Abstract
Increasing evidence indicates that volatile compounds emitted by bacteria can influence the growth of other organisms. In this study, the volatiles produced by three different strains of Burkholderia ambifaria were analysed and their effects on the growth of plants and fungi, as well as on the antibiotic resistance of target bacteria, were assessed. Burkholderia ambifaria emitted highly bioactive volatiles independently of the strain origin (clinical environment, rhizosphere of pea, roots of maize). These volatile blends induced significant biomass increase in the model plant Arabidopsis thaliana as well as growth inhibition of two phytopathogenic fungi (Rhizoctonia solani and Alternaria alternata). In Escherichia coli exposed to the volatiles of B. ambifaria, resistance to the aminoglycoside antibiotics gentamicin and kanamycin was found to be increased. The volatile blends of the three strains were similar, and dimethyl disulfide was the most abundant compound. Sulfur compounds, ketones, and aromatic compounds were major groups in all three volatile profiles. When applied as pure substance, dimethyl disulfide led to increased plant biomass, as did acetophenone and 3-hexanone. Significant fungal growth reduction was observed with high concentrations of dimethyl di- and trisulfide, 4-octanone, S-methyl methanethiosulphonate, 1-phenylpropan-1-one, and 2-undecanone, while dimethyl trisulfide, 1-methylthio-3-pentanone, and o-aminoacetophenone increased resistance of E. coli to aminoglycosides. Comparison of the volatile profile produced by an engineered mutant impaired in quorum-sensing (QS) signalling with the corresponding wild-type led to the conclusion that QS is not involved in the regulation of volatile production in B. ambifaria LMG strain 19182.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade, the importance of volatile organic compounds in mediating interactions between bacteria and other organisms has become increasingly evident, although the vast majority of the studies published so far have dealt with bacteria growing in controlled laboratory conditions rather than in their natural environment. Bacteria produce a wide variety of volatile compounds (reviewed in Schulz and Dickschat 2007) depending on their taxonomic identity, their growth stage, as well as on the culture media they are grown in. Studies investigating the effects of bacterial volatiles on plants have reported significant plant growth promotion (Han et al. 2006; Ryu et al. 2003) or in contrast plant killing (Blom et al. 2011b; Kai and Piechulla 2010; Vespermann et al. 2007) upon exposure to volatiles from different bacteria. It was later shown that alteration of plant growth by volatiles from rhizosphere bacteria is a widespread phenomenon, which strongly depends on culture medium, as well as on inoculation doses (Blom et al. 2011a). Few candidate molecules have been suggested to account for the plant growth promoting effects of volatiles, such as 2,3-butanediol (Han et al. 2006; Ryu et al. 2003), dimethylhexadecylamine (Velazquez-Becerra et al. 2011), 2-pentylfuran (Zou et al. 2010), or indole (Blom et al. 2011a), while ammonia, dimethyl disulfide (Kai and Piechulla 2010) and hydrogen cyanide (HCN) (Blom et al. 2011b) seem to account, at least partly, for the observed volatile-mediated plant killing.
In addition to their influence on plant growth and development, bacterial volatiles also have been shown to reduce the growth of fungi. The best known example of such volatile-mediated fungal inhibition is the production of HCN by some Pseudomonas species such as P. fluorescens CHA0, whose direct involvement in biocontrol of Thielaviospis-induced root rot of tobacco has been demonstrated (Voisard et al. 1989). In the last decade, many reports, mostly studying Bacillus strains, have confirmed these early findings (for recent reviews, see (Campos et al. 2010; Effmert et al. 2012). In addition to HCN, dimethyl di- and trisulfide (Fernando et al. 2005; Kai et al. 2010; Li et al. 2010; Wang et al. 2013) as well as benzothiazole (Fernando et al. 2005; Zhao et al. 2011; Zou et al. 2010) have been shown to cause fungal growth inhibition, suggesting that they might partially explain the overall antifungal effect of specific bacterial strains, although the concentrations used in some of these studies were extremely high and far away from the ranges in which those volatiles would occur in nature. More recently, bacteria themselves have been reported to be influenced by volatile compounds from physically separated cells from the same, or from a different bacterial species. While it is at present still unknown whether bacterial growth would be altered upon exposure to volatiles, independent reports indicate that antibiotic resistance might be increased by exposure to volatile compounds such as indole (Lee et al. 2010) or hydrogen sulfide (Shatalin et al. 2011). Finally, quorum-sensing (QS) [a population-density dependent regulation mechanism in bacteria, (Whitehead et al. 2001)] also has been suggested to be impaired by volatile exposure in Pseudomonas or Chromobacterium strains (Chernin et al. 2011).
