Abstract
The novel water-soluble poly(vinyl alcohol) with pendant rhodamine B moiety as colorimetric and fluorescene chemosensor for Hg2+ ions was prepared by grafting poly(vinyl alcohol) using rhodamine B hydrazide and hexamethylenediisocyanate as fluorescent dye and coupling agent, respectively. Because of their good water-solubility, the polymers binding rhodamine B can be used as chemosensors in aqueous media. With the addition of Hg2+ ions into the aqueous solution, visual color changes and fluorescence enhancements were detected. In addition, we also noticed that other metal ions such as Ag+, Cd2+, Co2+, Cu2+, K+, Mg2+, Ba2+, Fe2+, Ni2+, Pb2+, Cr3+, Fe3+ and Zn2+ cannot induce obvious changes to the fluorescence spectra of the polymer chemosensors. The combination of water solubility and positive fluorescence response as well as color change are hence particularly promising for the practical utility of the sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is one of the most toxic heavy metal elements, which could cause nervous disorders such as acrodynia, Hunter-Russell syndrome, and Minamata disease [1], while the mercury element also have wide applications in many industrially products, such as medicine, cosmetics, and optics. Therefore, it gives rise to the increased risk associated with mercury poisoning [2]. As a result, it is necessary to design and develop a novel fluorescent sensor for the Hg2+ ions detection, which have attracted many attentions [3, 4]. One advantage of this technique is that sensing phenomena can be readily used to select a quantified chemical species without using sophisticated, expensive, and time-consuming instruments [5–12]. Rhodamine-based fluorescent chemosensors have recently witnessed great development for sensing of mercury ions [13], owing to their excellent spectroscopic properties such as large molar extinction coefficient and high fluorescence quantum yields [2, 14, 15]. Moreover, it is well-known that many derivatives of rhodamine undergo equilibrium between spirolactam and a ring-opened amide, and both conformations always behave with completely different spectroscopic properties. The rhodamine with spirolactam structure is non-fluorescent, whereas ring-opening of the spirolactam gives rise to a strong fluorescence emission. This property provides an ideal mode to construct OFF–ON molecular switch [10]. Actually, the vast majority of sensing routes uses “conventional” spirolactam-based isomers to achieve the switch functionality. Recently, rhodamine-based sensors for cations and other analytes have received ever-increasing attention in many areas such as sensors for Pb2+, Cu2+, Hg2+, Fe3+, Cr3+, and NO [2, 16–18], whereas the corresponding ring-opened amide provides both chromogenic and fluorogenic responses that facilitate “naked eye” analyte detection [1].
Organic and organometallic rhodamine-based receptors are commonly used as sensory molecules for the detection of various chemicals. However, these small molecule chemosensors typically exhibit poor water solubility and usually only function in a medium of pure organic solvent or an aqueous solution containing at least 50 % organic cosolvent [12]. The lack of water solubility greatly limits the potential applications of rhodamine-based small molecules in biological systems and in environmental analyses [19, 20]. To solve the problem, covalent methods have been developed for incorporating organic dyes by attaching them to the backbone of the amphiphilic polymers [2]. Polymer system has advantages over low-molecular-weight system due to its stability, easy handling, good film-forming ability and reuse. As reported by researchers, there are some fluorescent polymer sensors with different macromolecular structure were designed for the detection of metal cations in the environment [21]. In order to obtained a successful chemsensor, the high quantum yields of fluorescence, high extinction coefficients, long excitation and emission wavelengths, long life times, and photostability are requirements [22]. Considering all those facts, the choice of the fluorophore and polymer become the most important aspect for attaining an appropriate chemosensor [23, 24].
Poly(vinyl alcohol) (PVA) is a kind of commercial polymer material, which has excellent water-solubility, good film forming ability, and miscibility properties. PVA can be modified easily due to the presence of abundant -OH functional groups in their backbone. Recently, increasing investigations and reports show that PVA was widely used as a superior polymer matrix material in the photoluminescence field [25]. The integration of the small molecular dye and PVA forms a kind of new multifunctional material which owns some applications in the field of optics and material science [26].
