Abstract
A monomer 7-hydroxy-4-methyl-8-(4′-allyloxypiperazin-1′-yl) methylcoumarin with blue fluorescence was synthesized. The present investigation dealt with the synthesis and characterization of a coumarin monomer, containing a piperazine group. Then it was copolymerized with N-vinylpyrrolidone to obtain a water-soluble fluorescent copolymer (poly(Al-HMPC-co-VP)). The fluorescence characteristics of the polymer as a function of pH sensor were investigated in aqueous solution. It was found that the polymer displayed sensitive fluorescence signal amplification over a wide pH scale, which was ascribed to a photoinduced electron transfer from the piperazine receptor to the coumarin fluorophore. In addition, the influence of metal cations on the fluorescence intensity of poly(Al-HMPC-co-VP) were also studied. Obvious fluorescence enhancement was due to the photophysical response of the polymer to the presence of Ni2+ ion. The results suggest that copolymer may offer potential application as a reusable polymer for sensor protons and Ni2+ ion in aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the recent years, the environmental pollution has been a major concern of the present industrial societies. The development of different sensors able to detect metal cations in the soil and water sources has become a very important scientific goal. In particular, special attention has been paid to the polymer sensors able to produce photoinduced electron transfer (PET) hence to serve as sensors for metal cations and protons in the environment. The co-polymers of traditional monomers with some polymerizable fluorescent units display intensive fluorescence [1]. Thus, using appropriate fluorophores, polymer sensors of a different fluorescent color can be obtained. On the other hand, photo stability of polymers is of great importance for their use. The covalent bonding of the fluorophores to the polymer chain provided a good stability to solvents and migration, improving their environmental behaviors [2, 3].
Among the coumarin dyes, we chose a coumarin fluorophore because of its bright color and good dye ability with synthetic polymers. Materials containing a coumarin component have been useful in many fields due to their characteristics of high emission yield, excellent photostability, and extended spectral range, such as fluorescent images, [4] non-linear optical materials, [5] liquid crystals [6] and fluorescent labels for fluorescence energy transfer experiments [7]. 7-Hydroxy-4-methyl coumarin (7-HMC) is a derivative of coumarin, which is also used as an important fluorescent indicator for pH measurements and fluorescent probes because of its relatively large variation in its fluorescence intensity versus pH and its low toxicity [8]. Given the fact that piperazine ring is a strong electron donor [9] and it has virtue that can potentially bind to various metals. Thus, it is expected to design a new fluorescence chemosensor based on coumarin having a piperazine group as a strong electron donor, which can operate as a PET “on-off” switch, and having allyl group, which can polymerize with some commercial monomers like styrene, methyl methacrylate and N-vinylpyrrolidone.
As we all know, polyvinylpyrrolidone (PVP) has the advantage of excellent water-solubility, good film-forming and biocompatibility [10, 11]. Therefore, N-vinylpyrrolidone is quite appropriate to be selected as the monomer to copolymerize with the fluorophore having allyl group. The fluorophore firmly bonded to the main chain, which eliminates any migration from the matrix and no crystallization processes occur during their exploitation. Thus, it can produce reusable sensors for monitoring pollution in water sources.
The present investigation deals with the synthesis and characterization of a coumarin monomer, containing a piperazine group. Then its radical polymerization with N-vinylpyrrolidone was carried out to produce a blue fluorescent polymer. The responsive properties of the copolymer on the presence of protons and metal cations have been investigated by fluorescent spectra as well.
Experimental
Materials
7-HMC was supplied from Zhixin Chemical Industrial Co. Ltd (Shanghai, China), purified by crystallization from ethanol solution, and dried under vacuum. Commercially available NaH was purchased. Allyl bromide purchased from Tianjin Chemistry Reagent Company in China, were used after further purification. N-Vinylpyrrolidone (VP), obtained from Acros Co., was distilled under reduced pressure to remove the stabilizer prior to use. Tetrahydrofuran (THF) (Tianjin Chemistry Reagent Company) was refluxed with metallic sodium for 5 h followed by distillation and stored over molecular sieves. Azo-bis-isobutyronitrile (AIBN) was recrystallized from ethanol. Organic solvents, dichloromethane (CH2Cl2) and ethanol were dried and distilled before being used. Other reagents were all analytical pure and the water used in experiment was distilled twice.
