Abstract
An acrylic monomer bearing xanthene group, N-oxethyl acrylate-N′-rhodamine B hydrazide (ARBHE) was synthesized from N-hydroxyl ethyl-N′-rhodamine hydrazide (RBHE) and acryloyl chloride (Ac) in the presence of triethylamine in dry dichloromethane (CH2Cl2) at room temperature. The synthesized ARBHE was identified by FTIR, 1H NMR spectra and elementary analysis. Copolymer poly(AM-ARBHE) of ARBHE and AM was synthesized with thermal initiator by free radical precipitation polymerization and it was characterized by the method of FTIR and 1H NMR. Its molecular weights (Mη) was 7.03 × 103 g mol−1 and the content of rhodamine units in the polymer chains was 1.44 % in mole fraction. The ability of the poly(AM-ARBHE) to detect different metal cations (Ag+, Ba2+, Cd2+ Co2+, Cr3+, Cu2+, Co2+ K+, La3+, Mg2+, Na+, Ni2+, Pb2+, Fe2+, Fe3+, Hg2+ and Zn2+) in water was investigated. Upon addition of Cr3+, Fe3+ or Hg2+ ions to the aqueous solution, visual color change and fluorescence enhancement were observed. Moreover, other metal ions did not induce obvious changes to the fluorescence spectra except to Fe2+. The detection limit of poly(AM-ARBHE) was less than 1 × 10−11 M. The results suggest that this copolymer may offer potential as a polymeric sensor for Cr3+, Fe3+ and Hg2+ ions in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhodamine scaffold is an ideal template for the construction of OFF–ON fluorescent chemosensors because they are excellent chromophores/fluorophores and have attracted considerable interest due to their very good photophysical properties [1, 2] such as large molar extinction coefficient, long excitation and emission wavelengths (over 500 nm) and high fluorescence quantum yields [3–5]. As is well known, rhodamine derivatives exist in a spirocyclic form which is nonfluorescent and colorless. The addition of specific cation leads to ring-opening of the spirolactam via coordination or irreversible chemical reaction [2, 6–9] giveing rise to both a chromogenic and fluorogenic response to facilitate an OFF/ON-type fluorescent detection [5]. In fact, a longer wavelength emission (∼550 nm) was often preferred to serve as a sensing signal to avoid the background fluorescence influence (below 500 nm) [10, 11]. Recently, several rhodamine-based probes as fluorescent chemosensors for metal ions have been developed [12].
Rhodamine B and rhodamine 6G are the less expensive dyes of this family and consequently they have been the most employed for further applications. A great interest in the development of new synthetic procedures for preparation of rhodamine derivatives based Rhodamine B and rhodamine 6G have arisen in recent years because for most applications the probe must be covalently linked to another (bio)molecule or surface [13]. However most fluorescent sensors for metal ions are based on small probing molecules [14–16]. Unfortunately, most of these small fluophores have poor water solubility, and usually work well in a pure organic solvent media, an aqueous solution containing at least 20 % organic cosolvent or in pure water are very rare [14]. The lack of water solubility greatly restrict the potential applications of rhodamine-based small molecules in biological systems and for environmental analyses [14, 17]. This inconvenience can be overcome by using hydrophilic copolymers that also contain small amounts of the hydrophobic organic receptors [16]. The water-soluble copolymers of traditional monomers with some polymerizable fluorescent units display intensive fluorescence. Thus, using appropriate fluorophores, water-soluble polymeric sensors of a different fluorescent color can be obtained. On the other hand, the stability of polymers is of great importance for their use. The covalent bonding of the fluorophores to the polymer chain provided a good stability to solvents and migration, improving their environmental behaviors [18]. During the past decade, research on polymers combined with fluorophore has attracted increasing interests. At present, there are many fluorescent polymer sensors with different macromolecular structure for the detection of metal cations in the environment [19]. Stomphorst et al. indicated that the use of polymer matrixes can avoid clustering of the dye molecules to aggregate the chromophores, often displayed in water and organic solvents [20].
In order to be used as a matrix for fluorescence chemosensor purposes in aqueous solution, the polymer must be amorphous, water-soluble and transparent so that the fluorescent light could propagate through it [21]. Among the polymer utilized, polyacrylamide (PAM) has been quite appropriate for the purpose, because it is amorphous and water-soluble [19].
The spacer structure [20] and length [21–23] from the copolymer main chains to fluorophore moiety have notable influences on sensory pattern [20, 22], analyte species [20], color changes [20], fluorescence quantum efficiency [21], max emission wavelength (λmax) [21], shape of emission band [21], fluorescent intensity [21, 23] and stability [24]. During recent years, our research group has focused on the design of new fluorimetric and colorimetric chemosensors of water-soluble polymer for the recognition of various metal ions in water [25–32]. Now, we are focused on designing new systems with different space between mainchains and fluorophore and researching the influences on fluorescent properties [20]. Herein, we report the design, synthesis and spectral characteristics of a new rhodamine B based polymeric chemosensor (poly(AM-ARBHE)) through the precipitation polymerization of acrylamide (AM) and polymerizable monomer ARBHE. RBHE was obtained by the reaction of rhodamine B hydrazide derivative and 2-bromo-1-ethanol firstly, then ARBHE was synthesized by the reaction of RBHE and acryloyl chloride (Ac). In this respect, the poly(AM-ARBHE) with oxethyl as spacer was investigated and observed to exhibit selective colorimetric and fluorescence sensing abilities for Cr3+, Fe3+ and Hg2+ in water. The signalling mechanism of the sensor follows a straight forward protocol, i.e. after binding of the analyte (metal ion) to spirolactum ring in which the rhodamine core breaks with an excellent enhancement in the fluorescence intensity and develops a strong colour and facilitates naked eye detection. The synthetic strategy of the monomer and polymer is shown in Scheme 1.