In this study, we have successfully synthesized a new kind of fluorescent polymeric material PVA-HRBH bearing rhodamine B via grafting reaction as shown in Scheme 1. The influences of different metal cations on the fluorescence intensity of the polymer sensors were also studied in aqueous solution. We expected that the affording water-soluble polymer has sensitivity and selectivity as small molecular sensor, which could be used in the field of metal cations pollution detection in the environment.
Experimental
Materials
Poly(vinyl alcohol) with the number average molecular weight of 7.50 × 104 and degree of hydrolysis (saponification) of 99.0 % was purchased from Anhui Wanwei updated High-tech material industry company limited (Anhui, China) and dried in vacuum at 40 °C for 24 h before use. Rhodamine B and hexamethylenediisocyanate (HDI) were purchased from Sigma-Aldrich Trading Co. Ltd. (Shanghai, China); Hydrazine hydrate, and dimethyl sulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and used without further purification; Anhydrous methanol and ethanol, ethylacetate, n-hexane and toluene were obtained from commercial suppliers and purified using distillation before use. Rhodamine B hydrazide (RBH) was prepared according to the literature method [27, 28].
Preparation of RBH-Mono-isocyanate (HRBH)
In a typical experiment, 0.168 g (1.00 mmol) of HDI in 10 mL of toluene was added to a three-necked flask. Then, 0.456 g (1.00 mmol) of RBH in 20 mL of toluene was added dropwisely into the flask at 100~110 °C. The mixture was stirred in a reflux condition for 12 h under the nitrogen atmosphere. After cooling the mixture to room temperature, the solvent was evaporated in vacuum to afford a purple precipitate. The crude was purified by column chromatography on alumina (ethylacetate/n-hexane, 15: 1) yielding the purple powders (yield: 85.94 %) [29, 30]. FT-IR of HRBH (KBr), cm−1: 3424.45 (s, ν-NH); 2970.78 (s), 2970.78 (s), 2934.67 (s), 2853.42 (s) [νC-H (CH2, CH3)]; 2271.10 (m, ν-N=C=O), 1715.86(s, νc=o); 1616.55 (s, νAr C = C); 1508.21 (s, δN-H), 1465.33 (m, νAr C = C), 1426. 96 (m, νAr C = C). 1H NMR of HRBH (400 MHz, CDCl3), (δ, ppm): 7.907 (m, 1H, Ph); 7.592–4.476(m, 2H, Ph); 7.257–7.164(m, 2H, NH); 7.111–7.084 (m, 4H, Ph); 3.260 (s, 8H, Me-CH2-N-); 3.242–3.180 (m, 4H, N-CH2-); 2.557–2.284 (m, 8H, −CH2-); 0.909–0.766 (t, 12H, CH3). Anal. calc. for C36H44O4N6 (%): C, 69.21; H, 7.10; N, 13.45. Found (%): C, 69.45; H, 7.32; N, 13.36.
