Abstract
Natural enemies locate their herbivorous host and prey through kairomones emitted by host plants and herbivores. These kairomones could be exploited to attract and retain natural enemies in crop fields for insect pest control. The parasitoid Encarsia formosa preferentially parasitises its whitefly host, Trialeurodes vaporariorum, a major pest of tomato Solanum lycopersicum, thus offering an effective way to improve whitefly control. However, little is known about the chemical interactions that occur in E. formosa-T. vaporariorum-S. lycopersicum tritrophic system. Using behavioural assays and chemical analyses, we investigated the kairomones mediating attraction of the parasitoid to host-infested tomato plants. In Y-tube olfactometer bioassays, unlike volatiles of healthy tomato plants, those of T. vaporariorum-infested tomato plants attracted E. formosa, and this response varied with host infestation density. Coupled gas chromatography/mass spectrometric analyses revealed that host infestation densities induced varying qualitative and quantitative differences in volatile compositions between healthy and T. vaporariorum adult-infested tomato plants. Bioassays using synthetic chemicals revealed the attractiveness of 3-carene, β-ocimene, β-myrcene and α-phellandrene to the parasitoid, and the blend of the four compounds elicited the greatest attraction. Our results suggest that these terpenes could be used as an attractant lure to recruit the parasitoid E. formosa for the control of whiteflies in tomato crop fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural enemies exploit kairomones from plants and herbivores to locate oviposition sites and feeding resources for their survival, growth and reproductive success (Afsheen et al. 2008; Colazza and Wanjberg 2013). Kairomones are chemical signals mediating interspecific interactions that are beneficial to organisms that detect them. Although healthy plants emit volatile organic compounds, herbivory results in the release of herbivore-induced plant volatiles (HIPVs), which serve as long-range kairomones for natural enemies seeking host and prey (Kessler and Baldwin 2001; Mumm and Dicke 2010; Takabayashi and Shiojiri 2019). Kairomones have been exploited to attract parasitoids for the biological control of insect pests (Kaplan 2012; Peri et al. 2018). For example, 4′-ethyl-acetophenone, a castor bean (Ricinus communis L. (Euphorbiaceae)) plant-emitted HIPV induced by Apolygus lucorum (Meyer-Dür) (Hemiptera: Miridae), has been applied to attract the parasitoid Peristenus spretus Chen & van Achterberg (Hymenoptera: Braconidae) for the control of the insect pest in the field (Xiu et al. 2019). However, HIPV blend compositions are influenced by biotic factors such as the herbivore-infesting species, its infestation density, infesting instar, and the host plant species, which in turn influence parasitoid responses (De Moraes et al. 1998; McCormick et al. 2012). Besides HIPVs, chemical cues from the host or prey, or its by-products such as feces and honeydew may also serve as kairomone signals that enable natural enemies to locate their host and prey (Afsheen et al. 2008). For example, the parasitoid Psyllaephagus pistaciae Ferrière (Hymenoptera: Encyrtidae) is attracted to volatiles from honeydew excreted by its host, Agonoscena pistaciae Burckhardt and Lauterer (Hemiptera: Psylloidae) (Mehrnejad and Copland 2006). Kairomones identified from tritrophic interactions have been used for the recruitment of insect parasitoids to increase parasitism rates in field crops (Murali-Baskaran et al. 2018; Peñaflor and Bento 2013).
Encarsia formosa Gahan (Hymenoptera: Aphelinidae) is one the most efficient parasitoids used for controlling Trialeurodes vaporariorum (Westwood) and Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) whiteflies (De Vis and van Lenteren 2008; Hoddle et al. 1998; Liu et al. 2015). The parasitoid preferably parasitises third and fourth nymphal instars of both T. vaporariorum and B. tabaci (Antony et al. 2003; Hu et al. 2002). Trialeurodes vaporariorum and B. tabaci are devastating insect pests of tomato (Solanum lycopersicum L. (Solanaceae)) crops, responsible for 30 to 100% yield losses in both open fields and greenhouses worldwide (Gamarra et al. 2016; Hanssen and Lapidot 2012; Perring et al. 2018). Damage is mainly due to whitefly-vectored viruses which cause interveinal yellowing, irregular fruit ripening and poor plant growth (Hanssen and Lapidot 2012; Navas-Castillo et al. 2014). The phloem sap sucking feeding behaviour of whiteflies also causes plant wilting and defoliation, and their honeydew provides a medium which promotes sooty mold development on leaves, which in turn interferes with photosynthesis, and consequently limits plant growth (Gamarra et al. 2016; Palumbo et al. 2000). Trialeurodes vaporariorum is an invasive species in Europe and sub-Saharan Africa where it threatens tomato production (Hanssen and Lapidot 2012; Gamarra et al. 2016). Trialeurodes vaporariorum completes its life cycle (eggs, nymphs and adults) on tomato plants within 25 ± 2 days at 25 °C, with the completion of up to three to four generations before tomato harvest (Gamarra et al. 2016). Successful control of invasive insect pests is often challenging as their reproduction and population growth times are typically rapid, making them very destructive and more competitive than native pests (Wagh et al. 2014). However, the parasitoid E. formosa has been reported to successfully control T. vaporariorum on tomato plants grown in greenhouses (De Vis and van Lenteren 2008; Hoddle et al. 1998), and this performance could be achieved in the field with the application of kairomones to attract and retain the parasitoid in tomato crops.
Previous studies have demonstrated that kairomones attract E. formosa to various host plant species infested by whiteflies (Inbar and Gerling 2008; Zhang et al. 2013a). For example, E. formosa is attracted to volatiles from Phaseolus vulgaris L. (Fabaceae) infested by T. vaporariorum, and to volatiles of Arabidopsis thaliana (L.) (Brassicaceae) infested by B. tabaci (Birkett et al. 2003; Zhang et al. 2013a). Birkett et al. (2003) reported that the HIPVs (Z)-3-hexen-1-ol, 4,8-dimethyl-1,3,7-nonatriene, and 3-octanone released by T. vaporariorum-infested P. vulgaris attract E. formosa. However, E. formosa preferably parasitises its hosts, T. vaporariorum and B. tabaci on tomato (S. lycopersicum) plants compared to other host plants from Fabaceae, Brassicaceae and Solanaceae families (Kos et al. 2009; Zhang et al. 2005). These findings suggest that whitefly-infested S. lycopersicum plants release specific volatile compounds which attract E. formosa. Although, plant colour plays a role in host plant location by E. formosa (Romeis and Zebitz 1997), a field observation also reported that the density of the whitefly B. tabaci on plants influenced the abundance of Encarsia species (Kishinevsky et al. 2016). Hence, it would be interesting to investigate the kairomones allowing for a functional response to T. vaporariorum density on tomato plants. We thus assessed the attractiveness of E. formosa to tomato plant volatiles in relation to host infestation densities and identified the attractant compounds. We discuss our results in the light of the potential application of these attractants as kairomone-based lures to recruit the parasitoid for T. vaporariorum control in tomato crop fields.