Experimental
Materials and Instruments
Materials
Rhodamine B and 2,2′-azobis(2-methylpropionitrile) (AIBN, 98 %) were purchased from Sigma-Aldrich Trading Co. Ltd. (Shanghai, China). Triethylamine, hydrazine hydrate (85 %), natrium sulfuricum, sodium carbonate, NaNO3, KNO3, Mg(NO3)2·6H2O, Cr(NO3)3·9H2O, La(NO3)3·6H2O, Fe(NO3)3·9H2O, Fe(NO3)2, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Cu(NO3)2·3H2O, Zn(NO3)2·6H2O, Cd(NO3)2·4H2O, Hg(NO3)2·2H2O, Pb(NO3)2, Ba(NO3)2, AgNO3 were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without further purification. Acryloyl chloride (Ac), acrylamide (AM), 2-bromo-1-ethanol were supplied by Aladdin Chemistry Co. Ltd. Anhydrous methanol and ethanol, dichloromethane, chloroform, sodium carbonate, toluene and tetrahydrofuran (THF) were obtained from commercial suppliers. N-(rhodamine B) lactam-hydrazine (RBH) was synthesized according to the literature method [33, 34].
Instruments
All fluorescence studies were done using Shimadzu spectrofluorimeter (model RF–5301PC, lamp source: 150 W (Xe lamp), wavelength band: 220–950 nm, wavelength accuracy: ±1.5 nm, sensitivity: S/N>150 (tape width: 5 nm, water Raman peak)) with 1 cm quartz cuvettes. FTIR spectra were recorded on a FTIR spectrophotometer (model Nicolet Neus 8700) with KBr compressing tablet. 1H NMR spectra were recorded with a Bruker 400 MHz spectrometer. Elemental analyses (C, H and N) were carried out on an analyzer (model VarioELIII) for the intermediates and monomer RBH, RBHE and ARBHE. All pH measurements were carried out using Control Dynamics digital pH meter (Model pHS-3C).
Synthetic Procedures and Characterization Data
Synthesis of RBHE
0.2499 g (2.0 mmol) of 2-bromo-1-ethanol, 0.9132 g (2.0 mmol) of RBH and 0.424 g (4.0 mmol) of sodium carbonate were dissolved in toulene (12 mL). The mixture heated under reflux with N2 for 48 h. After the reaction was completed, the mixture was cooled down to room temperature and there were yellow precipitate. Water (15 mL) was added to the residue and the aqueous phase was extracted with CH2Cl2 (3 × 15 mL). The organic layer was washed twice with water, dried over Na2SO4 and concentrated by evaporation. The residue was subjected to column chromatography (silica, dichloromethane/methanol (5:1) affording RBHE as an amaranth powder (0.6027 g, 64.55 %) [35, 36]. FTIR of RBHE (KBr), ν/cm−1: 3447.65 (ν-NH2), 2968.63, 2941.44, 2869.21 (νCH3, CH2, CH-), 1697.81 (νC=O), 1625.58, 1517.24, 1454.04, 1354.73(νPh). Anal. calcd for C30H36N4O3 (%): C, 71.97; H, 7.25; N, 11.19. Found: C, 71.85; H, 7.16; N, 11.32.
Synthesis of ARBHE
A 100 mL round bottomed flask equipped with a magnetic stirrer was cooled to 0 °C in ice bath and charged with 0.7509 g (1.5 mmol) of RBHE, 1.00 mL (7.20 mmol) of triethylamine, and dichloromethane (10 mL). To this solution, 0.1358 g (1.5 mmol) of acryloyl chloride solution in dichloromethane (10 mL) was added dropwise over half an hour. After the addition was complete, the reaction mixture was stirred at room temperature for 6 h, the solution was washed with 1 × 10 mL of 4:1 HCl, 3 × 10 mL of 10 % Na2CO3, 3 × 10 mL of water, respectively. The organic layer was dried over anhydrous Na2SO4, and the solvent was removed at room temperature under vacuum. The product was collected and dried overnight in a vacuum oven at 50 °C (yield: 77.86 %, purple powder) [16, 17]. FTIR of ARBHE (KBr, ν/cm−1): 3437.99 (ν-NH2), 3085.29, 3035.97 (νCH2=C-H), 2997.55, 2941.44, 2869.412 (νCH3, CH2, CH-), 1683.78 (νC=O), 1652.58, 1517.24, 1463.07, 1363.76 (νPh). 1H NMR (400 MHz, CDCl3) of ARBHE (δ, ppm): 7.920–7.944 (m, Ph); 7.432–7.456 (m, Ph); 7.104–7.113 (m, Ph); 6.517–6.539 (d, NH); 6.300–6.468 (m, Ph); 6.255–6.284 (m, −CH = CH2); 3.613(s, NH); 3.304–3.365 (m, NH, NCH2 ); 1.115–1.822 (m, CH3). Anal. calcd for C33H38N4O4: C, 69.29; H, 6.91; N, 10.10. Found: C, 69.16; H, 7.03; N,10.14 %.