Preparation of PVA-HRBH
One gram of the PVA in 10 mL of DMSO was heated up to 80 °C. After a homogeneous solution was obtained, the temperature was then cooled down to 50 °C. HRBH (0.0586, 0.1171, or 0.2343 g) in 10 mL of DMSO were added dropwisely to the homogeneous solution under vigorous stirring. After 2 h of reaction at 80 °C, the solution was cooled down to room temperature. The resulting polymer was precipitated into anhydrous ethanol. The cycle of the precipitation was repeated three times in excess anhydrous ethanol. The final product was dried at 50 °C under vacuum, affording pink solid (yield: 65.09 %, 64.13 %, 55.88 %) [30]. FT-IR of PVA-HRBH (KBr), cm−1: 3380.06(ν PVA-OH), 2940.69; 2899.31(ν-CH2-, −CH-); 1682.76(νC=O); 1606.77, 1518.00, 1270.47, 1229.09(νaromaticC=C); 1098.18(νC-O); 1435.24 (δC-H). 1H NMR of PVA-HRBH (400 MHz, DSMO-d6), (δ, ppm): 0.941 (m, CH3); 1.040–1.074(m, CH2); 2.504(m, N-CH 2-CH3); 3.353 (s, −CH-OH); 3.457–3.410(m, −OOCCH 3); 3.631 (s, −CH-OOCCH3); 3.834, 4.570–4.339, 4.660 (m, OH); 4.215(s, −CH-HRBH); 6.281(s, Ф-H); 6.328 (s, Ф-H); 6.400(s, Ф-H); 7.017(s, Ф-H); 7.034–7.236(d, Ф-H); 7.527(s, Φ-H); 7.549–7.568(m, Φ-H); 7.811–7.828(m, Φ-H).
Preparation of Stock Solution
PVA-HRBH was hard to dissolve in water at room temperature, so the polymer solutions of desired concentrations were prepared by dissolving a known amount of polymer 0.1000 g in doubly distilled water with gentle stirring at 80 °C for 3 h to ensure homogenization. Then, the solution was transferred to 50.00 mL volumetric flask, achieved 2.000 mg/mL polymer aqueous solution [30]. The different ions stock solutions of 0.100 mol/L, including Ag+, Cd2+, Co2+, Cu2+, K+, Mg2+, Ba2+, Fe2+, Ni2+, Hg2+, Pb2+, Cr3+, Fe3+ and Zn2+ were prepared in doubly distilled water, respectively.
Instruments and Measurements
The 1H NMR spectra were recorded on a DRX 400 Bruker spectrometer (AVANCE AV 400, Bruker corporation, Switzerland) at 298 K in CDCl3 or DSMO-d6 with TMS as internal standard. FT-IR spectra were recorded on a Nicolet Neus 8700 FI-IR spectrophotometer (Thermo Scientific Instrument Co. U.S.A). Elemental analyses (C, H and N) were carried out on a VarioELIII analyzer (Elementar corporation, Germany) for the monomer HRBH. All pH measurements were made with a Model pHS-3C pH meter (Shanghai, China). Fluorescence spectra were acquired on a RF5301PC fluorescence spectrophotometer (Shimadzu Corporation, Japan). For fluorescence emission measurements, a 10 × 10 mm quartz cell was used for detection. The effect of the metal cations on fluorescence intensity was examined by adding a few microlitre of stock solution of the metal cations to a known volume of the polymer solution (2.00 mL). The addition was limited to 0.10 mL, so that the dilution of the polymer solution remained insignificant [21]. The excitation and the emission slit widths were 10 nm and 5 nm, respectively, excitation wavelength was 500 nm, scanning range were from 520 to 650 nm unless otherwise noted, scanning speed was medium, testing temperature was at 25 °C. The detection limit was calculated with the equation: detection limit = 3S/ρ, where S is the standard deviation of blank measurements and ρ is the slope between intensity versus sample concentration.
Results and Discussion
Design and Synthesis
PVA-HRBHs containing rhodamine B were prepared using post-functionalization strategy as shown in Scheme 1. RBH-mono-isocyanate (HRBH) was prepared by the reaction of HDI and the equal molar of RBH. The choice of HDI as the spacer is aimed to endow the PVA polymer and RBH with the -NCO functional group. The rhodamine moiety was linked to the HDI through the reaction between -NH2 of RBH and the –NCO of HDI. Then the HRBH was grafted to the PVA by means of the reactions of another -NCO functional group of HRBH with -OH group of PVA. The hexamethylene group [(CH2)6] was as a spacer. The urea bond (−NH-CO-NH-) formed by –NCO and NH2 provided N and O binding sites for selective recognition of Hg2+. The whole process is just by a simple extraction or re-precipitation cycles; it is possible to perform the treatment and purification of the polymers, avoiding the complex and tedious separation and purification steps of small molecules containing rhodamine. The resulted PVA-HRBHs were pink powders whose colors are derived from the HRBH. The more the feeding of HRBH was, the deeper the color of the powder was [11, 30].
