Abstract
Plants emit volatile compounds in response to insect herbivory, which may play multiple roles as defensive compounds and mediators of interactions with other plants, microorganisms and animals. Herbivore-induced plant volatiles (HIPVs) may act as indirect plant defenses by attracting natural enemies of the attacking herbivore. We report here the first evidence of the attraction of three Neotropical mirid predators (Macrolophus basicornis, Engytatus varians and Campyloneuropsis infumatus) toward plants emitting volatiles induced upon feeding by two tomato pests, the leaf miner Tuta absoluta and the phloem feeder Bemisia tabaci, in olfactometer bioassays. Subsequently, we compared the composition of volatile blends emitted by insect-infested tomato plants by collecting headspace samples and analyzing them with GC-FID and GC-MS. Egg deposition by T. absoluta did not make tomato plants more attractive to the mirid predators than uninfested tomato plants. Macrolophus basicornis is attracted to tomato plants infested with either T. absoluta larvae or by a mixture of B. tabaci eggs, nymphs and adults. Engytatus varians and C. infumatus responded to volatile blends released by tomato plants infested with T. absoluta larvae over uninfested plants. Also, multiple herbivory by T. absoluta and B. tabaci did not increase the attraction of the mirids compared to infestation with T. absoluta alone. Terpenoids represented the most important class of compounds in the volatile blends and there were significant differences between the volatile blends emitted by tomato plants in response to attack by T. absoluta, B. tabaci, or by both insects. We, therefore, conclude that all three mirids use tomato plant volatiles to find T. absoluta larvae. Multiple herbivory did neither increase, nor decrease attraction of C. infumatus, E. varians and M. basicornis. By breeding for higher rates of emission of selected terpenes, increased attractiveness of tomato plants to natural enemies may improve the effectiveness of biological control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants under attack by herbivorous arthropods emit complex blends of compounds called herbivore-induced plant volatiles (HIPVs), which are exploited by predators and parasitoids during searching for their prey/host (Dicke and Sabelis 1988; Turlings et al. 1990; Geervliet et al. 1997; Dicke and Baldwin 2010). Understanding the role of HIPVs in prey/host habitat location (Pels and Sabelis 2000) and in reducing searching time by natural enemies is important for improving the use of these beneficial organisms in biological control programs (Turlings et al. 1991; Vet and Dicke 1992; Ninkovic et al. 2001). The outcome of plant-mediated interactions between herbivores and natural enemies depends on many factors, including plant species and genotype (Bukovinszky et al. 2005; Gencer et al. 2009), herbivore species (De Moraes et al. 1998) and developmental stage (Takabayashi et al. 1995).
Plants are usually colonized by more than one species of arthropod herbivore (Dicke et al. 2009; Ponzio et al. 2013). Nevertheless, most of the literature on tritrophic interactions mediated by HIPVs is based on the attack by a single herbivore species. These studies have yielded important insights, but we also need to understand multitrophic interactions mediated by HIPVs in response to attack by more than one species of herbivore (i.e., multiple herbivory). Multiple herbivory can lead to a different composition of HIPV blends than single-species herbivory and, consequently, can affect attraction of natural enemies (Shiojiri et al. 2001; Cardoza et al. 2002; Moayeri et al. 2007; Rasmann and Turlings 2007; de Boer et al. 2008; Gosset et al. 2009; Errard et al. 2015; Pangesti et al. 2015). The effect of multiple infestation on HIPV emission and natural enemy attraction appears difficult to predict and is highly variable, especially when multiple herbivory is inflicted by different feeding guilds such as leaf chewers and phloem suckers (Moayeri et al. 2007; Zhang et al. 2009; Dicke et al. 2009), which each induce a different defense signal transduction pathway: the jasmonic acid (JA) and salicylic acid (SA) pathways respectively. Interaction between the JA and SA signaling pathways commonly known as cross-talk may affect the HIPV composition and can result in positive (Cardoza et al. 2003; Rodriguez-Saona et al. 2005; Cusumano et al. 2015), negative (Zhang et al. 2009; Schwartzberg et al. 2011) or neutral (Erb et al. 2010) responses by natural enemies.
Tomato (Solanum lycopersicon L.) is one of the most consumed vegetables around the world (FAOSTAT 2015). Tomato plants are attacked by many pests (Errard et al. 2015), among which the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), and the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) cause major yield reductions (Oliveira et al. 2001; Desneux et al. 2010, 2011). Currently, the major pests of tomato can successfully be controlled by augmentative releases of natural enemies of arthropod herbivores (Calvo et al. 2009, 2011, 2012; van Lenteren 2012). In Europe, the predatory mirid bugs Nesidiocoris tenuis (Reuter) and Macrolophus pygmaeus Rambour (Hemiptera: Miridae) proved effective in controlling B. tabaci and T. absoluta (Perdikis et al. 2008; Urbaneja et al. 2009, 2012; Calvo et al. 2012; Mollá et al. 2014). Both N. tenuis and M. pygmaeus (Ingegno et al. 2013; Lins et al. 2014; de Backer et al. 2015) exploit HIPVs emitted by tomato plants infested by either T. absoluta or B. tabaci to locate their prey.
Recently, three mirid species have been found in the field in Brazil, Macrolophus basicornis (Stal), Engytatus varians (Distant) and Campyloneuropsis, infumatus (Carvalho), which are able to consume large numbers of T. absoluta and other pests occurring on tomato in laboratory experiments and seem to be promising biological control agents (Bueno et al. 2013; Silva et al. 2016). Their capacity to search for and localize main tomato pests and the role HIPVs in prey searching has not yet been studied.