Instrumentation
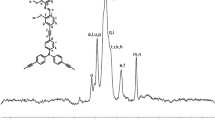
1H NMR experiments were performed on 300 MHz BB Bruker for the N-Boc-piperazine, N-Boc-HMPC, HMPC and Al-HMPC in CDCl3. MS was performed on ZAB-HS. FTIR spectra were recorded on a Nicolet Neus 670 FTIR spectrophotometer. UV-Vis spectra in THF solution were recorded with a Lambda 35 UV-Vis Spectrometer (Perkin Elmer). The mass concentration of the coumarin dye in the copolymer was estimated by the Lambert and Beer Law equation using a standard calibration procedure with Al-HMPC in THF solution.
Fluorescent excitation and fluorescence spectra were obtained from a LS 55 Luminescence Spectrometer (Perkin Elmer) for the solution samples. The fluorescence quantum yields (φfl) of Al-HMPC and the copolymer poly(Al-HMPC-co-VP) were determined with respect to quinine sulphate in 0.1 N H2SO4 solution (ФF = 0.54) [12].
For fluorescence measurements, a 10 × 10 mm quartz cell was used for detection. Fluorescence intensities against pH were obtained with pH-3B (made in Shanghai, China) by recording the emission in aqueous solution with different pH at fixed wavelength. The pH was adjusted by adding 0.1 mol l−1 HCl or 0.1 mol l−1 NaOH to the polymer aqueous solution. The pH range of the solution was changed from 3.04 to 12.06 by a digital pH controller. The effect of the metal cations on the fluorescence intensity was examined by adding a few microlitre of the metal cations solution (c = 10-3 mol l−1) to a known volume of the copolymer solution (3 ml). The total volume of the addition metal cations solution was limited to 0.16 ml, so that the dilution of the polymer solution remained insignificant. The metal ions are nitrate salts of K+, Na+, Ca2+, Ba2+, Al3+, Cr3+, Mn2+, Fe2+, Fe3+, Cd2+, Hg2+, Ag+, Pb2+, Ni2+, Cu2+, Co2+ and Zn2+.
Synthesis
Synthesis of the monomer
The monomer used in the synthesis of the polymers was presented in Scheme 1.
N-Boc-piperazine
N-Boc-piperazine was synthesised according to the literature [13].
7-Hydroxy-4-methyl-8-(4′-carboxylic acid tert-butyl ester piperazin-1-ylmethylcoumarin (N-Boc-HMPC)
To a solution of 1.76 g (10 mmol) 7-HMC and 1.86 g (10 mmol) N-Boc-piperazine in ethanol (20 ml) was added 0.86 g 37% formalin solution. The mixture was stirred at 80 °C for 4 h. The solution was concentrated in vacuo and the residue was purified by flash chromatography over silica using chloroform/ethylacetate (10:1) as an eluent to give N-Boc-HMPC (2.23 g) as a white solid. Yield: 58%, mp: 146–148 °C. 1H NMR (300 MHz, CDCl3), δ (ppm) = 7.42 (d, J = 8.7 Hz, H, arom), 6.86 (d, J = 8.7 Hz, 1H, arom), 6.12 (s, 1H, pyrone ring), 4.08 (s, 2 H, H in methylene of arom ring), 2.39–2.62 (br, 8 H, H in methylene of piperazine ring), 2.36 (s, 3 H, H in methyl of pyrone ring), 1.47 (s, 9 H, H in methyl of ter-butyl group). IR (KBr), cm−1: 3159, 3070, 1724, 1679, 1599, 1390, 833, 762. Anal.calc. for C20H26N2O5 (%): C, 64.15; H, 7.00; N, 7.48. Found (%): C, 64.22; H, 7.28; N, 7.30. MS (EI, m/z), 374 [M+], 318 (22.3), 189 (46.6), 55 (93.6).