Materials and Methods
Plant
The Kilele F1 tomato hybrid (Syngenta, Nairobi, Kenya) was used in the experiments. Seeds were sown in nurseries on a 1:1 mixture of soil: manure placed in a seedling plastic tray. Three weeks after sowing, the seedlings were individually transplanted in plastic pots (10 × 15 cm) containing 3:1 soil: manure mixture and grown in a screen house at 30 ± 5 °C, 65 ± 5% RH at the International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya. Plants were watered as needed, and water-dipped fertiliser containing 18% N, 20% P2O5, and 21% K2O (Easygrow, Osho Chemical Industries Ltd) was provided once a week. The plants were grown without insecticide application. Four-week-old plants with four developed leaves were used for behavioural assays and volatile chemical analyses.
Insects
Colonies of the experimental insect species, T. vaporariorum and E. formosa were initiated with field collected nymphs and adults from tomato fields at the Kenyan Agricultural and Livestock Research Organization (KALRO) in Kimbimbi (0°37′11.3”S 37°22′08.0″E), Mwea county, Kirinyaga region. Insects were reared in the laboratory and maintained at 25 ± 2 °C and 65 ± 5% RH under a 12:12 L:D photoperiod regime.
Trialeurodes vaporariorum was reared on tomato plants in laboratory Plexiglass cages (40 × 40 × 50 cm). Six-week-old plants were exposed to whiteflies for oviposition for three days. Infested plants were thereafter transferred into a separate screen house where the eggs developed into nymphs. Three-week post-infestation leaves with mature nymphs (mostly fourth instars) were cut from plants and returned to the original infestation cage where the adults emerged.
Encarsia formosa was reared on T. vaporariorum-infested tomato plants. Four infested plants at 14–21 days post infestation were offered to parasitoids in a laboratory Plexiglass cage (40 × 40 × 50 cm) for parasitism. Every three days, the oldest parasitoid-exposed plant was removed and replaced with a plant 14 days post-infestation. Plants with parasitised nymphs were placed in another Plexiglass cage where the insects emerged. Newly emerged parasitoid adults were returned to the original cage. Encarsia formosa adults were provided 80% honey solution and water twice a week. Naive 3–5-day-old E. formosa females were used in the experiments as the parasitoid fecundity is known to be higher within this age interval (Qiu et al. 2004).
Y-Tube Olfactometer Bioassays
The attractiveness of E. formosa to plant odours was assessed in dual choice assays using a vertically-oriented Y-tube olfactometer (0.5 cm internal diameter; 6 cm stem; two 6 cm side arms). A 10 L glass jar serving as an odour source container was connected to each side arm. A cardboard box (35 × 35 × 55 cm) served as observation chamber in which the Y-tube was placed, thus preventing insects from using plant visual cues. Uniform lighting was provided in the observation chamber using a 220–240 V cool white fluorescent light placed above the Y-tube. A unidirectional charcoal-filtered airflow was generated by an air pump (KNF lab LABOPORT N86KT.18, France) and passed through each odour source container toward the arms at a constant flow rate of 120 mL min−1 that was set using an AALBORG flow meter (Orangeburg, USA). Pots of growing test plants were wrapped in aluminium foil to avoid volatile contamination from the pot and soil. Experimental insects were individually introduced at the base of the Y-tube stem, and the insect was given 5 min during which its first choice was recorded. An insect was considered to have made a choice when it walked and reached the end of a given arm, and remained there for 30 s. Insects which did not make a choice within 5 min were considered as non-respondent and were subsequently not included in the data analysis. Ten insects were tested per plant per treatment per day, and the Y-tube was cleaned with dichloromethane after five insects and the volatile sources switched between the arms (left and right) to prevent contamination and positional bias. Overall, 80 insects were tested per choice test.
Encarsia formosa olfactory responses to volatiles from healthy and T. vaporariorum adult-infested tomato plants were investigated by performing the following dual choices (i) air vs. air (control); (ii) air vs. healthy plant; (iii) air vs. T. vaporariorum-infested plant; (iv) healthy plant vs. T. vaporariorum-infested plant. Infested plants were obtained by exposing a single 4-week-old plant to 50, 100 or 200 T. vaporariorum adults of mixed ages and sexes in a ventilated plastic box (35 × 25 × 35 cm) for four days which was enough to induce changes in volatile emission from tomato plants infested by B. tabaci adults (Su et al. 2018).
Collection of Volatiles
Tomato headspace volatiles were collected using a dynamic push-pull system, and trapped onto prepacked 30 mg super-Q adsorbent (Analytical Research Systems, Gainesville, FL, USA). Volatiles were collected from healthy and T. vaporariorum adult-infested tomato plants, as well as from empty volatile collection chamber and pot without plant (controls). Healthy or infested plants, and the pots, were separately placed in a 10 L glass jar used in the behavioural response assays. A charcoal-filtered airflow was directed into the containers at a rate of 200 mL min−1 using an air pump (KNF lab LABOPORT N86KT.18, France). Collection of volatiles was done for 24 h with 4 replications. Volatiles were eluted with 150 μL of dichloromethane, then the solution was concentrated to 50 μL to which 5 μL of biphenyl (20 ng/μL) was added as internal standard, and then stored at −80 °C until analysis.
