Abstract
Mating disruption of the carpenter moth, Cossus insularis (Staudinger) (Lepidoptera: Cossidae), with a synthetic version of its sex pheromone, a mixture of (E)-3-tetradecenyl acetate and (Z)-3-tetradecenyl acetate, was tested for three successive years in apple (Malus domestica Borkh.) orchards. Pheromone trap catches, percentage mating of tethered females and females enclosed with males in a mating cage, and tree damage were measured in both the pheromone-treated and untreated control orchards. The attraction of male moths to pheromone traps at heights of 1.5, 3, and 5 m was strongly disrupted when the pheromone dispensers were placed at 1.5 m height. Mating of tethered females placed at 1 m was completely inhibited, and the mating of tethered females at a height of 3 m was significantly reduced by the treatment in comparison to matings in an untreated control orchard. Similarly, mating of pairs of moths enclosed in mating cages was significantly reduced by the synthetic pheromone treatment in comparison to controls. The percentage of damaged trees in the pheromone-treated orchard also decreased significantly over the course of the experiment. These results suggest that mating disruption with the synthetic sex pheromone appears promising for reducing damage caused by C. insularis in apple orchards in Japan, and a commercial mating disruption product has been developed and registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The carpenter moth, Cossus insularis (Staudinger) (Lepidoptera:Cossidae) is found in Honshu, Kyushu, and Tsushima Island of Japan (Tadauchi and Inoue 2000). Although willow and poplar trees are native host plants of C. insularis (The Japanese Society of Applied Entomology and Zoology 2006), recently, larvae have been found infesting the main branches and trunks of Japanese pear trees (Pyrus pyrifolia var. culta) in the Tokushima prefecture of Japan, resulting in severe losses in fruit yields (Nakanishi 2005). Subsequently, C. insularis damage on apple and Japanese pear trees was found in many prefectures in central and northern Japan (Nakamuta et al. 2010). Therefore, effective tactics to control C. insularis are urgently required in Japanese apple and pear orchards.
In addition to apple and Japanese pear (sand pear), known host plants of C. insularis include poplar, willow, and gingko (Nakamuta et al. 2007). Adult moths are large, with wing spans of 40 to 60 mm, and they emerge from June to August. They mate in the evening or at night, usually on the day of emergence. Mated females lay egg masses consisting of an average of 85 eggs (range: 35–135) in cracks in the bark of host trees, and the newly hatched larvae bore into the branches or trunks of the trees, developing there until adult emergence (Nakamuta et al. 2007). Thus, all life stages except the adult are hidden in the bark or wood, and so insecticide sprays are not effective against the eggs and larvae.
Mating disruption (MD) with synthetic sex pheromone may represent an alternative control method because MD has been used successfully to control a number of lepidopteran pests of field crops, orchards, and vineyards (Cardé and Minks 1995; Hegazi et al. 2010; Matsumoto et al. 2007; Witzgall et al. 2010). In particular, successful control by MD has been reported for a number of other wood boring insects, including the cherry tree borer, Synanthedon hector Butler (family Sesiidae) (Aono et al. 1989; Yaginuma 1984) and several cossid moth species (Escofet et al. 2005; Natale and Pasqualini 1999).
The sex pheromone of C. insularis females consists of a 95:5 mixture of (E)-3-tetradecenyl acetate (E3–14:Ac) and (Z)-3-tetradecenyl acetate (Z3–14:Ac) (Chen et al. 2006). Preliminary trials testing MD of C. insularis in Japanese pear orchards in Tokushima prefecture resulted in trap shutdown, inhibition of mating in tethered females, and damage reduction (Nakanishi et al. 2013). In that study, the Japanese pear trees were trellised, and the canopy was less than 2.0 m high. Mating diruption dispensers were deployed near the top of the canopy, which probably contributed to the success of the trial. However, commercial apple trees in Japan generally are much taller than pear trees because apple trees are not trellis-trained. We, therefore, tested MD in apple orchards with 2 to 3.5 m tall trees in which the pheromone dispensers were placed at a height of ~1.5 m. It was important to determine whether or not mating occurred above the height of pheromone dispensers, and so we examined both trap shutdown and inhibition of mating above the dispenser level. We also assessed mating of mixed sex pairs enclosed in small cages, placed in MD and control orchards. Here, we report the outcome of these experiments.