7-Hydroxy-4-methyl-8-(4′-piperazin-1′-yl)methylcoumarin (HMPC)
Trifluoroacetic acid (10 ml) was added slowly at 0–5 °C under nitrogen to a solution of 0.76 g (2.0 mmol) N-Boc-HMPC in CH2Cl2 (10 ml). The mixture was stirred at ambient temperature for 1 h then poured into cold water (50 ml). The aqueous solution was made basic by the addition of aqueous ammonia solution, and then the product was extracted into dichloromethane (3 × 50 ml). The combined extracts were washed with brine (200 ml), dried over MgSO4, and then concentrated in vacuo. The residue was purified by flash column chromatography over silica using chloroform/ethyl acetate (6:1) as an eluent to give HMPC (0.52 g) as a pale yellow solid. Yield: 91%, mp: 153–155 °C. 1H NMR (300 MHz, CDCl3), δ (ppm) = 7.40 (d, J = 8.6 Hz, H, arom.), 6.76 (d, J = 8.6 Hz, 1H, arom), 6.12 (s, 1H, pyrone ring), 4.05 (s, 2H, H in methylene of arom ring), 2.63–2.98 (br, 8H, H in methylene of piperazine ring), 2.38 (s, 3H, H in methyl of pyrone ring). IR (KBr), cm−1: 3328, 2952, 2816, 1722, 1600, 1385, 1286, 1065, 833, 762. Anal.calc. for C15H18O3N2 (%): C, 65.68; H, 6.61; N, 10.21. Found (%): C, 65.82; H, 6.87; N, 10.10. MS (EI, m/z), 274 [M+], 232 (32.6), 189 (96.5), 218 (21.3), 84 (73.2).
7-Hydroxy-4-methyl-8-(4′-allyloxypiperazin-1′-yl)methylcoumarin (Al-HMPC)
0.41 g (1.5 mmol) of HMPC was dissolved in dry CH3CN (30 ml), 0.55 g (2.0 × 10−2) of K2CO3 was added to the solution at 0 °C. The mixture was stirred for 0.5 h, and then 0.36 g (3 mmol) allyl bromide dissolved in dry CH3CN (3 ml) was added dropwise. The resulting mixture was stirred at room temperature for 3 h. After the solvent was removed under vacuum, the crude product was purified by column chromatography on silica gel using chloroform/ethyl acetate (4/1) as an eluent to produce Al-HMPC (0.35 g) as a white colored solid. Yield: 46%, 1H NMR (300 MHz, CDCl3), δ (ppm) = 7.42 (d, J = 8.7 Hz, 1H, arom), 6.77 (d, J = 8.7 Hz, 1H, arom), 6.07 (s, 1H, pyrone ring), 5.23 (d, J = 15.3 Hz, 1H, H in allyl -CH2 =), 5.78–6.07 (m, 1H, H in allyl = CH-), 4.07 (s, 2H, H in methylene of arom ring), 3.04 (d, J = 6.3 Hz 2H, H in allyl N-CH2-), 2.41–2.69 (br, 8H, H in methylene of piperazine ring), 2.31 (s, 3H, H in methyl of pyrone ring). IR (KBr), cm−1: 3451, 3070, 1729, 1602, 1453, 1381, 1286, 1225, 1061, 1003, 932, 813. Anal.calc. for C18H22O3N2 (%): C, 68.77; H, 7.05; N, 8.91. Found (%): C, 69.01; H, 7.21; N, 8.76. MS (EI, m/z), 314 [M+], 217 (20.1), 189 (54.2), 125 (100.0).
Synthesis of polymers
Synthesis of PVP
A mixture solution of 1.1 g (10.0 mmol) VP and 8.9 mg (0.05 mmol) of AIBN in dry dioxane (10 ml) was introduced into a dry polymerization tube. The solution was deoxygenated by purging with purified N2 gas. The tube was sealed and placed in a regulated thermostat bath at 75 °C for 24 h. The solution was precipitated in excessive diethyl ether. The precipitate was collected by filtration and dried under vacuum to constant weigh. PVP was used for ultraviolet test and the experiment results were used to be compared with those of copolymer of VP with Al-HMPC.
Synthesis of poly(Al-HMPC-co-VP)
Poly(Al-HMPC-co-VP) was prepared by the copolymerization of VP with Al-HMPC using AIBN as the similar methods as the polymerization of PVP. A mixture solution of 0.02 g (0.05 mmol) Al-HMPC, 0.56 g (5.00 mmol) VP and 4.64 × 10−3 g (0.02 mmol) of AIBN in dry dioxane (10 ml) was introduced into a dry polymerization tube. The modified copolymer was dissolved in dioxane and precipitated with diethyl ether. The process was repeated five times in order to remove the unreacted monomer and the low-molecular weight oligomers. The precipitated copolymer was dried under vacuum to constant weight. Poly(Al-HMPC-co-VP) obtained in this way was used for further fluorescent test. The mass concentration of the dye in the copolymer was 0.96% via the ultraviolet spectrophotometry.