Synthesis of Poly(AM-ARBHE)
Poly(AM − ARBHE) was prepared by the precipitation polymerization of ARBHE and AM with AIBN as initiator. A solution of ARBHE (0.3328 g, 0.6 mmol), AM (1.379 g, 19.4 mmol) and AIBN (32.8 mg, 0.2 mmol, 0.25 % in mole) in 30 mL dry THF was introduced into a dry 100 mL round bottomed flask. The solution was deoxygenated by purging with purified N2 gas. The flask was sealed and placed in a regulated thermostat bath at 70 °C for 12 h. The mixture was cooled to room temperature and suction filtrated by Buchner funnel. The orange powder was washed with chloroform and ethanol until the solvent was not fluorescent, respectively. The precipitate was dissolve in 10 mL of distilled water and precipitated with 50 mL of methanol, then was filtered, dissolved in distilled water (10 mL) and again precipitated into methanol. The above dissolution–precipitation cycle was repeated three times. The precipitate was dried under vacuum to constant weigh (Yield: 74.08 %) [19]. FTIR (KBr, ν/cm−1): 3428.79, 3194.23 (ν−CONH2); 2923.39, 2860.19, 2787.96 (ΝCH3-, −CH2−, −CH−); 1661.69 (νC=O); 1616.55, 1463.07, 1336.68 (νPh). 1H NMR (400 MHz, D2O) (δ, ppm): 0.969–1.146 (m, −CH3), 1.284–1.730 (d, −CH2-), 2.147–2.289 (m,–CH-), 3.573–3.699 (m, −CH2-), 6.148 (m, −CO-NH2), 6.196–6.496 (m, Φ-H), 6.913 (m,–Φ–H, NH-), 7.657 (m, Φ-H).
Methods
A 1.00 mg mL−1 stock solution of poly(AM-ARBHE) was prepared by dissolving poly(AM-ARBHE) in distilled water. A standard stock solution of Fe3+, Cr3+ and Hg2+ (1.00 M) was prepared by dissolving an appropriate amount of ferric nitrate, chromium nitrate or mercuric nitrate in distilled water and adjusting the volume to 100.00 mL in a volumetric flask, which were further diluted to 0.10 M. Stock solutions of other metal ions were prepared in distilled water with a similar procedure. All measurements of spectra were carried out in an aqueous solution. For all measurements of fluorescence spectra, excitation was fixed at 500 nm with the emission recorded over the wavelength range of 520–650 nm. The excitation and the emission slit widths were 10.0 nm and 5.0 nm, respectively [14]. The detection limit was calculated with the equation: detection limit = 3S/ρ, where S is the standard deviation of blank measurements and ρ is the slope between relative fluorescence intensity versus sample concentration [14, 37–39]. The fluorescent intensities were mensurated for 3 min after adding of metal ions. For 0.5 min the incident light was falling on sample. The viscosity average molecular weight (Mη) of poly(AM-ARBHE) was estimated from intrinsic viscosity of the copolymer in distilled water at a constant temperature of 30 °C, using the Mark–Houwink–Sakurada (MHS) equation [η] = kM η α (K = 9.33 × 10−3, α = 0.75 for the polymer) [40, 41].
Results and Discussion
Synthesis and Characterization
The water soluble polymeric sensor poly(AM-ARBHE) was prepared in four steps from commercially available precursors following a convergent synthesis (Scheme 1). Briefly, intermediate RBH was first prepared by a condensation reaction between rhodamine B and hydrazide using rhodamine B as the starting material under N2 at reflux for 12 h [33, 34], Intermediate RBH and 2-bromo-1-ethanol were then combined together in a N-substitution reaction to produce intermediate RBHE which was obtained as a pure purple solid after chromatographic purification [42]. RBHE was then esterification reacted with acryloyl chloride in an ice bath to produce the target monomer ARBHE [43]. The structures of intermediate and the monomer were characterized with conventional techniques (1H NMR, FTIR and elemental analysis, Figs. S1, S2 and S3) and good agreement between the proposed chemical structures and the characterization data can be observed [16].
