Abstract
Electroantennogram (EAG) recordings showed that female Spodoptera exigua can detect their own sex pheromones (two single components and their mixture), displaying a similar dose–response pattern to that of males, although intensities of female responses were much less at all doses compared with males. Furthermore, the female calling behavior was inhibited and late-shifted by the presence of the female sex pheromone. When the pheromone components were presented, the calling female proportion in the peak calling period was significantly reduced and the calling peak time and calling termination time postponed, compared with controls. Although the calling behavior was inhibited, the pheromone titer of treated females was not different to the control, implying a reduced pheromone biosynthesis in the pheromone glands of treated moths. However, observations during the olfactometer experiments revealed that there were no obvious behavioral responses of females exposed to sex pheromone stimuli including whole gland extracts, 0.1, 1 or 10 μg binary pheromone mixtures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex pheromones play a vital role in insect communication between the sexes. It is generally accepted that sex pheromones detected by the opposite sex result in sequential behaviors which precede and make mating possible. In most moth species females are the sex pheromone releasers, and males detect the pheromone and respond with an oriented upwind flying from long distances. Male-produced sex pheromones appear to work at closer range and may also play various roles in the pre-mating and mating process (Cardé and Haynes 2004).
By definition a sex pheromone released by female moths provides their locations to interested males of the same species. However, the ability of females to detect and respond to their own sex pheromone has been reported in some moth species. For example, female antennal neurons can electrophysiologically respond to conspecific female sex pheromone components in some species of Tortricidae (DeLury et al. 2005; Stelinski et al. 2006; Gökçe et al. 2007), Noctuidae (Ochieng et al. 1995; Groot et al. 2005; Hillier et al. 2006), Arctiidae (Schneider et al. 1998; Grant and O’Connell 2000), and Sphingidae (Kalinova et al. 2001). However, these moths behaviorally respond to the conspecific sex pheromone in quite different ways including: (a) modifying the calling behavior (Palaniswamy and Seabrook 1985; Stelinski et al. 2006; Gökçe et al. 2007; Lim and Greenfield 2007); (b) escaping from (Saad and Scott 1981) or being attracted to (Birch 1977) the pheromone source; and (c) modifying the oviposition behavior (Palaniswamy and Seabrook 1978; Gökçe et al. 2007). More insect species need to be investigated to reveal the diversity and further the ecological and evolutional contexts of female responses to conspecific female sex pheromones.
The beet armyworm, S. exigua Hübner (Lepidoptera: Noctuidae), is a severe pest of various agricultural crops in Asia and North America. The sex pheromone of female S. exigua has been identified, and a lure with two components (Z9,E12-14:Ac and Z9-14:OH) has been successfully used in pest population monitoring and management (Mitchell and Tumlinson 1994; Yoshiyasu et al. 1995; Wakamura et al. 1989; Mitchell et al. 1997; Dong and Du 2002). Here, our current study showed a significant electrophysiological response by female S. exigua to the female sex pheromone components (Z9,E12-14:Ac, Z9-14:OH and their mixture). As a result, the female calling behavior was changed in females exposed to female sex pheromones, and meanwhile, pheromone titers in the sex pheromone glands were equivalent in pheromone exposed and control (unexposed) females. However, the female sex pheromone did not elicit any behavioral response in females.
Materials and Methods
Insects
The S. exigua laboratory colony was originally collected from soybean plants in Nanjing (32.0N, 118.5E), Jiangsu Province of China in August 2004, and was maintained for successive generations on an artificial diet (Huang et al. 2002) in the laboratory at 26 ± 1°C, 60–70% relative humidity and 14:10 h light/dark photoperiod. For this study, pupae were sexed based on the morphology of the abdominal terminal segments, and were placed in separate glass dishes (15 cm in diameter) with some moistened vermiculite to maintain the humidity. The dishes with female pupae were placed in wooden cages (24 × 26 × 34 cm), and kept in another room at the same temperature and humidity as stated above but with a reversed light schedule until eclosion. Following eclosion, daily transfers of uneclosed pupae to new cages were made to ensure that each cage contained adult females of the same age. Moths were fed with 10% sugar water solution saturated on cotton balls. Female moths aged 2–4 days were used in experiments.