Materials and Methods
Insects
Mature larvae of C. insularis were collected from damaged apple trees in Date city, Fukushima prefecture, in March 2013, and from damaged willow trees in Yamagata city, Yamagata prefecture, in April 2013. Larvae were reared on an artificial diet (Insecta LFS®, Nihon Nohsan Kogyo Co., Ltd., Yokohama, Japan) at 25 °C., 16 L: 8D photoperiod until pupation. The male and female pupae were separated, and the females were kept individually in plastic cups until adult emergence.
Chemicals
The sex pheromone was synthesized using the method described previously by Chen et al. (2006). This produced an 80:20 blend of E3–14:Ac and Z3–14:Ac, which was used in both MD dispensers and as a bait in monitoring traps, because Chen et al. (2006) had shown that the 80:20 blend was as effective as the natural blend for attracting male moths field tests.
Pheromone Dispensers for Mating Disruption
High-density polyethylene tubes (inner and outer diameters 0.84 and 1.54 mm, respectively; 20 cm length; Shin-Etsu Chemical Co., Ltd., Tokyo) were loaded with 130 mg of the synthetic sex pheromone, and the ends were sealed. The dispensers were hung from apple branches (one dispenser per tree) at a height of 1.5 m. One thousand dispensers were distributed per 1 ha, corresponding to 130 g of pheromone per hectare. The dispensers were deployed from the beginning of June until the end of August in 2011, 2012, and 2013.
Study Sites
The orchards used for the present study are summarized in Table 1. Trap shutdown and mating inhibition using tethered females were examined in orchards A and B. Damage assessments were conducted in orchards C and D from 2011 to 2013. Mating inhibition in screen cages was examined in orchard E.
Attraction to Monitoring Traps Baited with Synthetic Sex Pheromone (Trap Shutdown Test)
One mg of the synthetic pheromone blend (an 80:20 mixture of E3–14:Ac and Z3–14:Ac) dissolved in n-hexane (Wako Pure Chemical Industries, Osaka, Japan) was loaded onto gray halobutyl-isoprene blend rubber septa (8 mm O.D., West Co., Singapore), and the septa were kept at room temperature for 24 h, then at 5 °C until use. Traps (SE trap, Δ-shaped section, 29 × 32 × 8 cm, Sankei Chemical Co. Ltd., Tokyo, Japan) baited with pheromone-impregnated septa were hung from steel poles placed between trees (>10 m apart) at 1.5, 3, and 5 m above ground in triplicate inside MD orchard A and control orchard B, with lures being replaced monthly. From 18 June to 31 August 2012, the pheromone traps were set in orchards A and B (Table 1), and the numbers of males caught were checked every five days.
Mating of Pairs of Moths in Mating Cages
The effect of MD on mating success was evaluated using mating cages in which mixed sex pairs of adult moths were enclosed. The mating cages was modeled after the design of Minamishima et al. (2004) for the oriental fruit moth, Grapholita molesta. Two stainless steel sieve baskets (19 cm diam and 7 cm depth) were wired together to make a screen cage, and a pair of 1-d-old virgin male and female moths was enclosed in each cage every evening from 7 June to 9 July, 2013. One to six pairs (=cages) were placed in the experimental orchard (Orchard E, Table 1) at Yamagata Horticultural Experiment Station. The mating cages were left for one night, after which female moths were dissected to check for the presence of a spermatophore. Concurrently, paired moths in cages were placed in an untreated apple orchard 200 m from the edge of the MD-treated orchard (44 total).