Poly(AM-ARBHE) was synthesized by a conventional free radical precipitation polymerization of acrylamide (AM) and ARBHE at an AM: ARBHE molar ratio of 97: 3 (Scheme 1) at a temperature of 70 °C using THF as the solvent and AIBN as the radical initiator [16]. Because polyacrylamide (PAM) is a nonionic, water-soluble polymer without color, it has recently attracted attention as the water-soluble polymers. Thus, AM was appropriately selected as the hydrophilic monomer [17]. The structure of the obtained copolymer was confirmed by FTIR spectral analysis and 1H NMR spectroscopy. They were designed to chelate metal ions via its carbonyl O and amine N atoms. Characterization data of the synthesized polymeric sensors were shown in Fig. S4 and Fig. S5. FTIR was used to confirm that rhodamine B moiety was linked to the polymer. The new characteristic band centered at ~3008 cm−1 was Ar-H stretching vibrations attributed to rhodamine groups [44]. The prominent peak at 1661 cm−1 was attributed to the stretching vibration of an amide carbonyl, and the wide absorption band at 3429 cm−1 was indicative of the –NH functional groups [17]. The peaks characteristic of RBH at 1616, 1463 and 1337 cm−1 appeared. The presence of the C-O-C groups in poly(AM-ARBHE) was indicated by the peak at an asymmetric stretching of 1120 cm−1 [44]. The peak at 2928 cm−1 was attributed to symmetric –CH2– stretching vibrations. [45]. These results provided rich evidence for the successful in copolymerizing of ARBHE with AM, affording the target copolymer poly(AM-ARBHE) [14, 33, 44]. The 1H NMR spectra of ARBHE and poly(AM-ARBHE) in CDCl3 and D2O are shown in Fig. S3 and S5. The characteristic 1H NMR signals corresponding to the vinyl groups of the monomers (d(H) 6.255 and 6.284) disappeared completely in the polymer, and the signals corresponding to the xanthene and benzene rings of ARBHE (d(H) 6.0–8.0) appeared in the 1H NMR spectrum of poly(AM-ARBHE). This observation confirmed that the hydrophobic functional monomer ARBHE had been successfully incorporated into the polymer. The signals at 1.2–3.5 ppm were assigned to the aliphatic CH3 and CH2 groups [17]. The integration of the 1H NMR spectrum of the poly(AM-ARBHE) (Fig. S5) confirmed that the molar ratio of ARBHE to AM within the copolymer was lower (98.56: 1.44) than that the monomer feed ratio used in the synthesis (97: 3). It might be because the steric effect of ARBHE [16, 35].
The viscosity average molecular weight Mη was estimated for poly(AM-ARBHE) from intrinsic viscosity of the polymer in distilled water at a constant temperature of 30 °C, using the Mark–Houwink–Saknrada (MHS) equation [η] = kMη α (k = 9.33,α = 0.75 for the polymer) [40, 41]. The viscosity average molecular weight Mη for poly(AM-ARBHE) was 7.03 × 103 g mol−1 [14, 19] As the contents of the hydrophobic units and the viscosity average molecular weight Mη were reasonably low, poly(AM-ARBHE) could be easily dissolved in water, a key requirement for its sensing application [14, 16, 19].
Effect of pH Value
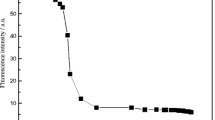
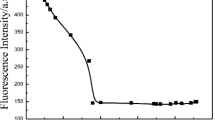
The rhodamine derivatives are usually pH sensitive, which interfere with the detection of the analytes [46]. Moreover, chemosensors containing nitrogen donors are highly sensitive to environmental pH as the protonation degree of the nitrogen are strongly dependent on the pH [47]. For practical application, the appropriate pH conditions for successful operation of the sensor were evaluated. The effects of pH on the fluorescence response of poly(AM-ARBHE) (1.00 mg mL−1) obtained in water are shown in Fig. 1 [48, 49]. As shown in Fig. 1, with the increase in solution acidity, the fluorescence (excited at 500 nm) of poly(AM-ARBHE) in water dramatically enhanced which implies that the spirolactam ring of poly(AM-ARBHE) was opened up due to protonation [45, 48]. Within the pH values of 7.1 to 11.6, The solution of poly(AM-ARBHE) itself exhibited very weak fluorescence and elicited negligible changes [49, 50], suggesting that the spirolactam form was still preferred in this condition and this tautomer was insensitive to such pH span [48]. Considering that higher pH range could lead to hydrolysis for transition metal ions, the proper pH span for poly(AM-ARBHE) to sense metal ions in aqueous solution was selected to be around 7.0 at room temperature [14, 48, 51].