Results and discussion
Design of the monomer (Al-HMPC)
By taking into consideration, the structure of the monomer should have the common format, fluorophore-spacer-receptor. So we have thought of designing the monomer by connecting a coumarin group to piperazine receptor by a methyl spacer, namely, Al-HMPC. The target Al-HMPC was carried out in four steps following Scheme 1. The synthesis of HMPC from 7-HMC and N-Boc-piperazine by Mannich reaction [14] was an important one to combine the piperazine group with the coumarin fluorophore. In order to obtain functional coumarin with polymerisable group, the intermediate HMPC thus was synthesized. The result obtained was good enough as we expected. Then HMPC was reacted with allyl bromide to obtain Al-HMPC that could polymerize with VP.
The choice of the piperazine group in Al-HMPC as the recognition site stemmed from its strong electron donating characteristic and its ability to bind several metal cations [15]. The monomer should be based on the “fluorophore-spacer-receptor” model, where the 7-HMC moiety was the fluorophore and the piperazine ring was the receptor. The benzylic methylene fragment served as spacer that covalently separated the two unites.
Characterization of the polymers
The fluorescence copolymer poly(Al-HMPC-co-VP) was obtained by free radical copolymerization of VP and Al-HMPC at a molar ratios (VP : Al-HMPC = 100:1). The process of copolymerization was conducted under conditions used for other similar monomeric fluorophore [16, 17]. Homopolymerization of VP was carried out as a blank reaction. The viscosity average molecular weight Mη was estimated for PVP and poly(Al-Flu-co-VP) from intrinsic viscosity of the polymer in distilled water at a constant temperature of 30 °C, using the Mark-Houwink-Saknrada (MHS) equation \( \left[ \eta \right] = {\hbox{k}}{{\hbox{M}}_{\eta }}^{\alpha }\left( {{\hbox{k}} = {1}.{4} \times {1}{0^{{ - {4}}}},\alpha = 0.{7}\,{\hbox{for}}\,{\hbox{the}}\,{\hbox{polymer}}} \right) \) [18]. The viscosity average molecular weight Mη for PVP and poly(Al-HMPC-co-VP) were 2.32 × 104 and 2.04 × 104 g mol−1, respectively.
FTIR spectra of VP (A), poly(Al-HMPC-co-VP) (B) and Al-HMPC (C) are shown in Fig. 1. Compared that of poly(Al-HMPC-co-VP) with PVP, the absorption peak at about 1,640 cm−1, corresponding to the stretching vibration of the carbonyl group (C = O), shows a shift to higher wavenumber (1,673 cm−1). New peaks at 1,374, 1,069, 927 cm−1 are connected with aromatic C-H bending vibrations of coumarin moiety. The peaks at 1,069 cm−1 are related to the stretching vibration of C-O band of coumarin moiety. However, other peaks related to the coumarin moiety are too low to be detected in the FTIR spectra of the copolymer. These results indicate that coumarin moiety is attached to the copolymer chain and the contents of coumarin moiety in the polymer backbone are too dilute to be detected.
UV-Vis absorption spectra of Al-HMPC (A), poly(Al-HMPC-co-VP) (B), and PVP (C) in THF solution are depicted in Fig. 2. The absorption peak of Al-HMPC around 324 nm can be assigned to the π → π* transitions of coumarin group. The absorption peak of at 323 nm can be due to the coumarin fluorophore by comparison with the absorption data of PVP, which shows no optical absorption in this region. As shown in Fig. 2, the absorption spectrum of poly(Al-HMPC-co-VP) is similar to that of Al-HMPC. The molar extinction coefficient (ε) of Al-HMPC and poly(Al-HMPC-co-VP) is 12,143 L mol−1 cm−1 and 12,526 L mol−1 cm−1, respectively. Apparently, the absorption of poly(Al-HMPC-co-VP) is due to the fluorophore moiety on PVP and the matrix did not change the optical characteristics of Al-HMPC. This fact also allows the use of the standard curved method according to the Lambert and Beer Law equation to estimate the percent of the monomer covalently bonded to the polymer chain. The mass concentration of the dye in the copolymer is 0.96%. The results imply that the polymer chain has no significant influence on the absorption maxima of the chromophore.