Chemicals
Z9,E12-14:Ac and Z9-14:OH were provided by Professor Du in Institute of Plant Physiology & Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The purity of these chemicals was checked by GC analysis to be >95%. The chemicals were diluted with redistilled hexane at a series of concentrations ranging from 0.1 to 1 μg/μl for each single compound, and from 0.1 to 10 μg/μl for the binary mixture of Z9,E12-14:Ac and Z9-14:OH (9:1 in ratio). All dilutions were kept at −20°C before use.
Electroantennogram Recordings
Antennal responses of virgin adults to two single components and their mixture were investigated using a standard electroantennogram (EAG) method. Prior to the experiment, 2–4 days old virgin moths were placed individually in glass tubes, which were then brought to the EAG laboratory using a lightproof hop-pocket for EAG analyses. Antennae of virgin adults (2–4 days old) were excised at the base from the moth head with a micro-scissors, and several terminal segments of the antenna were removed in order to keep better contact, then the ends of the isolated antenna were connected to the two recording electrodes by spectra 360 electrode gel (USA), respectively. The EAG signals were amplified and monitored with a head-stage preamplifier and further amplified and processed with a PC-based signal processing system (Syntech®, The Netherlands).
The odor stimuli were prepared by applying 10 μl of chemical solution on a filter paper strip (2.5 × 0.75 cm) in a Pasteur pipette. The tip of the pipette was inserted into the hole (3 mm in diameter, 120 mm upstream from the outlet) on the main airflow tube (12 mm in diameter, 170 mm long) in which a continuous, charcoal filtered and moistened airflow (4 ml/s) was blown onto the prepared antenna. Using an electronic controller a 0.5 s puff of charcoal filtered air (4 ml/s) was injected through the large end of the Pasteur pipette, transporting the volatiles to the antenna for stimulation. Each antenna was tested with a series of stimuli in an aptotic order: solvent, 0.0001, 0.001, 0.01, 0.1, 1 μg/μl, and solvent. In each test, a 10 μl volume of each stimulus was loaded onto the filter paper strip, and the strip was left for 1 min to let the solvent evaporate before it was used for EAG measurement. The EAG values to both solvent only puffs were averaged to serve as the control. At least 1 min was allowed between two stimuli to provide time for recovery of antennal responsiveness. All tests were conducted between the 6th and 9th hour into scotophase in three consecutive scotophases, with one pheromone component tested in anyone scotophase. The amplitude of the EAG response was recorded and analyzed with EAG-adapted software (Syntech®, The Netherlands).
Calling Observations
To test the influences of female sex pheromone on female calling behavior, a vitreous assay chamber (Fig. 1) was used. The chamber (11 cm in height and 7 cm in diameter) was sealed with a lid, not allowing for airflow to pass through it. The chamber was divided into two parts with wire mesh, with moths restricted in the upper part, and a pheromone dispenser in the lower part. The dispenser was a filter paper strip (3 × 2 cm) fixed on an insect pin, impregnating with 10 μl binary pheromone mixture or 10 μl hexane (as control). The insect pin with the filter paper was inserted in a rubber septum to keep it in position. The solvent was allowed to volatilize for 1 min before the filter paper was placed into assay chamber.
Two hours prior to the onset of scotophase, female moths (2–4 days old) were introduced into the chamber (three females/chamber) for acclimation to the environment. A cotton ball soaked with sugar water solution was put into the chamber to feed the moths. One hour later, a pheromone dispenser was put into each chamber. The observations were carried out from 1 h prior to until 2 h after the scotophase at 30 min intervals with a flash light having a red light filter. A calling female was defined as a moth with protruding ovipositor, but not exhibiting oviposition behavior. The observation room was controlled at 26 ± 1°C, 60–70% relative humidity, and with a reversed 14L:10D light schedule. An exhaust on the ceiling was used to keep clean air in the room.
Two separate experiments with different pheromone usage (100 and 1 μg) were conducted for the observations. In each experiment, observations were conducted on three consecutive days. In the first experiment (with 100 μg pheromone), seven, 12 and ten chambers for both control and treatment were observed in the first, second and third day, respectively. In the second experiment (with 1 μg pheromone usage), the chamber numbers for the observations were 11, 11 and eight in the first, second and third day, respectively.