We investigated the olfactory responses of M. basicornis, E. varians and C. infumatus to HIPVs released by tomato plants infested with T. absoluta (eggs or larvae) or B. tabaci (eggs, nymphs and adults), in olfactometer assays. Also, we examined whether infestation of tomato plants with both B. tabaci and T. absoluta affects the olfactory response of the mirids to HIPVs. Furthermore, we chemically characterized the volatile compounds emitted by single- and double-infested tomato plants to assess how changes in HIPV blend might affect the behavior of the three mirid species. As feeding by B. tabaci suppresses JA-regulated defenses by inducing the SA-signaling pathway to manipulate the plant’s immune system (Zarate et al. 2007; Estrada-Hernández et al. 2009; Zhang et al. 2009, 2013), we predicted that B. tabaci co-occurring with the chewing herbivore T. absoluta in tomato plants would release lower amounts of volatiles than plants infested with T. absoluta singly, negatively affecting the attraction of mirid predators to dual-infested tomato plants.
Methods and Materials
Plants and Insects
Tomato plants, Solanum lycopersicon L. cv. Santa Clara, were grown in pots (volume 3 L) containing formulated substrate mixed with 200 g NPK 4–14-8 complex fertilizer and placed in a greenhouse. Plants that were 30–35 days old, 20–25 cm high with 5–6 expanded leaves were used in the experiments.
Mirid predators were collected in tobacco (Nicotiana tabacum L.) fields located in the municipalities of Ribeirão Vermelho and Lavras (MG, Brazil, 21°08.596′S and 045°03.466′W, 808 m of altitude). Nymphs and adults were identified based on the family- specific dichotomous key of Ferreira and Enry (2011). Identification of the three mirids found in the field were confirmed by P.S.F Ferreira (Federal University of Viçosa, Viçosa-MG, Brazil) as Campyloneuropsis infumatus, Engytatus varians and Macrolophus basicornis. Stock colonies of the three mirid species were kept under laboratory conditions following the methodology of Bueno et al. (2013). Female adults were individually maintained in acrylic cages (60 × 30 × 30 cm) containing eggs of Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) for ad libitum feeding, and tobacco plants (N. tabacum L. cv. TNN) as oviposition substrate and water source. We used tobacco for maintaining the mirids to avoid conditioning effects to volatiles released by tomato plants. After seven days, tobacco plants containing eggs were transferred to new cages where nymphs hatched and fed on E. kuehniella eggs until reaching the adult stage. Females of 1–7 days old from the second generation in the laboratory were used in behavioral assays.
Eggs, larvae and pupae of T. absoluta were collected in a tomato field in an experimental area at the Federal University of Lavras (Lavras, MG, Brazil, 21°14′S 45°00′W, 918 m of altitude). Newly-formed pupae in the laboratory were sexed (Coelho and França 1987) and placed in cages (60 × 30 × 30 cm) containing tomato plants (cv. Santa Clara). Larvae and adults were kept separately in cages (60 × 30 × 30 cm) covered with fine mesh and regularly supplied with tomato plants for feeding and oviposition.
Whiteflies, B. tabaci biotype B, were provided from the rearing maintained at the Agronomic Institute of Campinas - AIC (Campinas, SP, Brazil). Colonies were kept in fine mesh cages (60 × 40 × 40 cm) with cabbage plants (Brassica oleracea L. var. acephala DC. cv. Manteiga) for feeding and oviposition.
All insects and plants were maintained at the Department of Entomology and Acarology, at ESALQ/ USP (Piracicaba, SP, Brazil). Colonies of the three mirids and T. absoluta were maintained under laboratory conditions at 25 ± 2 °C, RH 70 ± 10% and 12 L: 12D. The tomato plants and the whitefly colony were kept at ambient conditions (light, temperature and humidity) in different greenhouses.
Olfactometer Assays
Responses of female mirids to HIPVs were assessed in a glass Y-tube olfactometer (3.0 cm diameter, main arm 20 cm long, side arms 23 cm long, 70° angle between the side arms). The olfactometer device was vertically positioned, following the methodology used with other mirids (Moayeri et al. 2006; Ingegno et al. 2011, 2013; Lins et al. 2014) and connected to a volatile collection system (Analytical Research Systems, Gainesville, FL, USA). Each olfactometer side arm was connected to a 15-L glass vessel with a single tomato plant. Plastic pots in which the tomato plants were growing were wrapped with aluminum foil. Inlet air flow was adjusted to 0.8 L.min−1 for each side arm. The glass vessels were kept behind a black panel to prevent insects from visually detecting the plants.
Predator females used for the assays were considered naïve, i.e. insects that had not been exposured to tomato volatiles, nor had preyed on B. tabaci or T. absoluta before tests. A single naïve mirid female 1–7 days old since the adult moult was introduced in the main arm of the olfactometer and observed for up to 10 min. Females were considered to have made a choice when they crossed a line drawn 13 cm from the branching point of the Y-tube. Females not choosing a side arm within 10 min were considered as non-responsive and were excluded from data analysis. Each female was tested only once. Thirty replicates (responses) in total were performed for each treatment and each mirid species, using at least three pairs of tomato plants on three experimental days. Every two replicates, the olfactometer side arms were switched to minimize positional bias. After testing ten females, the Y-tube and glass vessels were washed with neutral soap and ethanol (70%) and dried. Bioassays were carried out in a climatized room at 24 ± 1 °C and 70 ± 10% RH between 10 and 12 am and 2–4 pm.
Olfactory responses of the three mirid predators were assessed to volatiles emitted from: (i) uninfested tomato plants (‘uninfested’), (ii) plants on which T. absoluta deposited eggs (‘T. absoluta eggs’), (iii) T. absoluta larvae-infested plants (‘T. absoluta larvae’), (iv) B. tabaci egg-nymph-adult-infested plants (‘B. tabaci’), and (v) T. absoluta larvae + B. tabaci (double infestation – ‘T. absoluta + B. tabaci’).