Fluorescent Sensing Performance of Poly(AM-ARBHE) for Cr3+, Fe3+ and Hg2+
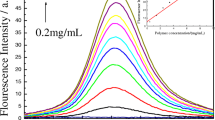
The application of poly(AM-ARBHE) as a chemosensor for metal ions was investigated by fluorescence spectroscopies under excitation at 500 nm. The sample was waited for 3 min before the fluorescence was recorded. Each experiment was performed at the optimal pH value [17, 45]. As shown in Fig. 2, the aqueous solution of poly(AM-ARBHE) (1.00 mg mL−1) showed only a weak fluorescence in the absence of ions, indicating that the spirolactam structure of rhodamine is predominant. However, with the gradual addition of Cr3+, Fe3+ or Hg2+ ions, a significant enhancement of fluorescence (with an emission maximum at 582 nm) was observed that corresponded to the fluorescence emission of rhodamine B [17, 52], which was reasonably assigned to the delocalized xanthene tautomer of the rhodamine group indicating the ring-opened process of rhodamine B unit in poly(AM-ARBHE). The increment saturated after adding 7.5 × 10−5, 3.5 × 10−5, 6.0 × 10−5 M of Cr3+, Fe3+ or Hg2+, up to 3.32-, 3.97- and 3.49-fold enhancement in the fluorescence intensity was noted, respectively. (Fig. 3) [14, 53]. Here I is the fluorescence emission intensity of poly(AM-ARBHE) actually measured at a given metal concentration, I0 is the fluorescence emission intensity of the free poly(AM-ARBHE) and [Mtn+] represents the concentration of Cr3+, Fe3+ or Hg2+ added [14]. In the meantime, the visible color change occurred of the solution from colorless changed to amaranth, yellowish-brown and purply, respectively [5, 17]. This results indicate that the Cr3+, Fe3+ or Hg2+ induces a highly conjugated rhodamine system via formation of opened-spirolactam to give a strong fluorescence emission. The possible mechanism is briefly described in Scheme 2. The color change and off-on fluorescence support our expectation that poly(AM-ARBHE) could serve as a sensitive fluorescent switcher as well as a naked-eye chemosensor for Cr3+, Fe3+ or Hg2+ in water [14].
The Fig. 4 shows the plots of the relative fluorescence intensity of poly(AM-ARBHE) at 582 nm versus the concentration of added Cr3+, Fe3+ and Hg2+, and a good linearity relationship was obtained in the range of 0–2.0 × 10−4, 0–6.0 × 10−4 and 0–1.6 × 10−4 M [14] with a very good regression coefficient as R = 0.9935, 0.9879 and R = 0.9946 and could be expressed by the following Eqs. (1–3) of the calibration line [54]:
The detection limit, which was calculated as three times the standard deviation of the background noise from the calibration curve, for the determination of Cr3+, Fe3+ and Hg2+ ions in the same medium was found to be 2.20 × 10−11, 3.88 × 10−11 and 3.03 × 10−11 M [53, 55]. These results confirmed that the probe could detect Cr3+, Fe3+, and Hg2+ in the water with a low detection limit [5].
Selectivity and Competitiveness of Poly(AM-ARBHE)
The effects of the different metal cations on the fluorescence spectra of the poly(AM-ARBHE) are quite interesting to investigate with regard to their ability to detect metal cations in the real environment pollutants [5, 56]. The change in the fluorescence intensity induced by the metal cations (5.00 × 10−4 M) was investigated and presented in Fig. 5. Relative fluorescent intensity (I/I0) has been used as a quantitative measure of the effect of metal cations on fluorescent intensity [19]. The synthesized copolymer is exposed to different transition metal ions such as Ag+, Ba2+, Cd2+ Co2+, Cu2+, K+, La3+, Mg2+, Na+, Ni2+, Pb2+, and Zn2+ have negligible influence on the polymer emission, as well as no color change [5, 54]. This can be ascribed to the poor coordination capability of these abundant cations with RBH moieties [57]. However, the addition of Fe2+, Cr3+, Fe3+ or Hg2+ ions into the solution of the poly(AM-ARBHE) lead to a prominent fluorescence enhancement [54]. The results indicate that the poly(AM-ARBHE) has a good selectivity for Cr3+, Fe3+ or Hg2+ among the metal cations tested under the same conditions. Therefore, we can conclude that the fluorescence enhancement is caused by the selective interaction between the Cr3+, Fe3+ or Hg2+ and polymer containing rhodamine derivative side chain in water [5, 49].
Metal-ion selectivity of poly(AM-ARBHE) in water (1.00 mg mL−1). The red bars represent the relative fluorescence intensity (I0/I) of a solution of poly(AM-ARBHE) (1.00 mg mL−1) and 5.0 × 10−4 M of other metal ions. The blue bars show the I0/I after the addition of 5.0 × 10−4 M of Fe3+ to the solution containing poly(AM-ARBHE) (1.00 mg mL−1) and different metal ions (5.0 × 10−4 M). The cyan bars show the I0/I after the addition of 5.0 × 10−4 M of Hg2+ to the solution containing poly(AM-ARBHE) (1.00 mg mL−1) and different metal ions (5.0 × 10−4 M)
Furthermore, the competition experiments were also conducted by adding 5.00 × 10−4 M Cr3+, Fe3+ or Hg2+ ions to the solution of poly(AM-ARBHE) (1.00 mg mL−1) in the presence of 5.00 × 10−4 M other metal ions, respectively. As shown in Fig. 5, despite the presence of miscellaneous competitive cations, the Cr3+, Fe3+ or Hg2+ still resulted in similar fluorescence changes at 582 nm. These results indicated that the sensing of Cr3+, Fe3+ or Hg2+ using poly(AM-ARBHE) are unaffected by these common coexistent metal ions [43, 44]. The color and fluorescence changes of poly(AM-ARBHE) upon the addition of various cations were shown in Fig. 6 [43]. The Cr3+, Fe3+ or Hg2+-induced color and fluorescence emission changes could also be visualized by naked eye in natural light and UV lamp (365 nm) respectively, as shown in Fig. 6. Distinctive colorimetric transition from colorless to amaranth, yellowish-brown or pink under visible light and the orange, dark and bright pink under ultraviolet at 365 nm appeared for Cr3+, Fe3+ or Hg2+, respectively [58]. These results suggested that poly(AM-ARBHE) can serve as a “naked-eye” and fluorescence chemosensor for Cr3+, Fe3+ or Hg2+ [14, 17, 43, 44].