Characterization
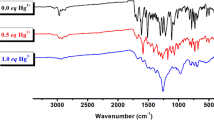
FT-IR and 1H NMR were used to prove a link between rhodamine B moiety and the PVA. Comparing the FT-IR spectra of PVA-HRBH with those of HRBH, we found that the peaks at 2271.10 cm−1 characteristic of -NCO group, which present to the FT-IR spectra of HRBH, disappeared completely in the FT-IR spectra of PVA-HRBH. There are the characteristic peaks of RBH at 1518, 1435 and 1229 cm−1 appeared in the FT-IR spectra of both HRBH and PVA-HRBH. These are clear evidence of success in grafting RBH to PVA, affording the target polymer PVA-HRBH. The 1H NMR spectra of HRBH and PVA-HRBH in CDCl3 and DSMO-d6 are shown that characteristic signals corresponding to the xanthene and benzene rings of RBH (δ(H) 6.0–8.0) appeared in the spectrum of PVA-HRBH. This observation also confirmed that the HRBH had been successfully incorporated into the PVA. The peaks at 1.2–5.0 ppm were assigned to the aliphatic CH3, CH2 and CH groups of PVA.
Solubility and Fluorescence Properties of PVA-HRBH
The solubility of PVA-HRBHs with different amount of HRBH was shown in Table 1. It can be seen that all of PVA-HRBHs (feed of HRBH (molar fraction): 0.5, 1.0 and 2.0 %) dissolved in DSMO. A series of fluorescence curves of PVA-HRBHs in DSMO are shown in Fig. 1 (The scanning range were from 520 to 700 nm. The concentration of polymer was 2.00 mg/mL. The concentration of Hg2+ was 2.5 × 10−5 mol/L). The fluorescence intensity of both PVA-HRBH and complex-Hg2+ increased with the increase of the amount of HRBH. In contrast, the solubility of PVA-HRBH in water present an opposite tendency caused by the hydrophobic properties of HRBH. While the feed of HRBH was run up to 2.0 % (molar fraction), the PVA-HRBH might be insoluble in water. It is also worth notice that PVA is hydrophilic polymer which provides a good solubility of PVA-HRBH with HRBH of 0.5 and 1.0 % in water. It is a key requirement for its sensing application.
Response Time
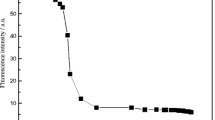
It is well known that the response time is an important matter for an excellent chemical sensor. A short response time is necessary for a fluorescent chemosensor to monitor Hg2+ in aqueous samples. The time course of the response of PVA-HRBH (1.0 %, 0.70 mg/mL) to Hg2+ (2.5 × 10−5 mol/L) in neutral aqueous solution was investigated (Fig. 2). The experimental results suggested that the recognition interaction was completed within 5 min after the addition of Hg2+ [2, 5].
Effects of pH Value
The effects of pH value on the fluorescence intensity of PVA-HRBH in the absence of Hg2+ were investigated in the pH range from 2.39 to 11.09 (Fig. 3). The aqueous solution of PVA-HRBH (1.0 %, 0.10 mg/mL) did not display any obvious and characteristic fluorescence (excited at 500 nm) at pH value range from 5.57 to 11.09, suggesting that it was stable over the pH value range of 5.57–11.09 and could work in real environmental and biological samples with low background fluorescence. When pH value was lower than 5.57, the fluorescence intensity increased rapidly with decreasing pH values, which might be caused by the ring-opened form of rhodamine B. This result suggests that no buffer solutions are required for the detection of Hg2+ in the basic or neutral media as optimum experimental condition, and this is convenient for practical application [11, 12, 30].