Analysis of Volatiles
An aliquot (1 μL) of headspace tomato plant volatile extract was analysed by gas chromatography/mass spectrometry (GC/MS) on a Shimadzu QP2010 Ultra GC/MS (GL Sciences, Tokyo, Japan). The mass spectrometer was equipped with an Inert Cap 5MS/NP capillary column (5% diphenyl and 95% dimethylpolysiloxane, 30 m × 0.25 mm × 0.25 μm film thickness). Analysis was performed in the splitless mode using helium as carrier gas at a constant flow rate of 1 mL min−1. The oven temperature was set at 35 °C for 5 min and then programmed to increase at 10 °C min−1 until reaching a final temperature of 280 °C at which it was held for 10.5 min. The ion source temperature was set at 250 °C with an interface temperature of 270 °C, and spectra were recorded at 70 eV. Compound identification was done using retention time, library mass spectra (NIST11, Wiley9), and Kovats retention indices (RIs) determined using retention times of n-alkane (C8-C23) standards. All peaks detected in the control were considered as contaminants and therefore discarded in the volatile analysis. Compounds were quantified relative to peak area and concentration of the internal standard.
Response of E. formosa to Synthetic Plant Volatile Compounds
A total of 11 compounds were found to be highly associated with 100-T. vaporariorum adult-infested tomato plants (see Results) which attracted the parasitoid. These VOCs included: β-myrcene, α-phellandrene, 3-carene, p-cymene, β-phellandrene, (E)-β-ocimene, terpinolene, allo-ocimene, β-elemene and (E)-β-caryophylene and α-humulene. They were therefore used in the bioassays with synthetic compounds, except β-phellandrene which was not commercially available. Based on the natural release rate (ng/plant/h) of the compounds (Table 1), each compound was tested at doses corresponding to release rates of 10, 100 and 1000 equivalent plants in an hour. Based on the results obtained, four of these compounds (3-carene, β-ocimene, β-myrcene and α-phellandrene), found to be attractive to the parasitoid were formulated into a blend (B1) using their individual optimal attractant doses (100 ng 3-carene, 200 ng α-phellandrene, 600 ng β-ocimene and 800 ng β-myrcene). Two other blends B2 (100 ng 3-carene, 200 ng α-phellandrene, 60 ng β-ocimene and 80 ng β-myrcene) and B3 (1 ng 3-carene, 20 ng α-phellandrene, 6 ng β-ocimene and 8 ng β-myrcene) were tested. The test compounds were diluted in dichloromethane and a 10 μL aliquot of the test solution was applied on a filter paper (2 × 2 cm) (Whatman, UK) and tested against the control (i.e. filter paper loaded with 10 μL dichloromethane). The solvent was allowed to evaporate for 30 s. Thereafter, the impregnated filter papers were placed in Pasteur pipettes which were directly connected to the olfactometer arms. The test aliquot was used for a single insect with a 5 min observation period, and 80 insects were tested per choice test.
Chemicals
The β-myrcene, α-phellandrene, 3-carene, p-cymene, β-ocimene, terpinolene, allo-ocimene, β-elemene, (E)-β-caryophylene and α-humulene synthetic standards used in the bioassays were all purchased from Sigma-Aldrich (France). All the chemicals were at 90–99% purity, except β-elemene (80% purity) and α-phellandrene (85% purity). Dichloromethane (99.9% purity) was purchased from Merck, Germany.
Statistical Analyses
Encarsia formosa preference for odours in the Y-tube olfactometer was determined by comparing the recorded frequencies of choice of either of the olfactometer arms using a chi-squared test. Analysis of concentrations of volatile compounds between healthy and whitefly-infested plants was performed using a non-parametric Kruskal-Wallis test to account for distribution normality and variance homogeneity, and when a significant difference was noted a post-hoc Dunn’s test associated with Bonferroni’s adjustment was applied for mean separation (Dinno 2015). The Random Forest (RF) analysis (Breiman 2001) was used to select the most discriminant volatiles between healthy and T. vaporariorum adult-infested tomato plants, as used in previous studies (Ranganathan and Borges 2010; McCormick et al. 2014, 2016). The most predictive variables (VOCs in our case) were defined using the RF “importance” function which provides the mean decrease in accuracy (MDA), where a higher MDA indicates higher importance in the classification (Liaw and Wiener 2002; Ranganathan and Borges 2010). Using concentrations of the most discriminant volatile compounds, a Sparse Partial Least Square Discriminant Analysis (sPLS-DA) biplot was performed to highlight variations in the emission of compounds between healthy and herbivore-infested plants, and to identify VOCs highly associated with the attractant plants (i.e. 100-T. vaporariorum adult-infested plants) using the mixOmics package (Hervé et al. 2018; Lê Cao et al. 2011). The sPLS-DA model was validated using the “perf” function and the leave-one-group-out cross-validation method in the mixOmics package (Rohart et al. 2017). All statistical analyses were performed using R statistical software, version 4.0.2 (R Core Team 2020).
Results
Olfactory Response of E. formosa to Plant Volatiles
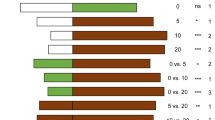
The whitefly parasitoid E. formosa was attracted to volatiles from tomato plants infested with 100 T. vaporariorum adults compared to clean air (control) (χ2= 9.59, df = 1, P = 0.002) and healthy tomato plant volatiles (χ2= 4.63, df = 1, P = 0.031) (Fig. 1). However, volatiles from tomato plants infested with either 50 (χ2= 0.63, df = 1, P = 0.428; χ2= 0.01, df = 1, P = 0.91) or 200 T. vaporariorum adults (χ2 = 0, df = 1, P = 1; χ2 = 0.11, df = 1, P = 0.737) did not attract the parasitoid when compared to clean air or healthy tomato plant volatiles, respectively. Similarly, the parasitoid was not attracted to volatiles of healthy tomato plants compared to clean air (χ2= 1.04, df = 1, P = 0.308).