Recent studies have demonstrated that the volatiles produced by closely related strains can be very different (Blom et al. 2011a, b; Nawrath et al. 2012). However, whether the isolation origin of a given strain, and hence the lifestyle of the strain prior to isolation, influences the spectrum of volatiles produced, is still an open question. In this study, we address this question by comparing three Burkholderia ambifaria strains. The genus Burkholderia contains highly versatile strains adapted to clinical as well as to natural environments (Mahenthiralingam et al. 2005), and members of this genus have been shown previously to produce highly bioactive volatiles (Blom et al. 2011b; Weisskopf and Bailly 2013). Burkholderia ambifaria was selected as the model organism to test whether the strain’s origin has an effect on volatile production, taking advantage of the breadth of niches it can adapt to and of its available genome sequence (B. ambifaria LMG 19182, NC 008390–008392, NC 008385). Three types of environments were chosen to compare strains from: i) the roots of maize (LMG 17828), ii) the rhizosphere of pea (LMG 19182), and iii) the lungs of a cystic fibrosis patient (LMG 19467). We compared the composition of the volatiles emitted by B. ambifaria strains from the three different niches and determined their bioactivity using relevant bioassays that reflect important biological features in the respective original strains’ environments, such as promotion of plant growth, inhibition of fungal growth, or modulation of antibiotic resistance in bacteria. Moreover, since many Gram-negative bacteria are known to regulate a variety of virulence-related traits by QS, e.g., the emission of non-volatile antifungal molecules, we hypothesized that production of volatile compounds also would be under QS control in B. ambifaria. To address this question, a QS deficient mutant was constructed in the rhizosphere strain B. ambifaria LMG 19182 and its volatile emission profile and its volatile-mediated impact on plants, fungi, and other bacteria were compared with the wild-type strain.
Methods and Material
Organisms and Culture Conditions
Bacterial strains used in this study were Burkholderia ambifaria LMG 17828, isolated from maize roots; B. ambifaria LMG 19182, isolated from pea rhizosphere; B. ambifaria LMG 19467, isolated from a cystic fibrosis patient; B. ambifaria LMG 19182 delta bafI (bafI deletion mutant); E. coli DH5α. Fungal strains used were Alternaria alternata, Fusarium solani, and Rhizoctonia solani Kühn strain 160, all obtained from the culture collection of the Phytopathology group of the Institute of Plant Sciences (Federal Institute of Technology, Zurich, Switzerland). For all plant experiments, Arabidopsis thaliana Col-0 was used. Bacterial strains were routinely grown on Luria-Bertani (LB) medium (Difco), 1.6 % agar, fungal strains on 1.5 % Malt Extract agar (Difco) supplemented with 1.2 % agar, and plants on half-strength Murashige and Skoog (MS) Basal Medium Sigma Aldrich, 1.5 % sucrose, 0.8 % agar, pH 5.7. For antibiotic resistance assays, E. coli was grown on Mueller-Hinton medium (Difco), 1.7 % agar. Unless otherwise specified, Burkholderia strains were incubated at 30 °C, E. coli at 37 °C, fungal strains at room temperature, and plants at 20 °C in a growth chamber with 50 % relative humidity, 12 hr:12 hr day/night period, and a light intensity of approximately 100 μE. Arabidopsis seeds were sterilized as described earlier (Blom et al. 2011a).
Analysis of Headspace Volatiles from B. ambifaria Strains
B. ambifaria LMG 17828, LMG 19182, and LMG 19467 as well as uninoculated medium as control were cultivated at 30 °C in 6 ml LB liquid media for 24 hr. LB medium 1.6 % agar plates were inoculated with 300 μl of the preculture, cultivated for 24 hr at 30 °C, and then analyzed by closed-loop stripping analysis (CLSA) at room temperature as described by (Schulz et al. 2004). In this system, air is continuously pumped (MB-21E, Senior Flextronics, USA) through the closed system that contains an activated charcoal filter (Chromtech GmbH, Idstein, Precision Charcoal Filter, 5 mg) and the agar plate for 24 hr. Trapped volatiles were extracted from the charcoal filter by rinsing the filter × 3 with 15 μl dichloromethane (≥ 99.8 %, Merck, Germany). The headspace extracts subsequently were analyzed by GC/MS. Each experiment was repeated three times. Media were analyzed without inoculation as control.
GC/MS Analyses
GC/MS analyses were performed on an HP7890A GC connected to an HP5975C mass selective detector fitted with an HP-5 ms fused silica capillary column (30 m, 0.22 mm i.d. 0.25 μm film, Agilent Technologies, USA). Conditions were as follows: inlet pressure 67 kPa, He 23.3 ml/min, injection volume 1 μl, transfer line 300 °C, injector 250 °C, electron energy 70 eV. The gas chromatograph was programmed as follows: 5 min at 50 °C, then increasing with 5 °C/min to 320 °C. Linear retention indices were determined from a homologous series of n-alkanes (C8-C32). Compounds were identified by comparison of mass spectra to database spectra (Wiley 7, NIST 08 and our own created from synthesized reference compounds), by comparison of the retention index data to standards [(own database and NIST Chemistry WebBook (2013)] and by synthesis of a reference compound.