Behavioral Bioassay
The effect of the female sex pheromone on female moth behavior was investigated in an I-tube olfactometer set-up. The I-tube was 20 cm long and 3.5 cm in diameter. At the upwind end of the olfactometer, there was a flask (5 cm long × 0.75 cm ID) used for placing the stimulus source. The experiments were performed in a dark room at 26–27°C, 60–70% relative humidity, illuminated with a 15 W red light bulb. The four stimuli sources in 10 μl hexane were prepared for the bioassay, and 10 μl hexane served as the control. The four treatments were 0.1, 1 and 10 μg pheromone mixtures (Z9,E12-14:Ac and Z9-14:OH in 9:1 ratio), and one female equivalent pheromone gland extraction. The pheromone gland extraction was prepared with same method in the determination of pheromone titers of this paper. The 10 μl stimulus or solvent control was loaded onto a piece of quadrate filter paper (2.5 × 0.8 cm). After the solvent volatilized for 1 min, the filter paper was introduced into the flask. A charcoal purified and humidified airflow of approximately 250 ml/min was employed to send the volatile through the I-tube. The females (2–4 days old) were transferred individually to the testing point 4 cm apart from the downwind end of the olfactometer, and allowed to respond for 2 min. After 2 min, the female was removed and a new female was introduced for the next observation. The response type for each moth was recorded as flying or walking upwind or downwind or no obvious response (quiescence or activity within a limited range).
The experiment was conducted between the 5th and 9th hours of scotophase. Ten to 12 moths (as one replicate) were tested for each treatment in one scotophase and repeated for four consecutive scotophases. Therefore, there were total of four replicates for each treatment (one replicate at each scotophase). The treatment order was randomized for each replicate. The apparatus was cleaned thoroughly after a treatment of stimuli by rinsing with hexane.
Determination of Pheromone Titers
According to the results from calling behavior experiments, sex pheromone titers in the sex pheromone glands at 5, 7 and 9 into and 1 h after scotophase were determined. Females were treated as in the experiment of calling behavior. The method for pheromone extraction was similar to that used by Dong and Du (2001). Briefly, the pheromone gland was extruded by applying gentle pressure to the abdomen, and the gland was dissected surgically using micro-scissors. Three glands were immersed in 10 μl distilled hexane containing 10 ng of undecyl acetate as an internal standard in a conical vial. Four replicates (vials) were made for both treated and control moths. After about 1 h extraction, the extract was transferred to a clean conical vial and was concentrated to 1–2 μl using a gentle stream of nitrogen.
The extract was injected into a gas chromatograph (GC–14B, Shimadzu, Kyoto, Japan) fitted with an FID, a split/splitless injector and a fused silica capillary column (SPB–50, 30 m × 0.25 mm ID, film thickness 0.25 μm, Supelco, USA) in splitless mode. The time for splitless injection was 1 min. Nitrogen was used as the carrier gas. The oven temperature was set initially at 100°C for 2 min, increased at 10°C/min to 250°C, and held for 5 min. Injector and flame ionization detector (FID) temperatures were set at 220°C and 250°C, respectively. Two major pheromone components, Z9,E12–14:Ac and Z9–14:OH in the extracts were verified from the GC retention time relative to that of internal standard, previously measured with the authentic chemicals. The quantity of each compound was calculated based on the peak area and calibrated by comparing it with that of the internal standard undecyl acetate.
Statistical Analyses
For the EAG and the behavioral bioassay data, a one-factor analysis of variance (ANOVA) was performed, followed by Tukey’s test to examine differences (P < 0.05) among dosages (EAG experiment) or female treatments (behavioral bioassay). Differences (P < 0.05) between the proportions of calling females and pheromone titers in pheromone treated moths versus the control were analyzed using paired t-tests. All values are mean ± SD unless otherwise designated.
Results
EAG Recordings
Females exhibited EAG responses to two main female sex pheromone components applied singly or in combination (Fig. 2A). Compared to the hexane control, the binary mixture, Z9,E12-14:Ac, and Z9-14:OH elicited significant responses in female antenna at the doses ≥0.01, ≥1.00 and ≥0.10 μg, The dose–response curves of these components showed an overall increasing trend in EAG response as the dose increased. However, a 10 μg dose did not elicit significantly higher EAG responses compared to the 1 μg dose for all two components and mixtures.
Dosage–response relationships for S. exigua female (A) and male (B) antennae to two single components (Z9,E12-14:Ac, Z9-14:OH) and their 9:1 mixture (Z9,E12-14:Ac: Z9-14:OH). Data were presented as mean values ± SD (N = 10) and analyzed by one-factor analysis of variance, followed by Duncan’s multiple range test (P < 0.05). Significant differences among various dosages of same stimulant are indicated with different letters.