Conclusions
In conclusion, a copolymer poly(AM-ARBHE) was synthesized by free radical precipitation polymerization of acrylamide with ARBHE, which was incorporated into the polymer side chains with oxethyl as the spacer. The contents of rhodamine chromophore (mole fraction) in the water-soluble polymer was 98.56/1.44 and the viscosity-average molecular weight of poly(AM-ARBHE) was 7.03 × 103 g mol−1. This RBH-functionalized polymer poly(AM-ARBHE) had a excellent solubility in water. By means of fluorescence spectroscopy, we studied the sensibility for protons and metal cations in aqueous solution. The results showed that it displayed selectivity and high sensitivity toward the detection of Cr3+, Fe3+ and Hg2+ in water over a wide range of tested metal ions with remarkably enhanced fluorescent intensities and also clear color changes from colorless toamaranth, yellowish-brown and pale pink. The background metal ions showed small or no interference with the detection of Cr3+, Fe3+ and Hg2+.
References
Angupillai S, Hwang JY, Lee JY, Rao BA, Son YA (2015) Efficient rhodamine-thiosemicarbazide-based colorimetric/fluorescent ‘turn-on’ chemodosimeters for the detection of Hg2+ in aqueous samples. Sensors Actuators B Chem 214:101–110
Zhang DT, Ma YT, An RB (2015) New colorimetric chemosensor based on rhodamine hydrazide to detect Cu2+ ions by naked eye. Res Chem Intermed 41:5059–5069
Ni JK, Li QY, Li B, Zhang LMA (2013) Novel fluorescent probe based on rhodamine B derivative for highly selective and sensitive detection of mercury(II) ion in aqueous solution. Sensors Actuators B Chem 186:278–285
Wu C, Bian QN, Zhang BG, Cai X, Zhang SD, Zheng H, Yang SY, Jiang YB (2012) Ring expansion of spiro-thiolactam in rhodamine scaffold: switching the recognition preference by adding one atom. Org Lett 14:4198–4201
Shi DJ, Ni M, Luo J, Akashi M, Liu XY, Chen MQ (2015) Fabrication of novel chemosensors composed of rhodamine derivative for the detection of ferric ion and mechanism studies on the interaction between sensor and ferric ion. Analyst 140:1306–1313
Kwon JM, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127:10107–10111
Lee MH, Wu JS, Lee JW, Jung JH, Kim JS (2007) Highly sensitive and selective chemosensor for Hg2+ based on the rhodamine fluorophore. Org Lett 9:2501–2504
Ko SK, Yang YK, Tae J, Shin I (2006) In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J Am Chem Soc 128:14150–14155
Ghosh K, Sarkar T, Samadder A, Khuda-Bukhsh AR (2012) Rhodamine-based bis-sulfonamide as a sensing probe for Cu2+ and Hg2+ ions. New J Chem 36:2121–2127
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Minta A, Tsien RY (1989) Fluorescent indicators for cytosolic sodium. J Biol Chem 264:19449–19457
Gupta VK, Mergu N, Singh AK (2015) Rhodamine-derived highly sensitive and selective colorimetric and off–on optical chemosensors for Cr3+. Sensors Actuators B Chem 220:420–432
Mariana B, Carlos AMA, Jose MGM (2009) Synthesis and applications of rhodamine derivative as fluorescent probes. Chem Soc Rev 38:2410–2433
Luo J, Jiang SS, Qin SH, Wu HQ, Wang Y, Jiang JQ, Liu XY (2011) Highly sensitive and selective turn-on fluorescent chemosensor for Hg2+ in pure water based on a rhodamine containing water-soluble copolymer. Sensors Actuators B Chem 160:1191–1197
Patidar R, Rebary B, Paul P (2015) Colorimetric and fluorogenic recognition of Hg2+ and Cr3+ in acetonitrile and their test paper recognition in aqueous media with the aid of rhodamine based sensors. J Fluoresc 25:387–395
Kaoutit HE, Estévez P, Ibeas S, García FC, Serna F, Benabdelouaha FB, García JM (2013) Chromogenic and fluorogenic detection of cations in aqueous media by means of an acrylic polymer chemosensor with pendant rhodamine-based dyes. Dyes Pigments 96:414–423
Wang Y, Wu HQ, Luo J, Liu XY (2012) Synthesis of an amphiphilic copolymer bearing rhodamine moieties and its self-assembly into micelles as chemosensors for Fe3+ in aqueous solution. React Funct Polym 72:169–175
Wang BY, Liu XY, Ding SL, Su ZX (2011) Synthesis and photophysical properties of a blue water-soluble fluorescent polymer for Ni2+ and proton sensing. J Polym Res 18:1315–1322