Mating of Tethered Females
We determined mating disruption efficacy by examining mating rates of tethered females placed at heights of 1 m or 3 m in treated orchard C, and in untreated control orchard D. A cotton thread (30 cm long) was tied carefully to the right forewing of each virgin female as a tether. The other end of the thread was attached to the top of a vertical wooden pole, 1 or 3 m high. Two to six female moths were tethered per treatment replicate during 8–19 July 2013. The females were left on the pole for one night, after which they were dissected to check for the presence of a spermatophore. The percentage of mated females was calculated from four independent trials (1 m and 3 m heights in the MD and control orchards, respectively).
Damage Assessment
Trees were assessed for damage from 2011 to 2013 in the pheromone-treated (C) and untreated orchards (D). All trees in the orchards were surveyed by counting the masses of fresh frass pushed out of tunnels by boring larvae, and the number of pupal exuviae protruding from the tree or dropped to the ground under the tree. A mass of frass indicates the presence of a group of larvae (Kitajima et al. 1998). Trees having a mass of frass or a protruding pupal exuvia were judged to be damaged. The numbers of pupal exuviae were counted at the end of August in 2011, 2012, and 2013, when no male moths had been captured in baited traps for at least a week. The masses of frass newly pushed out of tunnels were counted in September or October from 2011 to 2013. Orchard D had 36 trees during the course of the study, whereas orchard C started with 87 trees in 2011, but decreased to 80 trees in 2012 and 53 trees in 2013 due to the removal of heavily damaged trees.
Statistics
Trap catch data and the rate of mating were analyzed to detect significant differences between the control and the MD orchard with the nonparametric Kruskal–Wallis test. Damaged and undamaged trees were compared by analysis of 2 × 3 contingency tables using Fisher’s exact probability test among years, in the control and the treated orchard, respectively, because direct comparisons between the MD and the untreated orchard were not possible due to the different histories of damage by the carpenter moth. Pairwise tests between the years in the MD orchards were conducted by Fisher’s exact probability test with the Bonferroni correction. Numbers of pupal exuviae were compared by ANOVA, and significantly different means were separated by Tukey–Kramer’s HSD test.
Results
Trap Shutdown
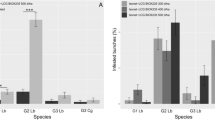
When MD pheromone dispensers were placed at a height of 1.5 m, monitoring traps placed at 1.5, 3, or 5 m high in the MD orchard captured significantly fewer male moths than the corresponding traps in the control orchard (Fig. 1). Even at the 5 m height, complete trap shutdown was observed in the MD orchard, whereas 16 male moths were captured in traps in the control orchard.
Number of male moths (Mean/trap ± SE) for the entire trapping period captured in traps placed at heights of 1.5, 3, and 5 m in the mating disruption (MD) (hatched bar) and control (white bar) orchards. Numbers of male moths captured were significantly different between the control and MD orchards (P < 0.05, Kruskal–Wallis test) at the same height
Reduction of Mating in Mating Cages
Most of the moth pairs eventually mated in cages placed in the control orchard, whereas in the MD orchard only about one third (34.5 %) of the pairs mated, which was significantly lower than in the control orchard, in which 87.5 % of the pairs mated (Kruskal–Wallis test, P < 0.05).
Reduction of Mating in Tethered Females
We also examined mating disruption efficacy in virgin females tethered at heights of 1 and 3 m in the MD and control orchards. No tethered females were mated in the MD orchard, whereas in the control orchard, one-third of the females (35.4 %) were mated (Kruskal–Wallis test, P < 0.05) at the 1 m height. Even at the 3 m height, mating of tethered females in the MD orchard still was reduced significantly, from 49 % in the control orchard to 4.2 % in the treated orchard (Kruskal–Wallis test, P < 0.05), even though the pheromone dispensers were placed at a height of 1.5 m.