The EAG responses of males to these pheromone components were also measured (Fig. 2B). The female and male antenna displayed similar EAG response patterns, but specific differences were noted. First, the EAG responses in male antennae were much higher than those in female antennae. For example, EAG values to three stimuli at the dose of 10 μg in male antennae were all about eight times of those in females. Secondly, the male antennae displayed an overall higher saturation dose and lower response dose than the female antennae. For male antennae, EAG saturation doses were 10 μg for both the pheromone mixture and Z9-14:OH, whereas the doses for female antenna were 1 μg for all three stimuli. Also, when compared with the hexane control, the doses inducing the significant EAG responses in males were lower than in females, especially for the binary mixture treatment.
Female Calling Behavior
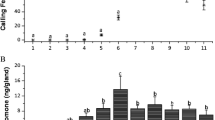
The bioassays indicated that pheromone treated females elicited significantly different calling behavior with respect to the calling female proportion, calling peak time and calling termination time, compared with the control (Fig. 3). In the first experiment (Fig. 3A), females with pheromone exposure showed lower proportions of females with calling behavior than control females during 6–10th hour of scotophase, and differences were significant from 6th to 8th hours of scotophase (paired t-test, P < 0.05). However, pheromone treated females showed a higher calling female proportion than did controls after 10th hour of scotophase, and the differences were significant within 2 h into photophase. Despite onsets of calling behavior for treatment and control being the same, treated females reached their calling peak 2.5 h later, and terminated the calling behavior 2 h later compared to the control. When the scotophase ended, control females terminated calling behavior within a half-hour, whereas a small proportion of treated females continued to exhibit calling behavior until the second hour into the photophase. Therefore, the overall calling behavior decreased and late-shifted in pheromone treated females.
Dynamics in proportions of calling females with presence of the binary pheromone mixture in the usage of 100 μg (A) and 1 μg (B). Data were showed as mean values ± SD and analyzed by a paired t-test (P < 0.05). Significant differences in calling female proportions between pheromone treatment versus the solvent control are indicated by asterisk. Scotophase was from 09:00 to 19:00 h. The picture in A showed two calling females (with extruded abdomen tip) in photophase when the females were exposed to the sex pheromone.
In the second experiment with 1 μg pheromone usage, similar changing trends in calling behavior of treated females were obtained, although some differences between the treatment and the control were slightly less than those in the first experiment (Fig. 3B).
Olfactometer Experiments
Compared to the hexane control, neither the female pheromone gland extract nor the binary pheromone mixture could elicit significant differences in behavior responses with respect to the three behavioral types: no response, downwind moving and upwind moving. In other words, females exhibited no directed behavior toward or away from their own sex pheromone (Fig. 4).
Behavioral responses of female S. exigua to one female equivalent of crude hexane extract and various doses of a 9:1 mixture of Z9,E12-14:Ac and Z9-14:OH. Data were presented as mean values + SD and analyzed by one-factor analysis of variance, followed by Duncan’s multiple range test (P < 0.05). Bars topped with same letters are not significantly different.
Pheromone Titer
Quantitative GC analysis showed that the titers of two major pheromone components (Z9,E12-14:Ac and Z9-14:OH) in the sex pheromone glands were not significantly different between pheromone treatment and solvent control at all 4 investigated time points (Fig. 5).
Comparison of the pheromone titers of Z9,E12-14:Ac (A) and Z9-14:OH (B) between pheromone treated and control moths. Data represented as mean values ± SD and analyzed by using a paired t-test. Bars topped with same letters are not significantly different (P > 0.05). L indicates photophase, and D indicates scotophase. D5, D7 and D9 are respectively corresponding to 5, 7 and 9 h into scotophase, and L1 is corresponding to 1 h into photophase.
Discussion
Our EAG measurements showed that female S. exigua can detect the sex pheromones of their own species. Similar results were demonstrated by EAG assay in several other noctuid species (Seabrook et al. 1987; Ljungberg et al. 1993; Fan et al. 2003; Groot et al. 2005; Hillier et al. 2006), tortricid species (Palaniswamy and Seabrook 1978; DeLury et al. 2005; Stelinski et al. 2006; Gökçe et al. 2007), arctiid species (Schneider et al. 1998; Grant and O’Connell 2000) and sphingid species (Kalinova et al. 2001). Therefore, it might be a common phenomenon for female moths to detect their own sex pheromone components.