Plants were transferred from the greenhouse to the olfactometer room just before the beginning of each assay series. To obtain tomato plants on which T. absoluta deposited eggs, plants were covered with organza bags containing five 1–3-day-old T. absoluta couples. Females were allowed to lay eggs for 48 h, and then the adults were removed. According to Silva et al. (2015), five T. absoluta females lay on average 125 eggs/day, resulting in an estimated 250 eggs after 48 h. Tomato plants with eggs (24 h after oviposition period) were used for the olfactometer tests.
Plants infested with T. absoluta larvae were obtained by hatching of larvae from eggs deposited by females on the plants. Egg survival at 25 °C is 98%, thus we obtained an estimated 245 first instar larvae (Silva et al. 2015), which were allowed to feed for 72 h (Lins et al. 2014).
Fifty B. tabaci adults (females and males) were released in a cage (60 × 30 × 40 cm) with one tomato plant for 10 days, in order to obtain tomato plants infested by mixed stages (i.e., eggs, nymphs and adults) of B. tabaci that were used in the tests (Lins et al. 2014). Tomato plants were also infested with both pests. We studied the effect of dual infestation by first infesting B. tabaci for 7 days and then introducing 30 first and second instar T. absoluta larvae on the same plants for another 3 days as a standard procedure. We chose this sequence of infestation (first B. tabaci then the chewer herbivore T. absoluta) to test the supposed negative cross-talk between SA and JA-signaling pathways elicited by B. tabaci feeding (Zarate et al. 2007).
Headspace Collection and Analysis of Plant Volatiles
Volatiles from different groups of uninfested and herbivore-infested tomato plants (treatments ii – v described above) and pots filled with soil (blank) were collected under laboratory conditions at 24 ± 1 °C, 70 ± 10% RH between 10 and 12 am and 2–4 pm, in a push-pull volatile collection system (ARS, Gainesville, FL, USA).
Prior to volatile collection, plant pots were carefully wrapped with aluminum foil to avoid trapping volatiles from plastic and soil and individually enclosed in a 15-L glass vessel. The volatile collections were randomly distributed between treatments. Six plants per treatment were sampled for 2 h (flow rate 0.8 L min−1) using a trap filled with 30 mg of HayeSep® (Supelco, Bellefonte, PA, USA). Volatile traps were immediately eluted with 150 μl dichloromethane (Merck, Kenilworth, NJ, USA) mixed with 30 μl of nonyl acetate solution (Sigma-Aldrich, Sto Louis, MO, USA) at 10 ng/μL used as internal standard. All extracts were stored at −80 °C until analyses. Immediately after the collection of volatiles, plant shoot fresh weight was determined.
The headspace analyses were performed by gas chromatography (Shimadzu, GC-2010 Gas Chromatograph) with flame ionization detection GC-FID operated at 280 °C. Quantification was based on comparison of area under the GC-FID peak with the internal standard and standardized per unit fresh shoot biomass (g) of each replicate. Briefly, a 2-μl aliquot of each sample was injected in the pulsed splitless mode into a HP-1 capillary column (Agilent J&W GC Columns, Santa Clara, CA, USA - 30 m, 0.25 mm ID, 0.25 μm film thickness). The carrier gas was high purity helium with a flow rate of 0.9 mL/min. The oven was programmed with an initial temperature of 40 °C for 5 min, increasing at 5 °C/min to 150 °C, and then held for 1 min subsequently to 200 °C at 20 °C/min followed by a post-run of 5 min at 250 °C. GCsolution (version 2.32.00, Shimadzu) was used for signal acquisition and peak integration.
The most representative sample of each treatment, selected based on the mean of quantity of compounds and the one which presented less contaminants compared to blank samples were also analyzed by a gas chromatograph (Agilent 6890 Series GC system G1530A) coupled to a mass spectrometer. The GC-MS operated in electron impact mode (Agilent 5973 Network Mass Selective Detector; transfer line 230 °C, source 230 °C, ionization potential 70 eV, scan range 33–280 amu). Briefly, a 2-μl aliquot of each sample was injected in the pulsed splitless mode into a HP-1 capillary column (Alltech Associates, Deerfield, IL, USA - 30 m, 0.25 mm ID, 0.25 μm film thickness). Helium (0.9 mL/min) was used as carrier gas. GC oven temperature was initially held at 40 °C for 3 min, raised to 100 °C at 8 °C/min and subsequently to 200 °C at 5 °C/min followed by a post-run of 5 min at 250 °C. Detected volatiles were identified by comparing their mass spectra with those of the NIST 11 library and with published retention times (López et al. 2012; Anastasaki et al. 2015; De Backer et al. 2015). In addition, the injection of authentic standards (except for carene, δ-elemene and β-elemene) and calculation of the linear retention index (LRI) of each compound were used as an additional criterion for the identification of the compounds.
Data Analysis
To investigate whether mirid female’s preference differed when various combinations of plant treatments were offered, data were analyzed by Generalized Linear Models with a binomial distribution and a logit-link function. The response variable was the proportion of insects responding to one of the volatile sources. In experiments of single infestation and double infestations, the effect of day was included in the GLM model. For all experiments, we fitted a separate binomial GLM to estimate the proportional response of each predator to test whether their choice was significantly different from a 50% distribution. The significance of the response was tested using a χ2 test. Volatile emission (relative amounts of individual compounds standardized per unit plant fresh weight) was tested for normality and homogeneity of variances using the Shapiro-Wilk and Bartlett tests, respectively. As distributions, even after log-transformation, did not meet the assumptions for parametric tests volatile emission data were analyzed by the non-parametric Kruskal-Wallis test followed by Bonferroni posthoc analysis (P < 0.05 and P < 0.01). The total emission of volatiles was submitted to one-way analysis of variance (ANOVA) and when significant differences among averages were found, Tukey’s HSD test at P < 0.05 level of significance was applied. Principal component analysis (PCA) was also applied. This projection method determines whether samples collected from different treatment groups can be separated by quantitative and/or qualitative differences in their volatile blends. All statistical analyses were performed using R statistical software (R Core Team 2014).