Responses of Encarsia formosa to volatiles from healthy tomato plants and others infested with different densities of Trialeurodes vaporariorum adults in a Y-tube olfactometer choice tests. 80 insects were tested per choice. nr = number of non-respondent insects (i.e. no choice). P = statistical significance level with ns = no significant difference (P > 0.05); *, ** = significant differences at P < 0.05 or P < 0.01 from χ2 test at α = 0.05
Analysis of Tomato Volatiles
A total of 25 volatile organic compounds (VOCs) belonging to six chemical classes: monoterpene (14), sesquiterpene (6), ketone (2), homoterpene (1), aldehyde (1) and benzenoid (1) were detected in the volatile profiles of both healthy and whitefly-infested tomato plants (Table 1; Fig. 2). Quantitative and qualitative differences were observed in volatile emission between healthy and T. vaporariorum adult-infested tomato volatiles (Table 1; Fig. 2). The monoterpenes 2-carene and β-phellandrene were the most abundant compounds and accounted for about 80% of the total volatiles released from healthy and whitefly-infested tomato plants (Table 1; Fig. 2). Six VOCs, i.e. benzaldehyde (aldehyde); β-pinene, 6-methyl-5-hepten-2-one, 3-carene, allo-ocimene (monoterpenes) and β-elemene (sesquiterpene) were specific to T. vaporariorum-infested tomato plants. Among these compounds, 3-carene, allo-ocimene and β-elemene were found only in volatiles of plants infested with 100 T. vaporariorum adults.
GC/MS profiles of headspace volatiles from healthy tomato plants and 100 Trialeurodes vaporariorum adult-infested tomato plants. Numbers correspond to volatile compounds indicated on Table 1. IS = internal standard (biphenyl)
Emission rates of some VOCs such as p-cymene, α-phellandrene, β-phellandrene, (E)-β-ocimene, α-pinene, β-myrcene, terpinolene and δ-elemene increased by ≥2 fold in plants infested with 100 T. vaporariorum adults than in healthy plants (Table 1). Differences in some VOCs such as the monoterpenes α-phellandrene and (E)-β-ocimene, and the sesquiterpenes (E)-β-caryophyllene and α-humulene were also observed between the different infestation densities, with a decline in the emission at the highest whitefly infestation density (Table 1). However, the emission rates of 3,7,7-trimethyl-1,3,5-cycloheptatriene, α-cedrene and γ-elemene did not change between healthy plants and T. vaporariorum adult-infested plants.
Determination of Discriminant Volatiles for Bioassays with Synthetic Compounds
The RF analysis and the sPLS-DA biplot highlighted the VOCs that mostly characterised the attractant 100-T. vaporariorum adult-infested plants compared to the unattractant plants (combination of healthy, 50 and 200-T. vaporariorum adult-infested plants). The results of the RF analysis revealed 13 VOCs discriminating T. vaporariorum adult-infested tomato plants and healthy plants (Fig. 3a). Using these VOCs, the sPLS-DA classified healthy and whitefly-infested plants into two clusters: one group composed of 100-T. vaporariorum adult-infested plants, which was separated from the other group composed of healthy, and 50 or 200-T. vaporariorum adult-infested plants (Fig. 3b). The sPLS-DA biplot displayed the correlation between the discriminant VOCs and the plants (Fig. 3c). The first two dimensions of the sPLS-DA explained 62% of the total variation (Fig. 3c). Dimension 1 explained 47% of the total variation and was highly associated mainly with (E)-β-caryophyllene, β-elemene, allo-ocimene, 3-carene and α-phellandrene. Whereas dimension 2 accounted for 15% of the total variation, and was closely correlated mainly with p-cymene, (Z)-β-ocimene and 6-methyl-5-hepten-2-one. Heatmap clustering was performed to illustrate variations in VOCs across replicates of healthy and infested tomato plants, and the findings showed that most of the discriminant VOCs were abundant in tomato plants infested with 100 T. vaporariorum adults (Fig. 3d). According to the horizontal direction of the heatmap, all samples were classified in two main groups, i.e. one composed of plants infested with 100 T. vaporariorum adults, and another group composed of the rest of the samples (i.e. combination of healthy plants and others infested with 50 and 200 T. vaporariorum adults). The results of the sPLS-DA biplot specifically showed that (E)-β-caryophyllene, β-elemene, allo-ocimene, α-phellandrene, (E)-β-ocimene, β-phellandrene, 3-carene, terpinolene, p-cymene, β-myrcene and α-humulene were highly associated with plants infested with 100 T. vaporariorum adults, which attracted the parasitoid. Therefore, we focused on these VOCs for testing the attractiveness of the parasitoid E. formosa to synthetic compounds.
Determination of the most discriminant volatiles and their correlation with healthy tomato plants and tomato plants infested by 50, 100 and 200 Trialeurodes vaporariorum adults (50Tv-inf, 100Tv-inf and 200Tv-inf, respectively). a The 13 most discriminant volatiles between healthy and infested tomato plants are listed in decreasing importance based on the mean decrease in accuracy (MDA > 1) in the random forest analysis. b A sPLS-DA plot displaying the distribution of healthy and infested tomato plants. c A sPLS-DA biplot showing the correlation of the most discriminant volatiles with healthy and infested tomato plants. d Heatmap clustering showing the abundance (in decreasing colour intensity) of the most discriminant VOCs across replicates of healthy and infested tomato plants
Bioassays with Synthetic Compounds
Encarsia formosa responded differently to the various doses of compounds tested individually (Fig. 4). The parasitoid was attracted to β-ocimene at 60 ng (χ2 = 5.06, df = 1, P = 0.024) and 600 ng (χ2 = 8.01, df = 1, P = 0.003) doses compared to the control (dichloromethane). The parasitoid was also attracted to β-myrcene at 80 ng (χ2 = 4.63, df = 1, P = 0.03) and 800 ng (χ2 = 6.13, df = 1, P = 0.013) doses compared to the control. Moreover, 3-carene attracted E. formosa only at the 100 ng dose (χ2 = 6.13, df = 1, P = 0.013) and α-phellandrene at 200 ng dose (χ2 = 7.81, df = 1, P = 0.005) compared to the control. On the other hand, terpinolene, p-cymene, α-humulene and (E)-β-caryophyllene were not attractive to the parasitoid at any of the tested doses (Fig. 4).
Olfactory responses of Encarsia formosa to three doses of selected synthetic volatile compounds. 80 insects were tested per choice. DCM = dichloromethane. nr = number of non-respondent insects (i.e. no choice). P = statistical significance level with ns = no significant difference at P > 0.05; *, ** = significant differences at P < 0.05 and P < 0.01 from χ2 test at α = 0.05
The parasitoid E. formosa was attracted to the blend of the four attractants 3-carene, α-phellandrene, β-myrcene and β-ocimene mixed at their optimal attractant doses (B1) compared to the control (dichloromethane) (χ2 = 6.28, df = 1, P = 0.012) (Fig. 5). However, the parasitoid was highly attracted to the mixture of the four attractants when blended at the intermediate attractant doses (B2) compared to the control (χ2 = 11.39, df = 1, P < 0.001). On the other hand, E. formosa was not attracted to the blend when the attractant compounds were mixed at their lowest doses (B3) compared to the control (χ2 = 3.24, df = 1, P = 0.072) (Fig. 5).