Reduction of Damage
Damage assessments in orchards C and D are shown in Fig. 2. Damage in the control orchard did not change during the course of the study, whereas damage in the MD orchard significantly decreased in 2013, the third year of MD treatment (Fig. 2). The numbers of new pupal exuviae per tree in the MD plot also decreased significantly in 2013, in contrast to the number of new exuviae in the control orchard, which did not change (Fig. 3).
Percentage of trees damaged by larvae of Cossus insularis in the mating disruption (MD) (■) and control orchards (●). Symbols with the same letters are not significantly different (P < 0.05, Fisher’s exact probability test with Bonferroni correction) within the treatment and the control groups, respectively
Discussion
Generally, in plots treated with MD, the number of males attracted to monitoring traps and the rate of mating are expected to decrease in comparison to untreated plots (Witzgall et al. 2010). In the current study, as expected, attraction of males to monitoring traps in the MD-treated orchard was reduced significantly in comparison to the control orchard (Fig. 1), and mating rates of tethered females and females in mating cages in the MD orchard were significantly lower than in the control orchard. These results suggest that treatment with the 80:20 mixture of synthetic E3–14:Ac and Z3–14:Ac, at the rate of 130 g/ha, strongly interfered with the reproductive behaviors of C. insularis males and females.
In the current study, placement of the MD pheromone dispensers at a height of 1.5 m reduced trap captures of C. insularis males at the same level or at higher levels in the canopy. Furthermore, C. insularis females tethered at a height of 3 m in the MD orchard mated significantly less than females placed at 3 m height in the control orchard. Taken together, these results suggest that pheromone released from the dispensers disperses upward to at least the top of the canopy. From the trap captures in the untreated orchard (Fig. 1), male moths prefer to fly at heights of ~1 to 3 m, below the top of the canopy of the apple trees, which are typically ~3.5 m high in commercial orchards in northern Japan. Therefore, placing pheromone dispensers at a height of 1.5 m appears to be effective for MD of C. insularis, i.e., the additional labor and other costs that would accrue from placing the dispensers higher in the canopy can be avoided.
Most of the C. insularis placed in small mating cages in the control orchard eventually mated, but in the MD orchard only about a third of the pairs mated. The fact that relatively few pairs mated in the treated plots was unexpected because the cages were small enough that the moths could easily find each other just by random movement. This suggests that relatively high levels of pheromone may inhibit mating or even movement of the moths. However, the normal activity levels of this moth during the scotophase are not well known, so the possible causes of this decreased mating rate remain speculative. In some other Lepidoptera, exposure to high concentrations of their pheromone blend components is known to reduce responses of males to their pheromone blends (Gökçe et al. 2007), and also to inhibit female calling behavior (Yang et al. 2009), both of which, separately or jointly, would result in the reduction of mating. Similar mechanisms may have been involved in the lowered mating rates seen in the caged pairs in the current study.
Although damage was decreased in the orchard treated with MD (Figs. 2, 3), it is not possible to attribute the decrease of damage only to MD, because heavily damaged trees were removed by the owner of the orchards used for the trials. However, the complete trap shutdown and reduction of mating of females placed in the MD orchard suggest that the MD treatment may at least have contributed to the reduction in damage.
From our results, we conservatively estimate that pheromone dispensers placed at 1.5 m high will disrupt orientation of C. insularis throughout tree canopies that are 2.5 to 3.5 m tall. Because most commercial apple trees are <3.5 m tall in northern Japan, pheromone dispensers can be placed easily at 1.5 m without requiring special equipment, facilitating grower adoption of mating disruption for the control of C. insularis.