Results
Response of C. infumatus, E. varians and M. basicornis to Tomato Volatiles
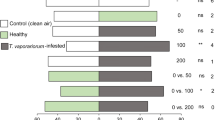
No influence of experimental day was found on the response of either predator in any of the treatments (GLM, P > 0.005). Macrolophus basicornis, E. varians and C. infumatus preferred volatiles from uninfested plants, plants carrying T. absoluta eggs, T. absoluta larvae-infested, B. tabaci infested and T. absoluta larvae + B. tabaci infested tomato over clean air (Fig. 1, Table 1). In addition, all three mirid predators oriented preferentially to volatiles emitted by T. absoluta larvae-infested tomato over uninfested tomato plants (Fig. 1, Table 1), but they did not discriminate between volatiles from plants carrying T. absoluta eggs and volatiles from uninfested plants (Fig. 1, Table 1).
Responses of Macrolophus basicornis (a), Engytatus varians (b) and Campyloneuropsis infumatus (c) females (n = 30/species) to volatiles from uninfested tomato plants (UP), plants carrying T. absoluta eggs (TE) or larvae (TL) of Tuta absoluta or Bemisia tabaci (BT) or double infestation (BT + TL) in a Y-tube olfactometer. The horizontal axis represents the number of predators that moved towards the volatile sources in the corresponding choice situations indicated on the left. NC indicates the number of tested individuals that did not make a choice. ** P < 0.01, * P < 0.05, ns P > 0.05 (GLM, binomial test)
Females of M. basicornis preferred odors from B. tabaci-infested plants over uninfested plants, but E. varians and C. infumatus did not discriminate between these two treatments (Fig. 1, Table 1). None of the three mirid species discriminated between volatiles from T. absoluta larvae + B. tabaci infested over T. absoluta larvae-infested tomato plants (Fig. 1, Table 1). However, M. basicornis, E. varians and C. infumatus preferred volatiles emitted from plants with double infestation (T. absoluta larvae + B. tabaci) over the volatile blend from B. tabaci infested plants (Fig. 1, Table 1).
Chemical Composition of the Headspace Volatile Blend of Uninfested and Infested Tomato Plants
Egg deposition by T. absoluta on tomato induced higher emission levels of eight terpenes: α-pinene, α-terpinene, β-phellandrene, β-ocimene, γ-terpinene, terpinolene, β-caryophyllene and α-humulene compared to uninfested plants (Table 2, Bonferroni, P < 0.05). Leaf feeding by T. absoluta larvae, in turn, promoted elevated amounts of volatile emissions, including green leaf volatiles and 12 terpenes when compared to uninfested plants (Table 2, Bonferroni, P < 0.05).
Infestation of tomato with B. tabaci induced a blend composed of higher concentrations of nine terpenes and the green leaf volatile (Z)-3-hexen-1-ol relative to the control (Table 2, Bonferroni, P < 0.05). The volatile blend from B. tabaci infested plants had six terpenes at lower concentrations, but one at a higher concentration (terpinolene), compared to the blend emitted by T. absoluta larvae-infested plants. δ – Elemene, which was present in the blend of T. absoluta larvae-infested plants, was not found in the volatile emission from B. tabaci infested plants.
Double infestation (T. absoluta + B. tabaci) of tomato resulted in augmented emissions of all terpenes found in the tomato HIPV blend, and of (Z)-3-hexen-1-ol, relative to the blend from uninfested plants (Table 2, Bonferroni, P < 0.05). The blend emitted by double infested plants contained higher concentrations of β-myrcene, limonene, γ-terpinene, terpinolene and β-elemene, but lower levels of α-pinene, compared to the blend from T. absoluta larvae-infested plants (Table 2, Bonferroni, P < 0.05). Besides β-myrcene, limonene, γ-terpinene and β-elemene, the emissions from T. absoluta + B. tabaci-infested plants also contained higher amounts of α-terpinene, β-phellandrene, β-ocimene, δ-elemene and α-humulene than the B. tabaci-infested plant blend (Table 2, Bonferroni, P < 0.05).
Tuta absoluta larvae-infested and T. absoluta + B. tabaci-infested plants emitted higher total amounts of volatiles compared to either uninfested plants (up to 32-fold difference) or plants carrying T. absoluta eggs (up to 6-fold difference) or B. tabaci (up to ca. 3-fold difference) (Table 2, Tukey HSD, P < 0.05).
The principal component analysis, showed three significant principal components (PC) with the first two explaining 55% and 13% of the total variance, respectively. A plot based on these first and second PC axes revealed a clear separation of blends from uninfested and T. absoluta egg- carrying plants from T. absoluta + B. tabaci-infested plants (Fig. 2).
Principal component analysis (PCA) on volatile composition emitted by uninfested tomato plants (UP), tomato plants infested with: Tuta absoluta eggs (TE), T. absoluta larvae (TL), B. tabaci (BT) or double infestation (DI). Vector numbers correspond with compound numbers in Table 1
Discussion
The three mirid predators, M. basicornis, E. varians and C. infumatus, were attracted to tomato plants infested with T. absoluta larvae. Although B. tabaci is used as prey by these mirids (Van Lenteren et al. 2016), only M. basicornis discriminated between B. tabaci-infested and uninfested tomato plants. The mirids also did not distinguish double infested (T. absoluta + B. tabaci) plants from T. absoluta larvae-infested plants, but preferred double infested (T. absoluta + B. tabaci) over B. tabaci-infested plants. This demonstrates that the HIPV-profile of T. absoluta larvae-infested plants is preferred and that simultaneous infestation with the alternative prey B. tabaci does not interfere with attraction.