Encarsia formosa attractiveness to mixtures of attractant compounds blended at the attractant doses of (B1): 100 ng 3-carene, 200 ng α-phellandrene, 600 ng β-ocimene and 800 ng β-myrcene and (B2): 100 ng 3-carene, 200 ng α-phellandrene, 60 ng β-ocimene and 80 ng β-myrcene, and at lowest tested doses of (B3): 1 ng 3-carene, 20 ng α-phellandrene, 6 ng β-ocimene and 8 ng β-myrcene. 80 insects were tested per choice test. DCM = dichloromethane. nr = number of non-respondent insects (i.e. no choice). P = statistical significance level with ns = no significant difference (P > 0.05); *, *** = significant differences at P < 0.05 and P < 0.001 from χ2 test at α = 0.05
Discussion
We have shown that the parasitoid E. formosa responded to volatiles of T. vaporariorum adult-infested tomato plants, and this response varied with the host infestation density. Encarsia formosa was attracted to volatiles of plants infested with 100 T. vaporariorum adults, and the parasitoid preferred 100-T. vaporariorum adult-infested tomato plant volatiles compared to healthy plant volatiles. Hence, plants infested with 100 T. vaporariorum adults produced specific induced plant volatiles which are detected by the parasitoid to discriminate between infested and healthy tomato plants. Our results support previous studies on other plant-host-parasitoid systems. For example, E. formosa was reported to be attracted to volatiles of T. vaporariorum adult-infested P. vulgaris plants and B. tabaci adult-infested A. thaliana plants compared to volatiles of healthy plants (Birkett et al. 2003; Zhang et al. 2013a). Similarly, E. formosa preferred Myzus persicae (Sulzer) (Hemiptera: Aphididae) adult-infested tomato plant volatiles relative to volatiles of healthy plants (Tan and Liu 2014). The attraction of parasitoids to specific host infestation density is the result of qualitative and quantitative differences in volatile emission between healthy and herbivore-infested plants (McCormick et al. 2012; Shiojiri et al. 2010). We found that the infestation density of 100 T. vaporariorum adults changed the quality and quantity of released volatile compounds in tomato plants. Our tomato volatile analysis showed that volatile emission reached a maximum at an intermediate host density (100 T. vaporariorum adults). Similarly volatile emission by Brasica nigra L. (Brassicaceae) plants infested with Myzus persicae (Sulzer) (Hemiptera: Aphididae) aphid increased at the infestation density of 50 aphid adults compared to infestation by lower (i.e. 25) and higher (i.e. 100) host densities (Ponzio et al. 2016). The VOCs β-pinene, 6-methyl-5-hepten-2-one, benzaldehyde, 3-carene, allo-ocimene and β-elemene were specific to 100-T. vaporariorum adult-infested tomato plants, and levels of p-cymene, α-phellandrene, (E)-β-ocimene and (E)-β-caryophyllene increased in the attractant 100 T. vaporariorum adult-infested tomato plants compared to healthy plants.
The parasitoid E. formosa was, however, not attracted to volatiles produced by tomato plants infested with 50 or 200 T. vaporariorum adults. Our findings on the host infestation density dependent-olfactory response in E. formosa corroborated the findings of previous studies on other parasitoid species of phloem-feeding herbivores, where an infestation threshold is generally required for the parasitoids for optimal response to herbivore-infested plants (Lou et al. 2005; Ponzio et al. 2016). Lou et al. (2005) reported that the parasitoid Anagrus nilaparvatae Pang and Wang (Hymenoptera: Mymaridae) was attracted to volatiles of Oryza sativa L. (Poaceae) plants infested with 10 or 20 Nilaparvata lugens Stål (Hemiptera: Delphacidae) adults, but there was no attraction to plants with lower (1 or 5) or higher (40 or 80) infestation densities. This density-dependent olfactory response was also reported for the parasitoid Dieaeretiella rapae (McIntoch) (Hymenoptera: Braconidae) which preferred volatiles of B. nigra plants infested with 25 M. persicae aphid nymphs over those of healthy plants, but it showed no attraction to plants infested with higher host infestation density, i.e. 50 or 100 M. persicae nymphs (Ponzio et al. 2016).
In our study, the relatively low attraction of E. formosa to plants infested by either low or high T. vaporariorum adult infestation densities could be explained by the results obtained from our volatile analysis. Phloem-feeding herbivores such as whiteflies and aphids which are salicylic acid pathway inducers may activate or suppress the plant defence mechanisms, thus emission of volatiles, depending on their density and the infestation or feeding duration (Lou et al. 2005; Walling 2008; Zhang et al. 2013b). We found that 50 whitefly adults induced minor changes in the plant volatiles, where benzaldehyde, β-pinene, and 6-methyl-5-hepten-2-one were only released in 50 whitefly adult-infested tomato plants compared to healthy plants. On the other hand, we found that volatile emission declined in tomato plants infested with 200 T. vaporariorum adults compared to plants infested with 100 T. vaporariorum adults. Specifically, α-phellandrene, p-cymene, (E)-β-caryophyllene and α-humulene decreased in 200-T. vaporariorum adult-infested plants compared to 100-T. vaporariorum adult-infested plants, and also (E)-β-caryophyllene decreased in 200-T. vaporariorum adult-infested plants compared to healthy plants. Darshanee et al. (2017) reported that α-humulene and (E)-β-caryophyllene emission decreased in tomato plants infested with 200 T. vaporariorum adults compared to healthy plants. The decrease of volatile emission at the highest T. vaporariorum infestation density could be explained by an inhibitory effect on the jasmonic acid-regulated pathway, as observed for B. tabaci feeding on Arabidopsis and tomato plants (Su et al. 2018; Zarate et al. 2007; Zhang et al. 2013b, 2019). Estrada-Hernandez et al. (2009) also reported that a high B. tabaci feeding intensity on tomato plants led to downregulation of genes associated with photosynthesis and secondary metabolites. Therefore, we speculate that the high T. vaporariorum infestation rate could have induced a reduction in photosynthetic activity and secondary metabolite synthesis. Moreover, it is likely that the high T. vaporariorum density which correlated with a high feeding intensity could have weakened the plant quality and thus its ability to maintain normal physiological processes, which could potentially decrease whitefly survival and fitness, which in turn affects the fitness of the parasitoid. It has been observed that a high infestation density and longer feeding period of B. tabaci whiteflies on cucumber crops resulted in a reduction in plant photosynthetic activity (Shannag and Freihat 2009). Palumbo et al. (2000) also reported that heavy Bemisia whitefly density led to a reduction in the growth and vigor of alfalfa (Medicago sativa L. (Fabaceae)) plants through the reduction in the plant crude protein content.