Wang BY, Guan XL, Hu YL, Su ZX (2008) Synthesis and photop hysical behavior of a water-soluble fluorescein-be aring polymer for Fe3+ ion sensing. J Polym Res 15:427–433
Geng TM, Wang X, Wang ZQ, Chen TJ, Zhu H, Wang Y (2015) Effects of single and double bonds in linkers on colorimetric and fluorescent sensing properties of polyving akohol grafting rhodamine hydrazides. J Fluoresc 25:409–418
Cheng JA, Chang CP, Chen CH, Lin MS (2005) The fluorescent quantum efficiency of copolymers containing coumarin-6 at the side-chain. J Polym Res 12:53–59
Chan CYK, Jacky WYL, Deng CM, Chen XJ, Wong KS, Tang BZ (2015) Synthesis, light emission, explosive detection, fluorescent photopatterning, and optical limiting of disubstituted polyacetylenes carrying tetraphenylethene luminogens. Macromolecules 48:1038–1047
Gao LN, Lü FT, Xia HY, Ding LP, Yu F (2011) Fluorescent film sensor for copper ion based on an assembled monolayer of pyrene moieties. Spectrochim Acta A 79:79437–79442
Sebra RP, Kasko AM, Anseth KS, Bowman CN (2006) Synthesis and photografting of highly pH-responsive polymer chains. Sensors Actuators B Chem 119:127–134
Wang BY, Guan XL, Hu YL, Su ZX (2007) Preparation and fluorescent properties of poly(vinyl alcohol) bearing coumarin. Polym Adv Technol 18:529–534
Serrano B, Baselga J, Piérola IF (2002) Fluorescence lifetime distributions of labeled amorphous polymers in bulk. Polym J 34:905–910
Geng TM, Wang Y, Huang RY (2014) Fluorescence sensors for selective detection of Hg2+ ion using a water-soluble poly(vinyl alcohol) bearing rhodamine B moieties. J Fluoresc 24:1207–1213
Geng TM, Wu DY (2015) Water-soluble polymeric chemosensor for selective detection of Hg2+ in aqueous solution using rhodamine-based modified poly(acrylamide–acrylic acid). Luminescence 8:1263–1268
Geng TM, Wu DY, Huang W, Huang RY, Wu GH (2014) Fluorogenic detection of Hg2+, Cd2+, Fe2+, Pb2+ cations in aqueous media by means of an acrylamide-acrylic acid copolymer chemosensor with pendant rhodamine-based dyes. J Polym Res 21:354–361
Geng TM, Wu DY, Huang W (2015) Dual turn-on fluorescent chemosensor for Cu2+ and Hg2+ in aqueous medium based on a water-soluble polyacrylamide containing rhodamine. J Polym Res 22:40–47
Geng TM, Huang RY, Wu DY (2014) Turn-on fluorogenic and chromogenic detection of Fe3+ and Cr3+ in a completely water medium with polyacrylamide covalently bonding to rhodamine B using diethylenetriamine as a linker. RSC Advances 4:46332–46339
Geng TM, Guo C, Dong YJ, Chen M, Wang Y (2015) Turn-on fluorogenic and chromogenic detection of cations in complete water media with poly(N-vinyl pyrolidone) bearing rhodamine B derivatives as polymeric chemosensor. Polym Adv Technol 1:90–97
Yang XF, Guo XQ, Zhao YB (2002) Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 57:883–890
Wu CM, Chen YH, Dayananda K, Shiue TW, Hung CH, Liaw WF, Chen PY, Wang YM (2011) Sensitivity evaluation of rhodamine B hydrazide towards nitric oxide and its application for macrophage cells imaging. Anal Chim Acta 708:141–148
Chen BY, Kuo CC, Huang YS, Lu ST, Liang FC, Jiang DH (2015) Novel highly selective and reversible chemosensors based on dual ratiometric fluorescent electrospun nanofibers with pH- and Fe3+ − modulated multicolor fluorescence emission. ACS Appl Mater Interfaces 7:2797–2808
Ma B, Wu S, Zeng F, Luo Y, Zhao J, Tong Z (2010) Nanosized diblock copolymer micelles as a scaffold for constructing a ratiometric fluorescent sensor for metal ion detection in aqueous media. Nanotechnology 21:195501–195509
Chen XQ, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 112:1910–1956
Zhou ZG, Yu MX, Yang H, Huang KW, Li FY, Yi T, Huang CH (2008) FRET-based sensor for imaging chromium(III) in living cells. Chem Commun 29:3387–3389
Kumar KS, Ramakrishnappa T, Balakrishna RG, Pandurangappa M (2014) A fluorescent chemodosimeter for Hg2+ based on a spirolactam ring-opening strategy and its application towards mercury determination in aqueous and cellular media. J Fluoresc 24:67–74