Although females can detect their own sex pheromone, the intensities of EAG responses are generally much lower in females than in conspecific males. In our study with S. exigua, for example, EAG values to three stimulants at the dose of 10 μg in male antenna were all about eight times of those in female’s; in Spodoptera littoralis, male EAG responses to Z9,E11-14:OAc were more than six times higher than female’s (Ljungberg et al. 1993); and in Trichoplusia ni, the EAG response of male antenna to the major pheromone component (Z7-12:OAc, at 100 μg dose) was more than three times higher than that of female (Seabrook et al. 1987). Two factors are probably involved in this low EAG intensity in females. Firstly, female antennae are morphologically different from male, possessing fewer chemoreceptor sensillae (Mochizuki et al. 1992; Ljungberg et al. 1993). As the EAG value is the summation of potentials produced by all pheromone-sensitive sensillae on the antennae (Howse 1998), fewer pheromone-sensitive sensillae in female antennae certainly will result in lower EAG intensities. Secondly, females have lower sex pheromone binding protein (PBP) levels in their sensillae lymph than that do males. Our previous study on S. exigua PBP expression indicated that the female expression levels for SexigPBP1 and SexigPBP2 are about 39% and 73%, respectively, relative to male levels (Xiu and Dong 2007). Lower expressions of PBP in females than in males have also been reported in some other moths, such as Bombyx mori (Krieger et al. 1996; Maida et al. 2005), Mamestra brassicae (Nagnan-Le Meillour et al. 1996), Helicoverpa zea and Spodoptera frugiperda (Callahan et al. 2000), Agrotis ipsilon (Picimbon and Gadenne 2002), and Sesamia nonagrioides (De Santis et al. 2006). As PBPs function to bind and transport the hydrophobic sex pheromone molecules across the aqueous antennal lymph onto the receptor proteins on the sensory neurons (Prestwich 1996), low PBP expression levels in female antennae may contribute to the low EAG response as well as the low saturation dose. Nevertheless, this lower response intensity in females compared to males is well in agreement with the characteristics of the moth sex communication system.
Published reports indicate there are several ways that conspecific pheromones can affect female calling behavior. The first is to advance the onset of calling periodicity and/or increase the total proportion of calling females as in some tortricid species (Palaniswamy and Seabrook 1985; Stelinski et al. 2006) and one arctiid species (Lim et al. 2007). This modification could be advantageous for the females, as it make the females obtain more chances to attract and mate with the males, especially under high female densities. The second way, as shown in our study, was to postpone both calling peak time and calling termination time of females. This might also be advantageous for the females to get mating chances, as it actually decreases the mating competition among conspecific females. The third way is that females reduce their calling proportions with the presence of conspecific female sex pheromone or neighboring calling females, which is indicated by our current study in S. exigua and observations in Choristoneura rosaceana and Argyrotaenia velutinana (Gökçe et al. 2007). A feedback mechanism might exist in female release (and even synthesis) of the pheromone. Through detecting of the sex pheromone around them, females can respond by adjusting their calling behavior (pheromone release). This feedback mechanism could also explain the delayed onset of calling in two tortricid species (Noguchi and Tamaki 1985), and shortened calling duration in C. rosaceana and A. velutinana (Gökçe et al. 2007).
Besides the modified calling behaviors, the female detection of their own sex pheromone may induce other behavioral changes which might be advantageous to the females or the whole population. Birch (1977) reported oriented movement of females toward virgin females in T. ni and suggested that the pheromone may bring about aggregation, thus increasing local chances of mating. In contrast, repelling among conspecific females was observed in Helicoverpa armigera and H. zea, which might decrease the competition and enhance mating success (Saad and Scott 1981). However, females did not show obvious tendency or escape behavior to various female sex pheromone sources in our study. It might be reasonable for females in this species to respond by modifying their current calling behaviors rather than a large-scale escaping, as the former is less energy consuming.
Although calling female proportions were significantly lower during calling peak times in pheromone treated females than in control females, the titers of two major pheromone components in the pheromone glands did not show significant differences between treated and control females. This may implicates that the pheromone synthesis or transportation to the pheromone gland in the treated females was relatively reduced, if the released amount of pheromone by females is positively correlated with the calling female proportions. Therefore, the result of pheromone titer measurement supports the proposed feedback mechanism on pheromone synthesis and release. More detailed and specifically designed experiments need to be conducted to clarify the female response on the level of pheromone biosynthesis and release when females are exposed to conspecific female sex pheromone.