Spectral properties of Al-HMPC and poly(Al-HMPC-co-VP)
Figure 3 illustrates the excitation and fluorescence spectra of the monomer Al-HMPC (A) and poly(Al-HMPC-co-VP) (B) in THF solution. Al-HMPC is colorless with excitation maxima at λex = 335 nm and emits a blue fluorescence with well-defined maxima at λem = 441 nm in THF solution. The fluorescence emission of the copolymer is observed around 434 nm. Apparently, there is no significant difference in the fluorescence spectra of the copolymer compared to that of the monomer.
The ability of the monomer and the copolymer to emit the absorbed light energy is characterized quantitatively by the quantum fluorescence yield (ФF). It is calculated using Eq. 1: [19, 20]
where the ФF is the emission quantum yield of the sample, Фst st is the emission quantum yield of the standard, Ast and Au represent the absorbance of the standard and sample at the excited wavelength, respectively, while Sst and Su are the integrated emission band areas of the standard and sample, respectively, and nst and nu is the solvent refractive index of the standard and sample, subscripts u and st refer to the unknown and standard, respectively.
The ФF values for Al-HMPC and poly(Al-HMPC-co-VP) in THF solution are 0.35 and 0.40, respectively. The ФF value of the copolymer decreases slightly compared with that for Al-HMPC. All of the results show that the chemical structure of the chromophorous system undergoes no change during the copolymerization process and the monomer is suitable for preparing the fluorescent copolymer with VP.
Effect of protons on the spectral properties of poly(Al-HMPC-co-VP)
To test the sensibility of the copolymer as a pH sensor, the influence of different pH conditions on the fluorescence emission of poly(Al-HMPC-co-VP) is studied. As shown in Fig. 4, the fluorescence intensity of the copolymer increases as the pH varied from 3.04 to 12.06 in aqueous solution. The result shows that the emission of the copolymer exhibits pH-responsive characteristics over a wide pH range as expected. After careful titration from pH 3.04 to 12.06, the fluorescence intensity enhances nearly nine times. The pH dependence of fluorescence intensity is analyzed using Eq. 2: [21]
Fluorescence spectra of poly(Al-HMPC-co-VP) (c = 2.0 × 10−5 g l−1) in aqueous solution at different pH value (λex = 324 nm). Curves a—j correspond to: (a) pH 3.04; (b) pH 4.01; (c) pH 5.04; (d) pH 6.01; (e) pH 7.08; (f) pH 8.05; (g) pH 9.08; (h) pH 10.06; (i) pH 11.08; (j) pH 12.06. The insert displays the pH dependence of the fluorescence intensity of poly(Al-HMPC-co-VP) in aqueous solution
The calculated pKa value is 7.96.
These fluorescence intensity changes at different pH conditions can be considered as representing two different “states” [22, 23], where the fluorescence emission is “switched off” in alkaline solution and “switched on” in acidic solution. The piperazine group of the coumarin fluorophore performs a dual role [24]. One is intramolecular hydrogen-bonding formation between the phenolic OH and the N-1′of piperazine ring. The other role is that it acts as a PET switch. In a strong alkaline medium, intramolecular H-bonding cannot exist so that the piperazine ring nitrogens can freely participate in the PET and lead to quenching of the coumarin fluorescence by the piperazine ring nitrogens (“switched off” state) (Scheme 2a). Thus, the fluorescence intensity reaches the minimum at pH 12.06. Under neutral conditions, because of the formation of intramolecular hydrogen-bonding between the phenolic OH and the N-1′ of piperazine ring, an electron transfer from the piperazine group to the excite state of the coumarin fluorophore is influenced and piperazine moiety cannot participate in the PET completely. Therefore, the fluorescence intensity is higher than that in alkaline solution. However, in acidic medium, the proton of the piperazine can increase the oxidation potential of the receptor, and as such, thermodynamically disallows the electron transfer [25]. Consequently, the fluorescence emission is “switched on” completely as it was expected (Scheme 2b). It is indicated that the polymer sensor may be suitable to act as potential efficient “off-on” switcher for pH.