Cossus insularis Pheromone Mating Disruptant Registration
Based on the scientific results, some of which are shown here, Shin-Etsu Chemical Co., Ltd. submitted an application to commercialize a mating disruptant for C. insularis to the Food and Agricultural Materials Inspection Center (FAMIC) of Japan. According to the Agricultural Chemicals Regulation Law in Japan, test results regarding efficacy, phytotoxicity, toxicity, and persistence of chemicals are required in applications to register new agrochemicals. Details of the application process are described on the web page of FAMIC (http://www.acis.famic.go.jp/eng/shinsei/index.htm). However, if the chemical is an attractant and used in an encapsulated condition, such as a tube dispenser, phytotoxicity tests, most of the toxicity tests, and persistence tests are waived. Toxicity tests consist of i) acute oral toxicity, ii) acute dermal toxicity, iii) mutagenicity assays, iv) fish acute toxicity, v) Daphnia spp. acute immobilization tests, and vi) Algal growth inhibition tests. At present, only acute oral toxicity and mutagenicity assays are required for the registration of a mating disruptant. This differs from the United States, where toxicity tests are exempted in the registration of straight chain lepidopteran pheromones (USEPA 2009), whereas regulations in EU (European Commission 2009) and Asian countries such as China, India, Indonesia, and Korea are similar to those in Japan (F. Mochizuki, Shin-Etsu Chemical Co., Ltd., Pers. Comm.).
For efficacy tests, results indicating trap shutdown in three different locations for two years are required as part of the application to FAMIC. However, in Japan, unregistered pheromone compounds cannot be used for pest control in the field even for experimental purposes, even though efficacy test results are a prerequisite for an application for registration. To get around this “Catch 22”, the applicant usually entrusts the efficacy tests to a local government experimental station or an equivalent organization through the Japan Plant Protection Association. Alternatively, the registrant can apply for a research project to a competitive research granting agency supported by the Ministry of Agriculture, Forestry, and Fisheries (MAFF) of Japan. In the case of C. insularis, the MD efficacy trials were supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industries from MAFF.
For the application to register a mating disruptant for C. insularis, we demonstrated trap shutdown in Yamagata, Fukushima, and Tokushima Prefectures from 2011 to 2013, as shown in Fig. 1, which fulfilled the one of the main registration requirements. Shin-Etsu Chemical Co., Ltd. then submitted an application, which included the physical and chemical properties of the pheromone, the storage stability, the composition, and method of manufacturing, and the active ingredient. After inspection, the application was accepted by FAMIC, and the commercial name of “Bokuto-con H″ was approved. A commercial product was released in March 2015, and is now available for control of C. insularis in apples and sand pears in Japan.
References
Aono N, Natsumi K, Yukawa Y (1989) Mating suppression to the cherry tree borer, Synanthedon hector Butler, in Japanese apricot by its synthetic sex pheromone. Plant Prot 43:329–332 (in Japanese)
Cardé RT, Minks AK (1995) Control of moth pests by mating disruption: Successes and constraints. Annu Rev Entomol 40:559–585
Chen X, Nakamuta K, Nakanishi T, Nakashima T, Tokoro M, Mochizuki F, Fukumoto T (2006) Female sex pheromone of a carpenter moth, Cossus insularis (Lepidoptera: Cossidae). J Chem Ecol 32:669–679
Escofet M, Boada J, Melo JC, Casals C, Barrios G, Palau G, Cavallé C, Pelaez M, Mateu J, Aymami A, Nubiola N, Ribé E, Ibáñez R, Santiveri C, Sans J, Pallarés J, Agustí JA (2005) Sexual disruption to control leopard moth (Zeuzera pyrina) in hazelnut. Acta Hortic 686:421–425