References
Birch MC (1977) Responses of both sexes of Trichoplusia ni (Lepidoptera: Noctuidae) to virgin females and to synthetic pheromone. Ecol Entomol 2:99–104
Callahan FE, Vogt RG, Tucker ML, Dickens JC, Mattoo AK (2000) High level expression of “male specific” pheromone binding proteins (PBPs) in the antennae of female noctuid moths. Insect Biochem Mol Biol 30:507–514
Cardé RT, Haynes KE (2004) Structure of the pheromone communication channel in moths. In: Cardé RT, Millar JG (eds) Advances in insect chemical ecology. Cambridge University Press, Cambridge, pp 283–332
DeLury NC, Judd GJR, Gardiner MGT (2005) Antennal detection of sex pheromone by female Pandemis limitata (Robinson) (Lepidoptera: Tortricidae) and its impact on their calling behaviour. J Entomol Soc B C 102:3–11
De-Santis F, François MC, Merlin C, Pelletier J, Maïbèche-Coisné M, Conti E, Jacquin-Joly E (2006) Molecular cloning and in situ expression patterns of two new pheromone binding proteins from the corn stemborer, Sesamia nonagrioides. J Chem Ecol 32:1703–1717
Dong S-L, Du J-W (2001) Diel rhythms of calling behavior and sex pheromone production of beet armyworm, Spodoptera exigua (Lepidoptera:Noctuidae). Entomol Sin 8:89–96
Dong S-L, Du J-W (2002) Chemical identification and field tests of sex pheromone of beet armyworm Spodoptera exigua. Acta Phytophylacica Sinica 29:19–24
Fan W-M, Sheng C-F, Su J-W (2003) Electrophysilogical and behavioral responses of both sexes of the cotton bollworm, Helicoverpa armigera Hübner to sex pheromones. Acta Entomol Sin 46:138–143
Gökçe A, Stelinski LL, Gut LJ, Whalon ME (2007) Conparative behavioral and EAG responses of female obliquebanded and redbanded leafroller moths (Lepidoptera: Tortricidae) to their sex pheromone components. Eur J Entomol 104:187–194
Grant AJ, O’Connell RJ (2000) Responses of olfactory receptor neurons in Utetheisa ornatrix to gender-specific odors. J Comp Physiol A 186:535–542
Groot A, Gemeno C, Brownie C, Gould F, Schal C (2005) Male and female antennal responses in Heliothis virescens and H. subflexa to consepcific and heterospecific sex pheromone compounds. Environ Entomol 34:256–263
Hillier NK, Kleineidam C, Vickers NJ (2006) Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviorally relevant odors. J Comp Physiol A 192:199–219
Howse PE (1998) Pheromones and behaviour. In: Howse PE, Stevens IDR, Jones OT (eds) Insect pheromones and their use in pest management. Chapman and Hall, London, pp 1–132
Huang C-X, Zhu L-M, Ni J-P, Chao XY (2002) A method of rearing the beet armyworm Spodoptera exigua. Entomological Knowledge 39:229–231
Kalinová B, Hoskovec M, Liblikas I, Unelius CR, Hansson BS (2001) Detection of sex pheromone components in Manduca sexta (L.). Chem Senses 26:1175–1186
Krieger J, Von Nickisch-Rosenegk E, Mameli M, Pelosi P, Breer H (1996) Binding proteins from the antennae of Bombyx mori. Insect Biochem Mol Biol 26:297–307
Lim H, Greenfield MD (2007) Female pheromonal chorusing in an arctiid moth, Utetheisa ornatrix. Behav Ecol 18:165–173
Lim H, Park KC, Baker TC, Greenfield MD (2007) Perception of conspecific female pheromone stimulates female calling in an arctiid moth, Utetheisa ornatrix. J Chem Ecol 33:1257–1271
Ljungberg H, Anderson P, Hansson BS (1993) Physiology and morphology of pheromone specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J Insect Physiol 39:253–260
Maida R, Mameli M, Müller B, Krieger J, Steinbrecht RA (2005) The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx mori. J Neurocytol 34:149–163
Mitchell ER, Tumlinson JH (1994) Response of Spodoptera exigua and S. eridania (Lepidoptera: Noctuidae) males to synthetic pheromone and S. exigua females. Fla Entomol 77:237–247
Mitchell ER, Kehat M, Tingle FC, Mclaughlin JR (1997) Suppression of mating by beet armyworm (Noctuidae: Lepidoptera) in cotton with pheromone. J Agric Entomol 14:17–28
