitting level of europium(III) ion is suitable for the effective energy transfer as governed by the Latva’s empirical rule [49]. The energy transfer mechanism involve the transfer of energy from S1 state to T1 state of ligands and from T1 state of ligand to the excited energy state of Eu(III) ion. The ancillary ligand first absorb the energy and then transfer it to the ligand m-EFBA which then transfer the energy to the excited state of europium(III) ion. The luminescence of complexes C3 is associated with the inverse energy transfer process occurs due to smaller energy gap between triplet state of ancillary ligand batho and excited level of Eu(III) ion [∆E (T1 − M3+)] compared to complex C4 and C5 in which the diminished rate of inverse energy transfer occurs between the triplet state of ligand phen, bipy to the emitting level of europium(III) ion. The energy transfer mechanism in binary and ternary europium(III) complexes endorsed with the help of Dexter electron exchange theory [50] and the thermal de-excitation theory [51] which is also concordant with the results observed from the emission spectra of complexes C1–C5.
4 Conclusion
In this work, a series of new binary and ternary europium(III) complexes Eu(m-EFBA)3·(H2O)2 (C1), Eu(m-EFBA)3·neo (C2), Eu(m-EFBA)3·batho (C3), Eu(m-EFBA)3·phen (C4), Eu(m-EFBA)3·bipy (C5) have been synthesized and analysed by elemental analysis, 1H-NMR, IR, TG/DTG, UV–visible and PL spectroscopy. All the complexes C1–C5 exhibit characteristic red emission upon excitation with UV light and considered as a potential applicant in the field of luminescent materials. The energy transfer mechanism of complexes clearly reveals that light harvesting ancillary ligands support the organic ligand in the transfer of energy to emitting level of europium(III) ion. The luminescence quantum efficiency of complexes estimated from the decay curves and emission spectra of complexes. The Judd–ofelt intensity parameters (Ω2 and Ω4) are determined from the emission spectra of complexes in solid state. The higher quantum efficiency and intensity parameter Ω2 value for complex C5 indicating the efficient energy transfer from ligand to metal ion as well as asymmetric coordination environment around europium ion. The energy transfer mechanism proves the significance of ligand as a good chromophore for the efficient energy transfer to the europium(III) ion.
References
S.I. Weissman, J. Chem. Phys. 10, 214 (1942)
L.R. Melby, N.J. Rose, E. Abramson, J.C. Caris, J. Am. Chem. Soc. 86, 5117 (1964)
K. Binnemans, P. Lenaerts, K. Driesen, C. Görller-Walrand, J. Mater. Chem. 14, 191 (2004)
J.C. Bünzli, C. Piguet, Chem. Soc. Rev. 34, 1048 (2005)
G. Muller, Dalton Trans. 44, 9692 (2009)
S.V. Elisseva, J.C. Bünzli, Chem. Soc. Rev. 39, 189 (2010)
J.Q. Liu, Y.Y. Wang, S.R. Batten, H. Sakiyama, D.Y. Ma, Inorg. Chem. Commun. 19, 27 (2012)
X. Zhu, H.Y. Zheng, X.F. Wei, Z.Y. Lin, L.H. Guo, B. Qiu, G.N. Chen, Chem. Commun. 13, 1276 (2013)
L.X. Shi, C.D. Wu, Chem. Commun. 10, 2928 (2011)
Y.G. Lee, H.R. Moon, Y.E. Cheon, M.P. Suh, Angew. Chem. Int. Ed. 40, 7741 (2008)
T. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 131, 13860 (2009)
J.R. Morrow, Comments Inorg. Chem. 29, 169 (2008)
E.R. Farquhar, J.P. Richard, J.R. Morrow, Analog Inorg. Chem. 46, 7169 (2007)
V. Bekiari, P. Lianos, Adv. Mater. 10, 1455 (1998)
B. Gao, Z. Qiao, T. Chen, Mater. Chem. Phys. 143, 1119 (2014)
K. Binnemans, Chem. Rev. 109, 4283 (2009)