Herbivore-induced plant volatiles serve as kairomones during host location by parasitoids. We found that synthetic 3-carene, β-myrcene, β-ocimene and α-phellandrene tested individually attracted E. formosa. These attractant compounds were either specific to or abundant in the attractant 100-T. vaporariorum adult-infested plants compared to unattractant plants, i.e. healthy plants and plants infested with 50 and 200 T. vaporariorum adults. Among these compounds, only β-myrcene has been previously identified as an attractant to E. formosa, from a study on HIPV of B. tabaci adult-infested A. thaliana plants (Zhang et al. 2013a). However, other E. formosa attractants: (Z)-3-hexen-1-ol, 4,8-dimethyl-1,3,7-nonatriene, and 3-octanone have been identified in T. vaporariorum adult-infested P. vulgaris plant volatiles (Birkett et al. 2003). The E. formosa-attracting compounds identified in our study have been reported to mediate olfactory responses in other parasitoid species. For instance, α-phellandrene and (E)-β-ocimene induced by phloem-feeding aphids were reported to be attractants for the aphid parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae) (Takemoto and Takabayashi 2015). Moreover, 3-carene attracted Chouioia cunea (Yang) (Hymenoptera: Eulophidae), a pupal parasitoid of Hypanthia cunea (Drury) (Lepidoptera: Arctiidae) (Pan et al. 2020). The dose-response assays in our study revealed that, except for α-phellandrene, the proportion of attracted parasitoids slightly increased as the test dose increased. Doses of compounds attractive to E. formosa in previous studies were: e.g. 360 ng β-myrcene in olfactometer (Zhang et al. 2013a), and 10 μg (Z)-3-hexen-1-ol in wind tunnels (Birkett et al. 2003), suggesting that the doses tested in our study were in relevant range to elicit attraction in the parasitoid. Natural enemies are likely to use blend of odours when foraging for host and prey (Conchou et al. 2019; Thomas-Danguin et al. 2014). Our results showed that the parasitoid E. formosa was highly attracted to the blend of the four attractants, i.e. 3-carene, β-myrcene, β-ocimene and α-phellandrene when tested against clean air. However, the parasitoid attractiveness was reduced when concentrations of two compounds, β-myrcene and β-ocimene, were increased by 10-fold, indicating that the parasitoid could be sensitive to the relative concentration and ratio of these compounds in the blend. A previous study reported that the mixture of (Z)-3-hexen-1-ol and 3-octanone elicited the greatest attraction by E. formosa in wind tunnel assays (Birkett et al. 2003).
In summary, our findings show that the density of the whitefly T. vaporariorum adults on tomato plants influences both the olfactory response of E. formosa and the volatile composition of infested tomato plants. We also demonstrate that terpenes are responsible for E. formosa attraction to T. vaporariorum adult-infested tomato plants. The 4-component terpene blend of 3-carene, β-myrcene, β-ocimene and α-phellandrene, could be used as a kairomone-based attractant lure to recruit and retain the parasitoid for the control of T. vaporariorum in tomato crop fields.
References
Afsheen S, Wang X, Li R, Zhu C-S, Lou Y-G (2008) Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci 15:381–397. https://doi.org/10.1111/j.1744-7917.2008.00225.x
Antony B, Palaniswami MS, Henneberry TJ (2003) Encarsia transvena (Hymenoptera: Aphelinidae) development on different Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) instars. Environ Entomol 32:584–591. https://doi.org/10.1603/0046-225x-32.3.584
Birkett MA, Chamberlaln K, Guerrieri E, Pickett JA, Wadhams LJ, Yasuda T (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29:1589–1600. https://doi.org/10.1023/A:1024218729423
Breiman L (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/A:1010933404324
Colazza S, Wanjberg E (2013) Chemical ecology of insect parasitoids: Towards a new area. In : Wanjberg E, Colazza S (eds) Chemical Ecology of Insect Parasitoids. First edit. Wiley-Blackwell, Chichester, UK: John Wiley & Sons, Ltd.