Itaya T, Honda T, Kusumoto N, Matsumoto A, Inoue K (2003) Fluorescence behavior of water-soluble copolymers with pendant (4-carboxylatophenoxy)- cyclotriphosphazene/europium ion complexes. Polymer 44:2927–2932
Liu YH, Meng LZ, Lu XJ, Zhang LF, He YB (2008) Thermo and pH sensitive fluorescent polymer sensor for metal cations in aqueous solution. Polym Adv Technol 19:137–143
Hamilton GRC, Fullerton L, McCaughan B, Donnelly RF, Callan JF (2014) A ratiometric fluorescent hydrogel sensor for zinc(II) based on a two fluorophore approach. New J Chem 38:2823–2830
Niamsa N, Kaewtong C, Srinonmuang W, Wanno B, Pulpoka B, Tuntulani T (2013) Hybrid organic–inorganic nanomaterial sensors for selective detection of Au3+ using rhodamine-based modified polyacrylic acid (PAA)-coated FeNPs. Polym Chem 4:3039–3046
Shi DC, Yan FY, Wang M, Zou Y, Zheng TC, Zhou XG, Chen L (2015) Rhodamine derivative functionalized chitosan as efficient sensor and adsorbent for mercury(II) detection and removal. Mater Res Bull 70:958–964
Liu JS, Liu GN, Liu WX, Wang YR (2015) Turn-onfluorescence sensor for the detection of heparin based on rhodamine B-modified polyethyleneimine– graphene oxide complex. Biosens Bioelectron 64:300–305
Mao Y, Hong MM, Liu AF, Xu DM (2015) Highly selective and sensitive detection of Hg(II) from HgCl2 by a simple rhodamine-based fluorescent sensor. J Fluoresc 25:755–761
Chereddy NR, Suman K, Korrapati PS, Thennarasu S, Mandal AB (2012) Design and synthesis of rhodamine based chemosensors for the detection of Fe3+ ions. Dyes Pigments 95:606–613
Yan FY, Cao DL, Wang M, Yang N, Yu QH, Dai LF, Chen L (2012) A new rhodamine-based “Off-On” fluorescent chemosensor for Hg (II) ion and its application in imaging Hg(II) in living cells. J Fluoresc 22:1249–1256
Chu KH, Zhou Y, Fang Y, Wang LH, Li JY, Yao C (2013) Rhodamineepyrene conjugated chemosensors for ratiometric detection of Hg2+ ions: different sensing behavior between a spirolactone and a spirothiolactone. Dyes Pigments 98:339–346
Liu K, Zhou Y, Yao C (2011) A highly sensitive and selective ratiometric and colorimetric sensor for Hg2+ based on a rhodamine–nitrobenzoxadiazole conjugate. Inorg Chem Commun 14:1798–1801
Sikdar A, Panja SS, Biswas P, Roy S (2012) A rhodamine-based dual chemosensor for Cu(II) and Fe(III). J Fluoresc 22:443–450
Yang Z, She MY, Yin B, Cui JH, Zhang YZ, Sun W, Li JL, Shi Z (2012) Three rhodamine-based “Off−On” chemosensors with high selectivity and sensitivity for Fe3+ imaging in living cells. J Org Chem 77:1143–1147
She MY, Yang Z, Yin B, Zhang J, Gu J, Yin WT, Li JL, Zhao GF, Shi Z (2012) A novel rhodamine-based fluorescent and colorimetric “off-on” chemosensor and investigation of the recognizing behavior towards Fe3+. Dyes Pigments 92:1337–1343
Kaya İ, Kamacı M (2013) Highly select ive and stable florescent sensor for Cd(II) based on poly(az omethine-urethane). J Fluoresc 23:115–121
Kim KN, Choi MG, Noh JH, Ahn S, Chang SK (2008) Rhodamine B hydrazide revisited: chemodosimetric Hg2+-selective signaling behavior in aqueous environments. Bull Kor Chem Soc 29:571–574
Grabchev I, Dumas S, Chovelon JM (2008) Studying the photophysical properties of a polymerizable 1,8-naphthalimide dye and its copolymer with styrene as potential fluorescent sensors for metal cations. Polym Adv Technol 19:316–321
Liu T, Liu SY (2011) Responsive polymers-based dual fluorescent chemosensors for Zn2+ ions and temperatures working in purely aqueous media. Anal Chem 83:2775–2785
Wan XJ, Liu HY, Yao S, Liu TQ, Yao YW (2014) A stimuli-responsive nanogel-based sensitive and selective fluorescent sensor for Cr3+ with thermo-induced tunable detection sensitivity. Macromol Rapid Commun 35:323–329
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (under Grant No. 21307002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 375 kb)
Rights and permissions
About this article
Cite this article
Geng, TM., Wang, X., Jiang, H. et al. Fluorescent Polyamide-Based Rhodamine Hydrazide Moieties with Oxethyl as Spacer for Detection of Cr3+, Fe3+, and Hg2+ Ions in Water. J Fluoresc 26, 977–985 (2016). https://doi.org/10.1007/s10895-016-1785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1785-4