Mochizuki F, Sugi N, Shibuya T (1992) Pheromone sensilla of the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Appl Entomol Zool 27:547–556
Nagnan-Le Meillour P, Huet JC, Maïbèche M, Pernollet JC, Descoins C (1996) Purification and characterization of multiple forms of odorant/pheromone binding proteins in the antennae of Mamestra brassicae (Noctuidae). Insect Biochem Mol Biol 26:59–67
Noguchi H, Tamaki Y (1985) Conspecific female sex pheromone delays calling behavior of Adoxophyes sp. and Homona magnanima (Lepidoptera: Tortricidae). Jpn J Appl Entomol Zool 29:113–118
Ochieng SA, Anderson P, Hansson BS (1995) Antennal lobe projection patterns of olfactory receptor neurons involved in sex pheromone detection in Spodoptera littoralis (Lepidoptera: Noctuidae). Tissue Cell 27:221–232
Palaniswamy P, Seabrook WD (1978) Behavioral responses of the female eastern spruce budworm Choristoneura fumiferana (Lepidoptera, Tortricidae) to the sex pheromone of her own species. J Chem Ecol 4:649–655
Palaniswamy P, Seabrook WD (1985) The alteration of calling behaviour by female Choristoneura fumiferana when exposed to synthetic sex pheromone. Entomol Exp Appl 37:13–16
Picimbon J-F, Gadenne C (2002) Evolution of noctuid pheromone binding proteins: identification of PBP in the black cutworm moth, Agrotis ipsilon. Insect Biochem Mol Biol 32:839–846
Prestwich GD (1996) Proteins that smell: pheromone recognition and signal transduction. Bioorganic Med Chem 4:505–513
Saad AD, Scott DR (1981) Repellency of pheromones released by females of Heliothis armigera and H. zea to females both species. Entomol Exp Appl 30:123–127
Schneider D, Schulz S, Priesner E, Ziesmann J, Francke W (1998) Autodetection and chemistry of female and male pheromone in both sexes of the tiger moth Panaxia quadripunctaria. J Comp Physiol A 182:153–161
Seabrook WD, Linn CE, Dyer LJ, Shorey HH (1987) Comparison of electroantennograms from female and male cabbage looper moths (Trichoplusia ni) of different ages and for various pheromone concentrations. J Chem Ecol 13:1443–1453
Stelinski LL, Il’Ichev AL, Gut LJ (2006) Antennal and behavioral responses of virgin and mated oriental fruit moth (Lepidoptera: Tortricidae) females to their sex pheromone. Ann Entomol Soc Am 99:898–904
Wakamura S, Takai M, Kozai S, Inoue H, Yamashita I, Kawahara S, Kawamura M (1989) Control of the beet army worm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) using synthetic sex pheromone. I. Effect of communication disruption in Welsh onion fields. Appl Entomol Zool 24:387–397
Xiu W-M, Dong S-L (2007) Molecular characterization of two pheromone binding proteins and quantitative analysis of their expression in the beet armyworm, Spodoptera exigua (Hübner). J Chem Ecol 33:947–961
Yoshiyasu Y, Yamagishi M, Katayama J (1995) Control of the beet army worm Spodoptera exigua (Hübner), on Welsh onion by synthetic sex pheromone in Yodo district Kyoto. Scientific reports of the Kyoto Prefectural University 47:1–8
Acknowledgments
Authors thank Professor Gregg Henderson (Louisiana State University, USA) for comments on and language polishing with this manuscript; and Professor Jia-Wei Du (Shanghai Institute of Plant Physiology and Ecology, CAAS, China) for providing the sex pheromone chemicals. The research is supported by funds from the National Natural Science Foundation of China (grant number: 30571220) and Jiangsu Province Natural Science Foundation (grant number: BK2004098).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, MW., Dong, SL. & Chen, L. Electrophysiological and Behavioral Responses of Female Beet Armyworm Spodoptera exigua (Hübner) to the Conspecific Female Sex Pheromone. J Insect Behav 22, 153–164 (2009). https://doi.org/10.1007/s10905-008-9162-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-008-9162-z