Effect of metal cations on the spectral properties of poly(Al-HMPC-co-VP)
The influence of various metal cations on the fluorescence intensity of the polymer was investigated in buffered aqueous solution (NaH2PO4/Na2HPO4 at pH 7). The copolymer has no response upon addition of K+, Na+, Ca2+, Ba2+, Al3+, Cr3+, Mn2+, Fe2+, Fe3+, Cd2+, Hg2+, Ag+ and Pb2+, respectively, for fluorescence spectra due to the poor ability of the receptors with these metal cations. There was a small fluorescence enhancement for Cu2+, Co2+ and Zn2+, but the highest for Ni2+ ion. Here Fig. 5 shows the fluorescence intensity changes in the presence of the four metal cations (Ni2+, Cu2+, Co2+ and Zn2+). Obviously, there is no notable enhancement like Ni2+ ion for the other three metal cations. Most probably, Ni2+ ion is more competitive in the complex forming between Ni2+ ion and piperazine group.
The fluorescence enhancement depends on the Ni2+ ion concentration in buffered aqueous solution (NaH2PO4/Na2HPO4 at pH 7). Figure 6 presents the sensibility of the copolymer in aqueous solution at different concentrations of Ni2+ ion. The increase in the fluorescence intensity occurs after the addition of Ni2+ ion in the concentration range from Ni2+-free solution to 7.67 × 10−5 mol l−1. The fluorescence intensity value remains constant above certain concentration (c = 7.67 × 10-5 mol l−1). The fluorescence enhancement (FE = I/I0) is determined from the ratio of maximum fluorescence intensity I (after addition of metal cations) and minimum fluorescence intensity I0 (before addition of metal cations). The emission intensity enhances nearly 2 times (FE = 1.96). However, we attempted addition of Ni2+ ion to aqueous solution of the parent 7-HMC and did not find any significant changes in the fluorescence intensity.
Fluorescence spectra of poly(Al-HMPC-co-VP) in buffered aqueous solution (NaH2PO4/Na2HPO4 at pH 7) (c = 2.0 × 10−5 g l−1) at various concentrations of Ni2+ ion. Curves a—f correspond to (a) 0, (b) 0.33 × 10−5, (c) 0.67 × 10−5, (d) 1 × 10−5, (e) 1.33 × 10−5, (f) 2 × 10−5 (g) 2.67 × 10−5 (h) 4 × 10−5 (i) 5.67 × 10−5 (j) 7.67 × 10−5 mol l−1 Ni2+ ion, respectively. (λex = 324 nm). The insert displays the relationship between the fluorescence intensity and the concentration of Ni2+ ion
The result attests the importance of the piperazine ring as the “recognition part” in the chemosensor. It is also believed that the piperazine ring in pendant coumarin moiety also performs a dual role in the presence of metal cations: it acts as a ligand for metal cations and as a PET switch. As mentioned above, a complex between Ni2+ ion and piperazine ring can form. Scheme 2 illustrates the “fluorophore-spacer-receptor” fluorescence enhancement mechanism. The interaction between the fluorophore (coumarin moiety) and the receptor (piperazine group) provoking PET from the nitrogen atoms to the fluorophore leads to quenching of fluorescence emission. Accordingly, when Ni2+ ion engages the lone electron pair of the receptor nitrogen atoms in coordination it weakens the electron donating ability of the piperazine group (Scheme 2c). Consequently, it leads to a “switched off” of the electron transfer and the fluorescence of the system is “switched on”. The above results imply the selectivity of the fluorescent material as a polymer sensor for Ni2+ and its potential prospect as a polymer sensor for detecting Ni2+ ion in aqueous solution.
The polymer sensor had selectivity for Ni2+ ion, but sensitivity was not excellent. It was found that the effect of fluorescence enhancement was valid only in the concentration range of Ni2+ ion from 0.33 × 10−5 to 7.67 × 10−5 mol l−1. At concentration higher than 7.67 × 10−5 mol l−1 the fluorescence intensity remained constant. It may be due to the low content of the fluorophore in the polymer backbone. Besides, Al-HMPC containing single piperiazine unit is not good enough for cation coordination. Both of the reasons may lead to the unsatisfactory sensitivity.