Chemical analysis of volatile blends emitted by herbivore-infested plants and intact plants demonstrated qualitative and/or quantitative changes in the emission of infochemicals in several plant-herbivore complexes (Dicke et al. 2009; Fatouros et al. 2012; Poelman et al. 2012; Weldegergis et al. 2015; Silva et al. 2017). Oviposition on tomato plants by T. absoluta triggered increased emission of eight terpenes (Table 1). Discrete differences between volatile blends of oviposited and uninfested plants can be perceived by egg parasitoids (Wei et al. 2007; Fatouros et al. 2012; Hilker and Fatouros 2015). However, the generalist mirid predators C. infumatus, E. varians and M. basicornis did not distinguish T. absoluta egg-carrying from uninfested plants. Other mirid species such N. tenuis, M. pygmaeus and Dicyphus errans Knight (Lins et al. 2014; Ingegno et al. 2013) were also not attracted to plants carrying T. absoluta eggs, suggesting that the lack of attraction of the mirid predators to such plants may be due to a low level of volatile emission. Mollá (2013) found N. tenuis attraction towards plants carrying T. absoluta eggs, but egg numbers per plant was about four times higher than in our experiments and in those reported by Lins et al. (2014).
Herbivore density and duration of herbivore induction might also result in different HIPV blends (López et al. 2012; Bawin et al. 2014). Tomato plants infested with 15 first instar T. absoluta larvae for 24 h emitted increased amounts of several terpenoids such as: α-phellandrene, isoterpinolene, α-cubebene, TMTT and (Z)-nerolidol and the aromatic compound methyl salicylate (Strapasson et al. 2014). In contrast, in the present study the same tomato variety infested with about 245 first instar T. absoluta larvae for 72 h did not emit those compounds, but increased the emission of 14 from 15 found compounds (Table 1). Tomato cultivar is another factor that can differentiate the quality and/or quantity of the compounds. For instance, tomato plants (cv. Moneymaker) infested with T. absoluta and/or B. tabaci released 80 organic compounds (Silva et al. 2017), nine of which were also found in the headspace of the cv. Santa Clara, but in lower quantities. Moreover, six terpenes, α-pinene, carene, β-phellandrene, γ-terpinene, δ-elemene and α-humulene found in the headspace of cv. Santa Clara were not found in the headspace of cv. Moneymaker (Silva et al. 2017). Interestingly, Megido et al. (2014) did not report the mono- and sesquiterpenes γ-terpinene and δ-elemene in the emissions of tomato cv. Moneymaker, but similar to our findings, they found α-pinene, carene, β-phellandrene and α-humulene. HIPVs and changes in the amounts of constitutive VOCs can also be observed in headspace of other tomato cultivars infested with T. absoluta or B. tabaci (López et al. 2012; Fang et al. 2013; Strapasson et al. 2014).
Immediately upon attack by herbivores, tomato plants enhanced the emission of fatty acid-derived volatile compounds, which are the result of the breakdown of lipids through the lipoxygenase pathway (Shen et al. 2014). Breakdown of plant cell membranes gives rise to free linoleic and/or linolenic acid, both of which are acted upon by lipoxygenase to form C5 volatile compounds and the C6 green leaf volatiles (Croft et al. 1993; McCormick et al. 2012; Shen et al. 2014). When released by the plant, these compounds can decrease herbivore feeding rates on tomato (Hildebrand et al. 1993) and can also trigger responses of natural enemies (Dicke et al. 2009).
Tomato infested with B. tabaci released also augmented amounts of several terpenes compared to uninfested plants. However, compared to the blend released by T. absoluta-larvae-infested-plants, the B. tabaci-infested plant blend emitted lower concentrations of six terpenes and a higher concentration of terpinolene. Differences in induction of plant volatiles can be the result of different insect feeding modes, where biting-chewing tomato borers induced higher amounts and higher numbers of compounds than phloem sucking whiteflies (Silva et al. 2017), which might influence mirid choices (Lins et al. 2014).
The three mirid species might benefit more from orientation to the T. absoluta-larvae infested HIPV blend than by the blend emitted by B. tabaci-infested plants, since T. absoluta larvae are much larger prey items than whitefly eggs and nymphs. Several studies have shown the benefit to mirid bugs of preying on lepidopteran eggs and larvae (Devi et al. 2002; Urbaneja et al. 2009; Hamdi and Bonato 2014; Silva et al. 2016). An alternative explanation might be that we tested naïve adults of the three mirids and that E. varians and C. infumatus will react to B. tabaci-infested plant volatiles after an associative learning experience like the mirid predator N. tenuis, which was attracted by volatiles from B. tabaci-infested plants only after experience with this prey (Lins et al. 2014). Moreover, in our study, mirids were maintained on tobacco plants, not being exposed to any type of conditioning to tomato volatiles. According to a recent study (Rim et al. 2017), mirids, in laboratory rearing, likely associate the availability of E. kuehniella eggs with the volatiles emitted from the host plant, in a way they become conditioned to the plant species. Therefore, it is possible that tomato-reared mirids may have their ability to recognize infested tomato volatile blends enhanced and, unlike tobacco-reared insects, would discriminate volatiles of B. tabaci-infested from uninfested tomatoes.