Conchou L, Lucas P, Meslin C, Proffit M, Staudt M, Renou M (2019) Insect odorscapes : from plant volatiles to natural olfactory scenes. Front Physiol 10:1–20. https://doi.org/10.3389/fphys.2019.00972
Darshanee HLC, Ren H, Ahmed N, Zhang Z-F, Liu Y-H, Liu T-X (2017) Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and eggplant. Front Plant Sci 8:1–13. https://doi.org/10.3389/fpls.2017.01285
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573. https://doi.org/10.1038/31219
De Vis RMJ, Van Lenteren JC (2008) Biological control of Trialeurodes vaporariorum by Encarsia formosa on tomato in unheated greenhouses in the high altitude tropics. Bull Insectology 61:43–57
Dinno A (2015) Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J 15:292–300. https://doi.org/10.1177/1536867X1501500117
Estrada-Hernandez MG, Valenzuela-Soto JH, Ibarra-Laclette E, John Paul Delano-Frier JP (2009) Differential gene expression in whitefly bemisia tabaci-infested tomato (Solanum lycopersicum) plants at progressing developmental stages of the insect’s life cycle. Physiol Plant 137:44–60. https://doi.org/10.1111/j.1399-3054.2009.01260.x
Gamarra H, Carhuapoma P, Mujica M, Kreuze J, Kroschel J (2016) Greenhouse Whitefly, Trialeurodes vaporariorum (Westwood 1956). In: Kroschel J, Mujica N, Carhuapoma P, Sporleder M (eds.) Pest distribution and risk Atlas for Africa. Potential, Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents under Current and Future Climates, 154:154–168. Lima, Peru: International Potato Center (CIP). ISBN 978-92-9060-476-1. https://doi.org/10.4160/9789290604761
Hanssen IM, Lapidot M (2012) Major tomato viruses in the mediterranean basin. Adv Virus Res 84:31-66. Elsevier Inc. https://doi.org/10.1016/B978-0-12-394314-9.00002-6
Hervé MR, Nicolè F, Lê Cao K-A (2018) Multivariate analysis of multiple datasets: a practical guide for chemical ecology. J Chem Ecol 44:215–234. https://doi.org/10.1007/s10886-018-0932-6
Hoddle MS, Van Driesche RG, Sanderson JP (1998) Biology and use of the whitefly parasitoid Encarsia formosa. Annu Rev Entomol 43:645–669. https://doi.org/10.1146/annurev.ento.43.1.645
Hu JS, Gelman DB, Blackburn MB (2002) Growth and development of Encarsia formosa (Hymenoptera: Aphelinidae) in the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae): effect of host age. Arch Insect Biochem Physiol 49:125–136. https://doi.org/10.1002/arch.10015
Inbar M, Gerling D (2008) Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu Rev Entomol 53:431–448. https://doi.org/10.1146/annurev.ento.53.032107.122456
Kaplan I (2012) Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire? Biol. Control. 60:77–89. https://doi.org/10.1016/j.biocontrol.2011.10.017
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291(5511):2141–2144. https://doi.org/10.1126/science.291.5511.2141
Khan M, Mousa AA, Syamasundar KV, Alkhathlan HZ (2012) Determination of chemical constituents of leaf and stem essential oils of Artemisia monosperma from Central Saudi Arabia. Nat Prod Commun 7:1079–1082. https://doi.org/10.1177/1934578x1200700829
Kishinevsky M, Keasar T, Bar-Massada A (2016) Parasitoid Encarsia spp abundance on plants: effects of host abundance, plant species, and plant fLowering state. Arthropod Plant Interact 11:1–7. https://doi.org/10.1007/s11829-016-9476-2
Kos K, Tomanović Ž, Rojht H, Vidrih M, Trdan S (2009) First massive occurrence of greenhouse whitefly parasitoid, Encarsia formosa Gahan (Hymenoptera : Aphelinidae) on greenhouse whitefly, Trialeurodes vaporariorum [Westwood] (Homoptera : Aleyrodidae) in Slovenia. Acta Agric Slov 93:285–291. https://doi.org/10.2478/v10014-009-0017-x
Lê Cao K-A, Boitard S, Besse P (2011) Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform 12:1–17. https://doi.org/10.1186/1471-2105-12-253
Liaw A, Wiener M (2002) Classification and regression by random Forest. R News 2:18–22
Liu T-X, Stansly PA, Gerling D (2015) Whitefly parasitoids: distribution, life history, bionomics, and utilization. Annu Rev Entomol 60:1–15. https://doi.org/10.1146/annurev-ento-010814-021101
Lou Y-G, Ma B, Cheng J-A (2005) Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J Chem Ecol 31:2357–2372. https://doi.org/10.1007/s10886-005-7106-z
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310. https://doi.org/10.1016/j.tplants.2012.03.012
Mccormick AC, Gershenzon J, Unsicker SB (2014) Little peaks with big effects: establishing the role of minor plant volatiles in plant–insect interactions. Plant Cell Environ 37(8):1836–1844
McCormick AC, Reinecke A, Gershenzon J, Unsicker SB (2016) Feeding experience affects the behavioral response of polyphagous gypsy moth caterpillars to herbivore-induced poplar volatiles. J Chem Ecol 42:382–393. https://doi.org/10.1007/s10886-016-0698-7
Mehrnejad MR, Copland MJW (2006) Behavioral responses of the parasitoid Psyllaephagus pistaciae (Hymenoptera : Encyrtidae) to host plant volatiles and honeydew. Entomol Sci 9:31–37. https://doi.org/10.1111/j.1479-8298.2006.00151.x
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667. https://doi.org/10.1139/Z10-032
Murali-Baskaran KR, Chander K, Kaushal P, Kumar J, Parthiban P, Senthil-Nathan S, Mankin RW (2018) Role of kairomone in biological control of crop pests-a review. Physiol Mol Plant Pathol 101:3–15. https://doi.org/10.1016/j.pmpp.2017.07.004
Navas-Castillo J, López-Moya JJ, Aranda MA (2014) Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann Appl Biol 165:155–171
Palumbo JC, Toscano NC, Blua MJ, Yoshida HA (2000) Impact of Bemisia whiteflies (Homoptera: Aleyrodidae) on alfalfa growth, forage yield, and quality. J Econ Entomol 93:1688–1694. https://doi.org/10.1603/0022-0493-93.6.1688
Pan L, Xiang W, Sun Z, Yang Y, Han J, Wang Y, Yan C, Li M (2020) CcOBP2 plays a crucial role in 3-carene olfactory response of the parasitoid wasp Chouioia cunea. Insect Biochem Mol Biol 117:103286. https://doi.org/10.1016/j.ibmb.2019.103286
Peñaflor MFGV, Bento JMS (2013) Herbivore-induced plant volatiles to enhance biological control in agriculture. Neotrop Entomol 42:331–343. https://doi.org/10.1007/s13744-013-0147-z
Peri E, Moujahed R, Wajnberg E, Colazza S (2018) Applied chemical ecology to enhance insect parasitoid efficacy in the biological control of crop pests. In: Tabata J (ed) Chemical ecology of insects: applications and associations with plants and microbes. Taylor & Francis, New York, pp 234–267