The Influence of Metal Cations on the Polymer Fluorescence Properties
The changes in the fluorescence intensity induced by alkali, alkaline earthmetal ions, and transition metal ions were investigated and the fluorescence responses of PVA-HRBH (1.0 %, 0.1 mg/mL) were presented in Figs. 4 and 5 [2, 11, 12, 18]. As seen, the addition of metal cations led to a change of the fluorescence intensity, which was different for each metal cation [26]. The titration of Hg2+ into the aqueous polymer solution, obvious fluorescence enhancement could be seen and the color of the solution was changed from colorless to pink. While the addition of other metal ions including Ag+, Ba2+, Cd2+, Co2+, Cu2+, Fe2+, K+, Mg2+, Ni2+, Pb2+, Cr3+, Fe3+ or Zn2+ did not induce any obvious fluorescence enhancement and color change. Therefore, PVA-HRBH was a highly selective chemosensor for Hg2+, which was probably due to the cooperating of several combined influences, such as the suitable coordination geometry, the proper radius and charge density of the Hg2+ ion, and the amide deprotonation ability of Hg2+ [11].
The competitive experiments were conducted in the presence of Hg2+ (5.0 × 10−5 mol/L) mixed with 1 equiv. of various cations, respectively. The fluorescence spectra were recorded at 500 nm within 5 min after the addition of these metal ions and the subsequent addition of Hg2+ to the above solutions. As shown in Fig. 6, the Hg2+-induced relative fluorescence intensity (I/I0, here I is the fluorescence emission intensity of PVA-HRBH actually measured at a given metal concentration, I0 is the fluorescence emission intensity of the PVA-HRBH without metal cations) enhancement was not obviously affected in the presence of Ag+, Ba2+, Cd2+, Co2+, Cu2+, Fe2+, K+, Mg2+, Ni2+, Pb2+, Cr3+, Fe3+ or Zn2+ cation. The competition experiments revealed that the Hg2+-induced luminescence response will be unaffected in a background of environmentally relevant other ions. Selectivity and competition experiments showed that PVA-HRBH has a remarkable selectivity toward Hg2+ [11, 18, 26].
The Influence of Hg2+ Ion Concentration on the Polymer Fluorescence Intensity
The emission spectra were also recorded under the same condition. Like most of the spirocycle RBH derivatives, the aqueous solution of PVA-HRBH (1.0 %, 0.1 mg/mL) was colorless and exhibited weak fluorescence at about 572 nm in neutral water, indicating that the spirolactam form was the predominant species. After the addition of Hg2+ into the water solution of PVA-HRBH, a new strong absorption band centered at about 578 nm was observed (Fig. 7) and the color of solution was changed from colorless to purple. As shown, the higher the concentration of added Hg2+ was, the deeper the color of the aqueous solution was (Fig. 8). Meanwhile, a dramatic change in the fluorescence spectra was also obtained. The fluorescence maximum wavelength was red-shift from 572 to 578 nm, and up to about 11-fold enhancement in the fluorescence intensity was noted. This indicates that the Hg2+ induces a highly conjugated rhodamine system via formation of opened-spirolactam to give a strong fluorescence emission [4]. The color change and OFF-ON fluorescence support our expectation that PVA-HRBH could serve as a sensitive fluorescent switcher as well as a naked-eye chemosensor for Hg2+ in aqueous solution [2, 11].