To locate prey in a complex system that undergoes changes in plant-derived odour cues due to single or multiple pest infestation, mirid females could rely on learning abilities to enhance responses. Insect learning is a well-known and widely studied experience-based modification of behavior (Steidle and van Loon 2003; De Boer et al. 2005; Glinwood et al. 2011; Rim et al. 2015), in particular for parasitoids (Vet and Dicke 1992). Predatory mirid bugs have only recently been studied, and indications for associative learning have been reported (Lins et al. 2014). Also, some studies show that the attack of multiple herbivores species can lead to a different composition of HIPV blends, and consequently being more attractive to natural enemies than plants infested by a single herbivore species (Rodriguez-Saona et al. 2005; Moayeri et al. 2007; Cusumano et al. 2015). Furthermore, simultaneous feeding by herbivores with different feeding modes (biting-chewing or piercing-sucking) may interfere with the attraction of natural enemies (Zhang et al. 2009; Schwartzberg et al. 2011). Results in this study do not confirm our initial hypothesis that the whitefly B. tabaci sharing the same host with the larvae of the tomato borer T. absoluta suppress plant volatile emission compared to the emission by plants infested with the tomato borer only. Indeed, infestation with T. absoluta + B. tabaci in tomato triggered an overall increase of terpene emissions compared to infestation by either of the two single herbivores. Although we infested tomato plants with 50 whitefly adults (or 8–10 adults/leaf), which fed and reproduced for 10 days on the same plants, the level and/or duration of whitefly infestation may still have not been enough for suppressing the JA-signaling pathway. Previous studies that detected the repression of JA-responsive genes by the activation of SA-signaling pathway infested plants with higher densities of B. tabaci adults (more than 13 adults/leaf) and for longer periods (12–14 days), coinciding with feeding by second- to third-instar nymphs (Estrada-Hernández et al. 2009; Zhang et al. 2009, 2013).
The three mirids did not distinguish between T. absoluta + B. tabaci-infested and T. absoluta-infested plant volatile blends, demonstrating that double infestation by T. absoluta + B. tabaci does not increase attraction to these mirid predators compared to plants infested with T. absoluta only when no previous experience with the plant-prey combinations had occurred. Similarly, two other mirid predators (M. pygmaeus and N. tenuis) did not distinguish double-infested (T. absoluta + B. tabaci) from single-infested (T. absoluta or B. tabaci) tomato plants, and this behavioral pattern did not change after experience (Lins et al. 2014).
The PCA analysis showed differences in blends of uninfested over double infested tomato plants, indicating an alteration of volatile blend after the oviposition and/or feeding by T. absoluta and by B. tabaci. The vector for β-myrcene, γ-terpinene, terpinolene, δ-elemene and β-elemene was correlated with the volatile samples from T. absoluta + B. tabaci-infested plants in the PCA plot (Fig. 2) which is in line with their higher quantities in headspace samples compared to the other treatments (Table 1). We suggest that higher amounts of those compounds on double infestation mostly contributed to the separation of these treatments in the PCA. In addition, the vector for α-pinene was correlated with the samples from T. absoluta -infested plants, and also presented higher quantities in headspace samples compared to the other treatments (Table 1). High amounts of this compound were also found in other studies (Degenhardt et al. 2010; Megido et al. 2014; Fang et al. 2013; Strapasson et al. 2014), but small quantities or absence of this compound have also been reported (López et al. 2012; Silva et al. 2017).
Due to the complexity of HIPV blends emitted by single- and double-infested tomatoes, it is difficult to select volatile compounds that potentially play a role in mirid attraction. In this study, combining the information on the attraction of mirids to T. absoluta larvae-infested and T. absoluta + B. tabaci-infested plants, and contrasting it with the lack of attraction to plants carrying T. absoluta eggs and B. tabaci-infested plants over uninfested plants, suggests that α-terpinene, limonene, β-phellandrene and δ-elemene are important compounds for attraction of these mirids. This hypothesis should be addressed in future studies by manipulating the concentration of these single terpenes in the blend from an otherwise non-attractive plant in behavioral assays, and/or testing different mixtures and concentrations of single synthetic compounds in such assays.
The results of this study show that volatiles of tomato infested with T. absoluta larvae attract all three mirid species. Engytatus varians and C. infumatus did not respond innately to HIPVs from B. tabaci-infested tomato. Multiple herbivory by herbivores of two different feeding guilds – chewing (T. absoluta) and phloem-sucking insects (B. tabaci) – did neither increase, nor decrease attraction of C. infumatus, E. varians and M. basicornis. In addition, M. basicornis is likely more efficient in finding infested tomato plants for control of B. tabaci, unless the two other mirids (C. infumatus and E. varians) quickly learn to associate HIPVs emitted by B. tabaci infested tomato plants with prey availability.