A.P. Souza, F.A.A. Paz, R.O. Freire, L.D. Carlos, O.L. Malta, S. Alves Jr., G.F. de Sà, J. Phys. Chem. B 111, 9228 (2007)
I.R. Lasker, T.M. Chen, Chem. Mater. 16, 111 (2004)
Z. Bao, A.J. Lovinger, J. Brown, J. Am. Chem. Soc. 120, 207 (1998)
C. Yang, L.M. Fu, Y. Wang, J.P. Zhang, W.T. Wong, X.C. Ai, Y.F. Qiao, B.S. Zou, L.L. Gui, Angew. Chem. Int. Ed. 43, 5010 (2004)
A. Dossing, Eur. J. Inorg. Chem. 2005, 1425 (2005)
M. Bala, S. Kumar, V.B. Taxak, P. Boora, S.P. Khatkar, J. Fluorine Chem. 178, 6 (2015)
E.J. Roh, J.M. Keller, Z. Olah, M.J. Ladarola, K.A. Jacobson, Bioorg. Med. Chem. 16, 9349 (2008)
D. Wang, C. Zheng, L. Fan, J. Zheng, X. Wei, Synth. Met. 162, 2063 (2012)
W. Zhang, C.H. Liu, R.R. Tang, C.Q. Tang, Bull. Korean Chem. Soc. 30, 2213 (2009)
M. Arvind, K. Sageed, Indian J. Chem. 25A, 589 (1986)
L. Fu, R.A.S. Ferreira, N.J.O. Silva, A.J. Fernandes, P. Ribeiro-Claro, S. Goncalves, V.D.Z. Bermudez, L.D. Carlos, J. Mater. Chem. 15, 3117 (2005)
Y. Zhang, H. Shi, Y. Ke, Y. Cao, J. Lumin. 124, 51 (2007)
D. Wang, C. Zheng, L. Fan, Y. Hu, J. Zheng, Spectrochim. Acta Mol. Biomol. Spectrosc. 117, 245 (2014)
M. Bala, S. Kumar, P. Boora, V.B. Taxak, A. Khatkar, S.P. Khatkar, J. Mater. Sci.: Mater. Electron. 25, 2850 (2014)
G. Shao, Y. Li, K. Feng, F. Gan, M. Gong, Sens. Actuators B 173, 692 (2012)
J. Kai, D.F. Parra, H.F. Brito, J. Mater. Chem. 18, 4549 (2008)
A.F. Kirby, D. Foster, F.S. Richardson, Chem. Phys. Lett. 95, 507 (1983)
F.S. Richardson, Chem. Rev. 82, 541 (1982)
R. Ilmi, K. Iftikar, Polyhedron 102, 16 (2015)
H. Wang, P. He, H. Yan, M. Gong, Sens. Actuators B 156, 6 (2011)
N. Sabbatini, M. Guardigli, J.M. Lehn, Coord. Chem. Rev. 123, 201 (1993)
H.F. Brito, O.L. Malta, L.R. Souza, J.F.S. Menezes, C.A.A. Carvalho, J. Non-Cryst, Solids 247, 129 (1999)
D.B.A. Raj, S. Biju, M.L.P. Reddy, Inorg. Chem. 47, 8091 (2008)
M.H.V. Werts, R.T.F. Jukes, J.W. Verhoeven, Phys. Chem. Chem. Phys. 4, 1542 (2002)
S. Stanimirov, I. Petkov, Spectrochim. Acta Mol. Biomol. Spectrosc. 72, 1127 (2009)
L.D. Carlos, Y. Messaddeq, H.F. Brito, R.A.S. Ferreira, V.D. Bermudez, S.J.L. Ribeiro, Adv. Mater. 12, 594 (2000)
R. Ferreira, P. Pires, B.D. Castro, R.A.S. Ferreira, L.D. Carlos, U. Pischel, N. J. Chem. 28, 1506 (2004)
G.F. de Sà, O.L. Malta, C. de Mello Donegà, A.M. Simas, R.L. Longo, P.A. Santa-Cruz, E.F. da Silva Jr., Coord. Chem. Rev. 196, 165 (2000)
M.C.F.C. Felinto, C.S. Tomiyama, H.F. Brito, E.E.S. Teotonio, O.L. Malta, J. Solid State Chem. 171, 189 (2003)
E.E.S. Teotonio, H.F. Brito, M.C.F.C. Felinto, C.A. Kodaira, O.L. Malta, J. Coord. Chem. 56, 913 (2003)
F. Cagnin, M.R. Davolos, E.E. Castellano, Polyhedron 67, 65 (2014)
P. Gawryszewska, J. Sokolnicki, J. Legendziewicz, Coord. Chem. Rev. 249, 2489 (2005)
M. Latva, H. Takalo, V.M. Mukkala, C. Matachescu, J.C.R. Ubis, J. Kankare, J. Lumin. 75, 149 (1997)
D.L. Dexter, J. Chem. Phys. 21, 836 (1953)
C.R.S. Dean, T.M. Shephred, J. Chem. Soc. Faraday Trans. 2(71), 146 (1975)
Acknowledgments
This work was financially supported in the form of senior research fellowship (SRF) from UGC, New Delhi, India (Award No: 2121210101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicated no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Devi, R., Dalal, M., Bala, M. et al. Synthesis, photoluminescence features with intramolecular energy transfer and Judd–Ofelt analysis of highly efficient europium(III) complexes. J Mater Sci: Mater Electron 27, 12506–12516 (2016). https://doi.org/10.1007/s10854-016-5760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5760-2