Figure 9 shows the plot of the relative fluorescence intensity (I/I0) of PVA-HRBH (excition wavelength at 500 nm) versus the concentration of added Hg2+, and a good linearity relationship is obtained in concentration range from Hg2+-free solution to 8.0 × 10−5 mol/L, which was described by a function \( \raisebox{1ex}{$I$}\!\left/ \!\raisebox{-1ex}{${I}_0$}\right.=1.1087+1.6735\times {10}^5\left[H{g}^{2+}\right] \) with the correlation coefficient R = 0.9976 [11]. The corresponding detection limits (3S/ρ: S, standard deviation; ρ, slope) were calculated to be 5.72 × 10−7 mol/L for Hg2+. According to the results, it could be assumed that the novel fluorescent polymer had potential prospects as a selective detector of Hg2+ ion in aqueous environment [2, 11].
Conclusions
A simple and low-cost post-functionalization strategy was adopted to prepare a novel fluorescent Hg2+ polymeric chemosensor by covalent coupling of organic fluorescent molecular RBH to a water-soluble polymer PVA with HDI as coupling agent. The fluorescence properties of PVA-HRBH in solution were evaluated in detail. The selectiveness of PVA-HRBH to various of metal cations including Ag+, Cd2+, Co2+, Cu2+, K+, Mg2+, Ba2+, Fe2+, Ni2+, Hg2+, Pb2+, Cr3+, Fe3+ and Zn2+ were determined. In the presence of these metal cations, the fluorescence intensity of the polymer was enhanced and the I/I0 of each cation was different. Moreover, clear color changes from colorless to pink were observed. The highest enhancing effect was noticed in the presence of Hg2+ ion. The results revealed that the aqueous solutions of the polymeric chemosensor can be used for the colorimetric, fluorogenic detection, as well as quantification of Hg2+. In particular, it represents one of the few fluorescent sensors that allow a selective and sensitive detection of Hg2+ in aqueous medium without any organic co-solvent required. We believe that PVA-HRBH can be used for many practical applications in chemical, environmental and biological systems.
References
Lee SH, Parthasarathy A, Schanze KS (2013) Based on amplified fluorescence quenching in a conjugated polyelectrolyte/spiro-cyclic rhodamine system. Macromol Rapid Commun 34:791–795
Wang Y, Wu H, Luo J, Liu XY (2012) Synthesis of an amphiphilic copolymer bearing rhodamine moieties and its self-assembly into micelles as chemosensors for Fe3+ in aqueous solution. React Funct Polym 72:169–175
Liu XF, Tang YL, Wang LH, Zhang J, Song SP, Fan C, Wang S (2007) Optical detection of mercury(II) in aqueous solutions by using conjugated polymers and label-free oligonucleotides. Adv Mater 11:1471–1474
Li D, Wieckowska A, Willner I (2008) Optical analysis of Hg2+ ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines. Angew Chem Int Ed 47:3927–3931
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107:3780–3799
Cheng TY, Wang T, Zhu WP, Chen XL, Yang YJ, Xu YF, Qian XH (2011) Fluorescent probe sensing cadmium and pyrophosphate selectively in aqueous solution. Org Lett 13:3656–3659
Wu JS, Hwang IC, Kim KS, Kim JS (2007) Rhodamine-based Hg2+-selective chemodosimeter in aqueous solution: fluorescent OFF-ON. Org Lett 9:907–910
Sanchez JF, Entwistle R, Hung JH, Yaegashi J, Jain SF, Chiang YM, Wang CCC, Oakley BR (2011) Genome-based deletion analysis reveals the prenyl xanthone biosynthesis pathway in aspergillus nidulans. J Am Chem Soc 133:4010–4017
Rosenthal J, Lippard SJ (2010) Direct detection of nitroxyl in aqueous solution using a tripodal copper(II) BODIPY complex. J Am Chem Soc 132:5536–5537