Perring TM, Stansly PA, Liu TX, Smith HA, Andreason SA (2018) Whiteflies : biology, ecology, and management. In: Wakil W, Brust GE, and Perring TM (eds) Sustainable management of arthropod pests of tomato,73–110. Elsevier Inc. Academic Press. https://doi.org/10.1016/B978-0-12-802441-6.00004-8
Ponzio C, Cascone P, Cusumano A, Weldegergis BT, Fatouros NE, Guerrieri E, Dicke M, Gols R (2016) Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim Behav 111:197–206. https://doi.org/10.1016/j.anbehav.2015.10.024
Qiu YT, Van Lenteren JC, Drost YC, Posthuma-Doodeman JAM (2004) Life-history parameters of Encarsia formosa, Eretmocerus eremicus and E. mundus, aphelinid parasitoids of Bemisia argentifolii (Hemiptera: Aleyrodidae). Eur J Entomol 101:83–94
R Core Team (2020) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. URL https://www.R-project.org/. Accessed 11 Aug 2020
Ranganathan Y, Borges RM (2010) Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biol 12:735–742. https://doi.org/10.1111/j.1438-8677.2009.00278.x
Rohart F, Gautier B, Singh A, Lê Cao K-A (2017) MixOmics : an R package for ‘Omics feature selection and multiple data integration. PLoS Comput Biol 13:e1005752. https://doi.org/10.1371/journal.pcbi.1005752
Romeis J, Zebitz CPW (1997) Searching behaviour of Encarsia formosa as mediated by colour and honeydew. Entomol Exp Appl 1990:299–309
Shannag HK, Freihat NM (2009) Gas exchange of cucumber impaired by tobacco whitefly, Bemisia tabaci (Gennadius) (Hemiptera : Aleyrodidae). Jordan J Agric Sci 5:295–305
Shiojiri K, Ozawa R, Kugimiya S, Uefune M, Wijk MV, Sabelis MW, Takabayashi J (2010) Herbivore-specific, density-dependent induction of plant volatiles: honest or ‘cry wolf’ signals? PLoS One 5:1–11. https://doi.org/10.1371/journal.pone.0012161
Su Q, Chen G, Mescher MC, Peng Z, Xie W, Wang S, Wu Q, Liu J, Li C, Wang W, Zhang Y (2018) Whitefly aggregation on tomato is mediated by feeding-induced changes in plant metabolites that influence the behaviour and performance of conspecifics. Funct Ecol 32:1180–1193. https://doi.org/10.1111/1365-2435.13055
Takabayashi J, Shiojiri K (2019) Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Curr Opin Insect Sci 32:110–117. https://doi.org/10.1016/j.cois.2019.01.003 2214-5745/ã
Takemoto H, Takabayashi J (2015) Parasitic wasps Aphidius ervi are more attracted to a blend of host-induced plant volatiles than to the independent compounds. J Chem Ecol 41:801–807. https://doi.org/10.1007/s10886-015-0615-5
Tan X-L, Liu T-X (2014) Aphid-induced plant volatiles affect the attractiveness of tomato plants to Bemisia tabaci and associated natural enemies. Entomol Exp Appl 151:259–269. https://doi.org/10.1111/eea.12190
Thomas-Danguin T, Sinding C, Romagny S, Mountassir FE, Atanasova B, Le Berre E, Anne-Marie L, Le Bon A-M, Coureaud G (2014) The perception of odor objects in everyday life : a review on the processing of odor mixtures. Front Physiol 5:1–18. https://doi.org/10.3389/fpsyg.2014.00504
Wagh VH, Dhote VW, Maimom S, Jha S (2014) Invasive insect pests: challenges of changing climate. J Interacademicia 18 : 695–700. https://www.cabdirect.org/cabdirect/abstract/20153061548
Walling LL (2008) Avoiding effective defenses : strategies employed by phloem-feeding insects. Plant Physiol 146:859–866. https://doi.org/10.1104/pp.107.113142
Xiu C, Dai W, Pan H, Zhang W, Luo S, Wyckhuys KAG, Yang Y, Lu Y (2019) Herbivore-induced plant volatiles enhance field-level parasitism of the mirid bug Apolygus lucorum. Biol Control 135:41–47. https://doi.org/10.1016/j.biocontrol.2019.05.004
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143:866–875. https://doi.org/10.1104/pp.106.090035
Zhang S-Z, Guo J-Y, Wan F-H, Zhang F (2005) Parasitic behavior and selectivity of Encarsia formosa (Hymenoptera: Aphelinidae) towards Bemisia tabaci (Homoptera: Aleyrodidae) on different host plants. Sheng Tai Xue Bao 25:2595–2600
Zhang P-J, Xu C-X, Zhang J-M, Lu Y-B, Wei J-N, Liu Y-Q, David A, Boland W, Turlings TCJ (2013a) Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct Ecol 27:1304–1312. https://doi.org/10.1111/1365-2435.12132
Zhang P-J, Li W-D, Huang F, Zhang J-M, Xu F-C, Lu Y-B (2013b) Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol 39:612–619. https://doi.org/10.1007/s10886-013-0283-2
Zhang P-J, Wei J-N, Zhao C, Zhang Y-F, Li C-Y, Liu S-S, Dicke M, Yu X-P, Turlings TCJ (2019) Airborne host–plant manipulation by whiteflies via an inducible blend of plant volatiles. PNAS 116:7387–7396. https://doi.org/10.1073/pnas.1818599116
Acknowledgments
We thank Prof Baldwyn Torto for critical review on a previous version of the manuscript and Beatrice Rhino for providing guidance on statistical analyses. We are grateful for the financial support provided by the following organisations and agencies: the French National Research Agency (ANR) through CIRAD under Award No. ANR-16-CE32-0010-01; the UK’s Foreign, Commonwealth & Development Office (FCDO); the Swedish International Development Cooperation Agency (SIDA); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Kenyan government. The University of Pretoria and the National Research Foundation (A.A.Y. and C.W.W.P) also provided financial support. P.M.A. was supported by a German Academic Exchange Service (DAAD) In-Region Postgraduate Scholarship (personal grant No. 91672680). The views expressed herein do not necessarily reflect the official opinion of the donors.
Author information
Authors and Affiliations
Contributions
P.M.A., A.A.Y., C.W.W.P., S.A.M., A.C. & E.D. contributed to the conception and design of the research work and provided intellectual inputs. P.M.A. conducted the experiments, analysed the data and drafted the manuscript. All authors proofread the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Rights and permissions
About this article
Cite this article
Ayelo, P.M., Yusuf, A.A., Pirk, C.W.W. et al. The Role of Trialeurodes vaporariorum-Infested Tomato Plant Volatiles in the Attraction of Encarsia formosa (Hymenoptera: Aphelinidae). J Chem Ecol 47, 192–203 (2021). https://doi.org/10.1007/s10886-021-01245-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01245-2