Conclusions
A blue fluorescent monomer based on PET enhancement mechanism was designed by connecting piperazine group to cuomarin derivative. Via radical copolymerization with N-vinylpyrrolidone, Al-HMPC was incorporated into the polymer side chains. The blue fluorescent poly(Al-HMPC-co-VP) had a good solubility in aqueous solution. The coumarin moiety in the polymer did not only act as a fluorophore but also as recognition site so that the sensor properties could be transduced into a measurable optical signal. By means of fluorescence spectroscopy, we studied the sensibility for protons and metal cations in aqueous solution. The result showed that it was an efficient “off-on” switcher for pH between 3.04 and 12.06. We attribute this effect to the protonation of the piperazine receptors, which disallow the PET in the pedant fluorophore.
Additionally, it was found that the copolymer was selective to Ni2+ ion and the increase in the fluorescence intensity depended on Ni2+ ion concentrations in the range of 0.33 × 10−5 to 7.67 × 10−5 mol l−1. The fluorescence enhancement in the presence of Ni2+ ion was due to the piperazine ring acting as a ligand for Ni2+ ion and as a PET switch. The results from the investigation allow the suggestion that the fluorescent polymer sensor has the potential application in detecting protons in aqueous solution. The polymer sensor had selectivity for Ni2+ ion, but sensitivity was not excellent, it is also a challenge for us to design new polymer sensor that has good sensitivity for metal ions in our future work.
References
Baraschkov N, Gunter O (1987) Fluorescent polymers. Chimia, Moskva (in Russian)
Shuhaibar K, Pasch H (1991) Dyes Pigm 15:57–67
Bojinov V, Grabchev I (2003) Dyes Pigm 59:277–283
Frenette M, Coenjarts C, Scaiano JC (2004) Macromol Rapid Commun 25:1628–1631
Moylan CR (1994) J Phys Chem 98:13513–13516
Tian YQ, Akiyama E, Nagase Y, Kanazawa A, Tsutsumi O, Ikeda T (2000) Macromol Chem Phys 201:1640–1652
Oh JK, Stőeva V, Rademacher J, Farwaha R, Winnik MA (2004) J Polym Sci A 42:3479–3489
Mallick A, Haldar B, Sengupta S, Chattopadhyay N (2006) J Lumin 118:165–172
Gan J, Chen K, Chang CP, Tian H (2003) Dyes Pigm 57:21–28
Villemson AL, Malykh EV, Shtilman MI, Larionova NI (2003) Biochemistry (Mosc) 68:869–874
Villemson AL, Kuskov AN, Shtilman MI, Galebskaya LV, Ryumina EV, Larionova NI (2004) Biochemistry (Mosc) 69:621–628
Demas JN, Crosby GA (1971) J Phys Chem 75:991–1024
Tahtaoui C, Parrot I, Klotz P, Guillier F, Galzi JL, Hibert M, Ilien B (2004) J Med Chem 47:4300–4315
Ghantwal SR, Samant SD (1999) Indian J Chem B 38:1242–1247
Guo ZQ, Zhu WH, Shen LJ, Tian H (2007) Angew Chem Int Ed 46:5549–5553
Campo LF, Rodembusch FS, Stefani V (2006) J Appl Polym Sci 99:2109–2116
Wu ZQ (2008) J Appl Polym Sci 110:777–783
Jin SP, Liu MZ, Chen SL, Chen Y (2005) Eur Polym J 41:2406–2415
Cho D, Mattice WL, Porter LJ, Hemingway RW (1989) Polymer 30:1955–1958
Williams ATR, Winfield SA, Miller JN (1983) Analyst 108:1067–1072
Grabchev I, Guittonneau S (2006) J Photochem Photobiol A 179:28–34
Callan JF, de Silva AP, Magri DC (2005) Tetrahedron 61:8551–8588
Kohno Y, Shiraishi Y, Hirai T (2008) J Photochem Photobiol A 195:267–276
de Silva AP, Fox DB, Huxley AJM, Moody TS (2000) Coord Chem Rev 205:41–57
Prasanna de Silva A, Nimal Gunaratne HQ, Habib-Jiwan JL, McCoy CP, Rice TE, Soumillion JP (1995) Angew Chem Int Ed 34:1728–1731
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, BY., Liu, XY., Ding, SL. et al. Synthesis and photophysical properties of a blue water-soluble fluorescent polymer for Ni2+ and proton sensing. J Polym Res 18, 1315–1322 (2011). https://doi.org/10.1007/s10965-010-9534-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9534-x