Huang W, Wu DY, Wu GH, Wang ZQ (2012) Dual functional rhodamine-immobilized silica toward sensing and extracting mercury ions in natural water samples. Dalton Trans 41:2620–2625
Luo J, Jiang SS, Qin SH, Wu HQ, Wang Y, Jiang JQ, Liu XX (2011) Highly sensitive and selective turn-on fluorescent chemosensor for Hg2+ in pure water based on a rhodamine containing water-soluble copolymer. Sens Actuators B Chem 160:1191–1197
Kaoutit HE, Estévez P, Ibeas S, García FC, Serna F, Benabdelouahab FB, García JM (2013) Chromogenic and fluorogenic detection of cations in aqueous media by means of an acrylic polymer chemosensor with pendant rhodamine-based dyes. Dye Pigment 96:414–423
Wang HH, Xue L, Yu CL, QianYY JH (2011) Rhodamine-based fluorescent sensor for mercury in buffer solution and living cells. Dye Pigment 91:350–355
Dujols V, Ford F, Czarni A (1997) A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J Am Chem Soc 119:7386–7387
Beija M, Afonso CAM, Martinho JMG (2009) Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem Soc Rev 38:2410–2433
Yang YK, Yook KJ, Tae J (2005) A Rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127:16760–16761
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127:10107–10111
Niamsa N, Kaewtong C, Srinonmuang W, Wanno B, Pulpoka B, Tuntulani T (2013) Hybrid organic–inorganic nanomaterial sensors for selective detection of Au3+ using rhodamine-based modified polyacrylic acid (PAA)-coated FeNPs. Polym Chem 4:3039–3046
Qi Y, Li NJ, Xu QF, Xia XW, Ge JF, Lu JM (2011) A cancer-targetable f containing tyrosine segments for labeling radioactive halogens. React Funct Polym 71:390–394
Zhu M, Zhou CJ, Zhao YJ, Li YJ, Liu HB, Li YL (2009) Synthesis of a fluorescent polymer bearing covalently linked thienylene moieties and rhodamine for efficient sensing. Macromol Rapid Commun 30:1339–1344
Wang BY, Guan XL, Hu YL, Su ZX (2008) Synthesis and photophysical behavior of a water-soluble fluorescein-be aring polymer for Fe3+ ion sensing. J Polym Res 15:427–433
Wang BY, Liu XY, Ding SL, Su ZX (2011) Synthesis and photop hysical properties of a blue water-soluble fluorescent polymer for Ni2+ and proton sensing. J Polym Res 18:1315–1322
Adhikari B, Majumdar S (2004) Polymers in sensor applications. Prog Polym Sci 29:699–766
Kaya İ, Kamacı M (2013) Highly selective and stable florescent sensor for Cd(II) based on poly (azomethine-urethane). J Fluoresc 23:115–121
Wang BY, Guan XL, Hu YL, Su ZX (2007) Preparation and fluorescent properties of poly(vinyl alcohol) bearing coumarin. Polym Adv Technol 18:529–534
Nithyaja B, Misha H, Radhakrishnan P, Nampoori VPN (2011) Effect of deoxyribonucleic acid on nonlinear optical properties of Rhodamine 6G-polyvinyl alcohol solution. J Appl Phys 109:23–110
Yang XF, Guo XQ, Zhao YB (2002) Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 57:883–890
Xiang Y, Tong AJ, Jin PY, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8:2863–2866
Zhou P, Meng QT, He GJ, Wu HM, Duan CY, Quan X (2009) Highly sensitive fluorescence probe based on functional SBA-15 for selective detection of Hg2+ in aqueous media. J Environ Monit 11:648–653
Hu QM, Huang GS, Zheng J, Su H, Guo C (2012) Synthesis and rheological properties of hydrophobically modified poly(vinyl alcohol). J Polym Res 19:6–15
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (under Grant No. 21307002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geng, TM., Wang, Y. & Huang, RY. Fluorescence Sensors for Selective Detection of Hg2+ Ion Using a Water-Soluble Poly(vinyl alcohol) Bearing Rhodamine B Moieties. J Fluoresc 24, 1207–1213 (2014). https://doi.org/10.1007/s10895-014-1402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1402-3