References
Anastasaki E, Balayannis G, Papanikolaou NE, Michaelakis AN, Milonas PG (2015) Oviposition induced volatiles in tomato plants. Phytochem Lett 13:262–266
Bawin T, De Backer L, Dujeu D, Legrand P, Megido RC, Francis F, Verheggen FJ (2014) Infestation level influences oviposition site selection in the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects 5:877–884
Bueno VHP, Lenteren VJ, Lins JC, Calixto AM, Montes FC, Silva DB, Pérez LM (2013) New records of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) predation by Brazilian Hemipteran predatory bugs. J Appl Entomol 137:29–34
Bukovinszky T, Gols R, Posthumus MA, Vet LEM, Van Lenteren JC (2005) Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellen). J Chem Ecol 31(3):461–480
Calvo J, Bolckmans K, Tansly P, Urbaneja A (2009) Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 54:237–246
Calvo J, Bolckmans K, Belda J (2011) Control of Bemisia tabaci and Frankliniella occidentalis in cucumber by Amblyseius swirskii. BioControl 56:185–192
Calvo J, Bolckmans K, Belda JE (2012) Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 57:809–817
Cardoza YJ, Alborn HT, Tumlinson JH (2002) In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J Chem Ecol 28:161–174
Cardoza YJ, Teal PE, Tumlinson JH (2003) Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ Entomol 32(5):970–976
Coelho MCF, França FH (1987) Biologia e quetotaxia da larva e descrição da pupa e adulto da traça-do-tomateiro. Pesq Agro Bras 22:129–135
Croft KP, Juttner R, Slusarenko AJ (1993) Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae sv phaseolicola. Plant Physiol 101:13–24
Cusumano A, Weldegergis BT, Colazza S, Dicke M, Fatouros NE (2015) Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecol 179:163–174
De Backer L, Megido RC, Fauconnier ML, Brostaux Y, Francis F, Verheggen F (2015) Tuta absoluta-induced plant volatiles: attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact 9:465–476
De Boer JG, Snoeren T, Dicke M (2005) Predatory mites learn to discriminate between plant volatiles induced by prey and nonprey herbivores. Animal Behav 69:869–879
De Boer JG, Hordijk CA, Posthumus MA, Dicke M (2008) Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J Chem Ecol 34:281–290
De Moraes C, Lewis W, Pare P, Alborn H, Tumlinson J (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE (2010) Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 71(17):2024–2037
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Catalán Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, history of invasion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84(4):403–408
Devi PK, Yadav DN, Anand J (2002) Role of Nesidiocoris tenuis Reuter (Hemiptera: Miridae) in natural suppression of tomato fruit borer, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Pest Manage Hortic Ecosyst 8:109–113
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15(3):167–175
Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38:148–165
Dicke M, Van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol 5:317–324
Erb MC, Foresti N, Turlings TC (2010) A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. Plant Biol 10:247
Errard A, Ulrichs C, Kühne S, Mewis I, Drungowski M, Schreiner M, Baldermann S (2015) Single-versus Multiple-Pest Infestation Affects Differently the Biochemistry of Tomato (Solanum lycopersicum ‘Ailsa Craig’). J Agric Food Chem 63:10103–10111
Estrada-Hernández MG, Valenzuela-Soto JH, Ibarra-Laclette E, Délano-Frier JP (2009) Differential gene expression in whitefly Bemisia tabaci-infested tomato (Solanum lycopersicum) plants at progressing developmental stages of the insect's life cycle. Physiol Plant 137(1):44–60
Fang Y, Jiao X, Xie W, Wang S, Wu Q, Shi X, Chen G, Su Q, Yang X, Pan H, Zhang Y (2013) Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci Rep 3
Faostat (2015) Food and Agriculture Organization of the United Nations. http://faostat3.fao.org/download/Q/QC/E. Accessed January 2016
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, Van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One 7:e43607–e43607
Ferreira PSF, Enry TJ (2011) Synopsis and keys to the tribes, genera, and species of Miridae (Hemiptera: Heteroptera) of Minas Gerais, Brazil Part I: Bryocorinae. Zoo 2920:1–41
Geervliet JBF, Posthumus MA, Vet LEM, Dicke M (1997) Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J Chem Ecol 23:2935–2954
Gencer NS, Kumral NA, Sivritepe HO, Seidi M, Susurluk H, Senturk B (2009) Olfactory response of the ladybird beetle Stethorus gilvifrons to two preys and herbivore-induced plant volatiles. Phytoparasitica 37(3):217–224
Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V (2011) Olfactory learning of plant genotypes by a polyphagous insect predator. Oecol 166:637–647
Gosset V, Harmel N, Göbel C, Francis F, Haubruge E, Wathelet JP, Du Jardin P, Feussner I, Fauconnier ML (2009) Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J Exp Bot 60:1231–1240
Hamdi F, Bonato O (2014) Relation entre sources trophiques et capacité de survie chez Macrolophus pygmaeus (Hemiptera: Miridae). Can Entomol 146(3):285–290
Hildebrand DF, Brown GC, Jackson DM, Hamilton TR (1993) Effect of some leaf emitted volatiles compounds on aphid population increase. J Chem Ecol 19:1875–1887
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515
Ingegno BL, Pansa MG, Tavella L (2011) Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol Control 58:174–181
Ingegno BL, Ferracini C, Gallinotti D, Alma A, Tavella L (2013) Evaluation of the effectiveness of Dicyphus errans (Wolff) as predator of Tuta absoluta (Meyrick). Biol Control 67:246–252
Lins JC Jr, Van Loon JJA, Bueno VH, Lucas-Barbosa D, Dicke M, van Lenteren JC (2014) Response of the zoophytophagous predators Macrolophus pygmaeus and Nesidiocoris tenuis to volatiles of uninfested plants and to plants infested by prey or conspecifics. BioControl 59:707–718
López YIA, Martínez-Gallardo RR, López MG, Sánchez-Hernández C, Délano-frier J (2012) Cross-Kingdom Effects of Plant-Plant Signaling via Volatile Organic Compounds Emitted by Tomato (Solanum lycopersicum) Plants Infested by the Greenhouse Whitefly (Trialeurodes vaporariorum). J Chem Ecol 38:1376–1386
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17(5):303–310
Megido RC, De Backer L, Ettaïb R, Brostaux Y, Fauconnier ML, Delaplace P, Lognay G, Belkadhi MS, Haubruge E, Francis F, Verheggen FJ (2014) Role of larval host plant experience and solanaceous plant volatile emissions in Tuta absoluta (Lepidoptera: Gelechiidae) host finding behavior. Arthropod-Plant Interac 8(4):293–304