European Commission (2009) Legislation on Plant Protection Products, http://ec.europa.eu/food/plant/pesticides/legislation/index_en.htm, Accessed 20 June, 2016
Gökçe A, Stelinski LL, Gut LJ, Whalon ME (2007) Comparative behavioral and EAG responses of female obliquebanded and redbanded leafroller moths (Lepidoptera: Tortricidae) to their sex pheromone components. Eur J Entomol 104:187–194
Hegazi, EM, Khafagi WE, Konstantopoulou MA, Schlyter F, Raptopoulos D, Shweil S, Abd El-Rahman S, Atwa A, Tawfik H (2010) Suppression of leopard moth (Lepidoptera: Cossidae) populations in olive trees in Egypt through mating disruption. J Econ Entomol 103:1621–1627
Japanese Society of Applied Entomology and Zoology (2006) Major insect and other pests of economic plants in Japan, Revised edition. Japan Plant Protection Association, Tokyo, Japan, p. 387 (in Japanese)
Kitajima H, Makihara H, Hasegawa M (1998) Morphology and injury forms of larvae of Cossus spp. (Lepidoptera: Cossidae). Trans Kanto Br Jpn For Soc 49:65–68 (in Japanese)
Matsumoto K, Nakamuta K, Nakashima T (2007) Mating disruption controls the cherry tree borer, Synanthedon hector (Butler) (Lepidoptera: Sesiidae), in a steep orchard of cherry trees. J For Res 12:34–37
Minamishima M, Arakawa A, Okazaki K, Mochizuki F, Fukumoto T (2004) An easy method for estimating the efficacy of mating disruption in the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Jpn J Appl Entomol Zool 48:201–205 (in Japanese)
Nakamuta K, Chen X, Kitajima H, Nakanishi T, Yoshimatsu S (2007) Ecology of three species of Cossus carpenter moths in Japan. For Pests 56:5–9 (in Japanese)
Nakamuta K, Itou S, Sasaki M, Nakanishi T, Minamishima M (2010) The carpenter moth, Cossus insularis, as an insect pest of the apple and Japanese pear trees in Japan. Plant Prot 64:779–781 (in Japanese)
Nakanishi, T (2005) First report of occurrence of Cossus insularis (Staudinger) on the Japanese pear. Jpn J Appl Entomol Zool 49:23–26 (in Japanese with an English summary)
Nakanishi T, Nakamuta K, Mochizuki F, Fukumoto T (2013) Mating disruption of the carpenter moth, Cossus insularis (Staudinger) (Lepidoptera: Cossidae) with synthetic sex pheromone in Japanese pear orchards. J Asia-Pacific Entomol 16:251–255
Natale D, Pasqualini E (1999) Il controllo di zeuzera e cossus mediante feromoni. Inf Agrar 16:79–83
Tadauchi O, Inoue H (2000) On MOKUROKU file based on “A Check List of Japanese Insects” on INTERNET. Esakia: Occasional papers of the Hikosan Biological Laboratory in Entomology, 40, pp.81–84, http://www.konchudb.agr.agr.kyushu-u.ac.jp/mokuroku/exec/, Accessed 20 June, 2016.
USEPA (2009) Straight Chain Lepidopteran Pheromones, https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-000004_27-Feb-09.pdf, Accessed 20 June, 2016.
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100
Yaginuma K (1984) Cherry tree borer. Experimental method of pheromones, vol. 2. Japan Plant Protection Association, Tokyo, pp. 116–120 (in Japanese)
Yang MW, Dong SL, Chen L (2009) Electrophysiological and behavioral responses of female beet armyworm Spodoptera exigua (Hübner) to the conspecific female sex pheromone. J Insect Behav 22:153–164
Acknowledgments
We thank Takehiko Fukumoto and Fumiaki Mochizuki of Shin-Etsu Chemical Co., Ltd. for their invaluable comments on the manuscript, and Masayuki Hayashi of Chiba University for his suggestions on the statistical analyses. This study was supported by Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (Project # 23060) from 2011 to 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoshi, H., Takabe, M. & Nakamuta, K. Mating Disruption of a Carpenter Moth, Cossus insularis (Lepidoptera: Cossidae) in Apple Orchards with Synthetic Sex Pheromone, and Registration of the Pheromone as an Agrochemical. J Chem Ecol 42, 606–611 (2016). https://doi.org/10.1007/s10886-016-0723-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0723-x