Moayeri HRS, Ashouri A, Brødsgaard HF, Enkegaard A (2006) Odour-mediated preference and prey preference of Macrolophus caliginosus between spider mites and green peach aphids. J Appl Entomol 130(9–10):504–508
Moayeri HRS, Ashouri A, Poll L, Enkegaard A (2007) Olfactory response of a predatory mirid to herbivore induced plant volatiles: multiple herbivory vs. single herbivory. J Appl Entomol 131(5):326–332
Mollá O (2013) Control biológico de la polilla del tomate Tuta absoluta (Lepidoptera: Gelechiidae) mediante la gestión de mıridos depredadores. Ph.D. Thesis, Faculty of Biological Sciences, University of Valencia, Spain
Mollá O, Biondi A, Alonso-Valiente M, Urbaneja A (2014) A comparative life history study of two mirid bugs preying on Tuta absoluta and Ephestia kuehniella eggs on tomato crops: implications for biological control. BioControl 59:175–183
Ninkovic V, Al Abassi S, Pettersson J (2001) The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol Control 21:191–195
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20(9):709–723
Pangesti N, Pineda A, Pieterse CMJ, Dicke M, Van Loon JJA (2015) Two-way plant mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front Plant Sci 4:1–11
Pels B, Sabelis MW (2000) Do herbivore-induced plant volatiles influence predator migration and local dynamics of herbivorous and predatory mites? Exp Appl Acarol 24:427–440
Perdikis D, Kapaxidi E, Papadoulis G (2008) Biological control of insect and mite pests in greenhouse solanaceous crops. Europ J Plant Sci Biotech 2(1):125–144
Poelman EH, Bruinsma M, Zhu F, Weldegergis BT, Boursault AE, Jongema Y, Van Loon JJA, Vet LE, Harvey JA, Dicke M (2012) Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol 10:e1001435
Ponzio C, Gols R, Pieterse CM, Dicke M (2013) Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct Ecol 27:587–598
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for statistical computing. R Development Core Team, Vienna
Rasmann S, Turlings TCJ (2007) Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett 10:926–936
Rim H, Uefune M, Ozawa R, Takabayashi J (2015) Olfactory response of the omnivorous mirid bug Nesidiocoris tenuis to eggplants infested by prey: Specificity in prey developmental stages and prey species. Biol Control 91:47–54
Rim H, Uefune M, Ozawa R, Yoneya K, Takabayashi J (2017) Experience of plant infestation by the omnivorous arthropod Nesidiocoris tenuis affects its subsequent responses to prey-infested plant volatiles. BioControl 62(2):233–242
Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS (2005) Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 143:566–577
Schwartzberg E, Beoreoczky K, Tumlinson J (2011) Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia faba. J Chem Ecol 37:1055–1062
Shen J, Tieman D, Jones JB, Taylor MG, Schmelz E, Huffaker A, Klee HJ (2014) A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J Exp Bot 65(2):419–428
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol 43:23–29
Silva DB, Bueno VHP, Lins JC Jr, Van Lenteren JC (2015) Life history data and population growth of Tuta absoluta at constant and alternating temperatures on two tomato lines. Bull Insectol 68(2):223–232
Silva DB, Bueno VHP, Montes FC, Van Lenteren JC (2016) Population growth of three mirid predatory bugs feeding on eggs and larvae of Tuta absoluta on tomato. Biol Control 61:545–553. https://doi.org/10.1007/s10526-016-9736-1
Silva DB, Weldegergis BT, Van Loon JJ, Bueno, VHP (2017) Qualitative and Quantitative Differences in Herbivore-Induced Plant Volatile Blends from Tomato Plants Infested by Either Tuta absoluta or Bemisia tabaci 43 (53): 1–13. doi:https://doi.org/10.1007/s10886-016-0807-7
Steidle JLM, Van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol Exp Appl 108:133–148
Strapasson P, Pinto-Zevallos DM, Paudel S, Rajotte EG, Felton GW, Zarbin PH (2014) Enhancing plant resistance at the seed stage: low concentrations of methyl jasmonate reduce the performance of the leaf miner Tuta absoluta but do not alter the behavior of its predator Chrysoperla externa. J Chem Ecol 40(10):1090–1098
Takabayashi J, Takahashi S, Dicke M, Posthumus MA (1995) Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol 21:273–287
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Turlings TCJ, Tumlinson JH, Eller FJ, Lewis WJ (1991) Larval-damaged plants: source of volatile synomines that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol Exp Appl 58(1):75–82
Urbaneja A, Montón H, Mollá O (2009) Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J Appl Entomol 133(4):292–296
Urbaneja A, González-Cabrera J, Arnó J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222
Van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biol Control 57:1–20
Van Lenteren JC, Hemerik L, Lins JC, Bueno VHP (2016) Functional responses of three Neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects. https://doi.org/10.3390/insects7030034
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wei J, Wang L, Zhu J, Zhang S, Nandi OI, Kang L (2007) Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS One 2:e852
Weldegergis BT, Zhu F, Poelman EH, Dicke M (2015) Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia 177:701–713
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143(2):866–875
Zhang PJ, Zheng SJ, Van Loon JJA, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc. Natl Acad Sci 106:21202–21207
Zhang PJ, Li WD, Huang F, Zhang JM, Xu FC, Lu YB (2013) Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol 39(5):612–619
Acknowledgements
We thank the CAPES/Nuffic Programme Project 044/12 of the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil); the National Institute of Science and Technology (INCT) Semiochemicals in Agriculture (CNPq Process 573761/2008-6 and FAPESP Process 2008/57701-2) and the National Council for Scientific and Technology Research (CNPq) for financial support of the project. We thank A. Prado (USP-ESALQ) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, D.B., Bueno, V.H.P., Van Loon, J.J.A. et al. Attraction of Three Mirid Predators to Tomato Infested by Both the Tomato Leaf Mining Moth Tuta absoluta and the Whitefly Bemisia tabaci . J Chem Ecol 44, 29–39 (2018). https://doi.org/10.1007/s10886-017-0909-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0909-x