Chemical Synthesis
Chemicals and solvents were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany) and ABCR (Karlsruhe, Germany) and were used without further purification. Solvents were distilled before use and, if necessary, dried after standard procedures. Purification of synthetic products was carried out by flash chromatography using Merck silica gel 60 (70–200 mesh). Thin-layer chromatography was performed with 0.2 mm pre-coated polyester sheets (Polygram SIL (G/UV254), Macherey-Nagel). NMR spectra were recorded on Bruker DRX-400 (400 MHz) or AV III-400 (400 MHz) spectrometers. 1-Methylthio-3-pentanone (20) was synthesized from commercially available 3-methylthiopropionyl chloride as reported by Ishibashi and colleagues, who described a similar reaction using 2-methylthioacetyl chloride as starting material (Ishibashi et al. 1989). A diethylaluminium chloride solution (2.7 ml, 4.8 mmol, 1.2 eq., of a 1.8 M solution in toluene) was added to 3-(methylthio)propionyl chloride (555 mg, 4 mmol, 1 eq.) in dry dichloromethane (10 ml) at −20 °C and stirred for 2 hr. The reaction mixture was poured carefully into water, and the organic layer was separated. The aqueous layer was extracted with dichloromethane, and the combined organic phases were dried with MgSO4. The solvent was removed, and the residue was purified by column chromatography on silica gel with pentane/diethylether (ratio 5:1) to give 407 mg (3.08 mmol) of the desired product (yield: 77 %). 1H NMR (400 MHz, CDCl3) δ (ppm): 1.07 (t, J = 7.33 Hz, 3 H), 2.10–2.14 (m, 3 H), 2.46 (q, J = 7.24 Hz, 2 H), 2.71–2.75 (m, 3 H); 13C NMR (101 MHz, CDCl3) δ (ppm): 7.56, 15.64, 27.93, 36.05, 41.84, 209.48; EIMS m/z: 132 [M+] (100), 57 (99), 75 (87), 61 (83), 85 (48), 103 (39), 47 (33, 45 (26), 41 (25), 55 (18); Rf (pentane/diethylether 5:1) 0.27; GC (HP-5MS) I = 1089. The other synthetic reference compounds were obtained either from commercial sources or from our in-house compound collection.
Bioassays Testing the Volatile-Mediated Effect of B. ambifaria or of Pure Compounds on Target Organisms
Effects on Plants: In order to test the effects of bacterial volatiles on plant growth and development, a set-up with square Petri dishes (120 × 120 cm, Greiner Bio-One) containing an autoclaved stainless steel ring were used. A steel ring was placed at the bottom of the plate, creating a compartment that then was filled with LB medium (see Fig. S3 for details). The surrounding was filled with solid ½ MS. A 20 μl drop of a bacterial overnight culture at OD600 of 1 was inoculated in the ring, and 15 sterile Arabidopsis thaliana seeds were placed at the top of the plate. The plates were sealed with Parafilm M, and the plants grown for 3 wk. After 3 wk, biomass was measured, and statistical analysis was performed using GraphPad Prism 5. Two independent experiments consisting each of 3 technical replicates (45 seedlings) were performed with similar results. Changes in the root architecture, such as root length and lateral root formation, were determined using the NIH ImageJ software (http://rsbweb.nih.gov/ij/).
Effects on Fungi: To test the effects of bacterial volatiles on fungal growth, two compartment Petri dishes (Greiner Bio-One) were used. On one half, Malt Agar was poured. On the other half, LB medium was poured, and 20 μl of an overnight culture of bacterial strains were spread evenly on the half plate surface. Plates were grown for 24 hr at 30 °C to allow bacteria to grow and produce volatiles. The following day, a 0.5 cm plug of either Rhizoctonia solani or Alternaria alternata was inoculated on the Malt Agar side. Plates were stored at room temperature in the dark for 3–5 d, depending on the growth speed of the fungus. Determination of inhibition was performed using NIH ImageJ for diameter measurements, followed by statistical analysis using GraphPad Prism 5. Three independent experiments consisting of 3 technical replicates were performed with similar results.
Effects on Bacteria: To test the effect of B. ambifaria volatiles on antibiotic resistance in target bacteria, two compartment Petri dishes were used. First, 10 ml of either LB (emitter) or Mueller-Hinton medium (receiver) were poured into each half. Thereafter, 100 μl of an overnight culture at OD600 of 1 of each emitter were mixed with 5 ml of LB half agar medium and poured onto the first layer of LB in the Petri dish. Control plates received 5 ml LB without bacterial cells. Plates were incubated for 24 hr at 30 °C prior to inoculating the receiver, to allow growth of the emitters and production of volatiles. The following day, 100 μl of an overnight culture at OD600 of 1 of the receiver were mixed with 5 ml of Mueller-Hinton half agar medium and poured onto the first layer of Mueller-Hinton in the Petri dish. After allowing to dry for a few minutes, a filter disc (SirSCan disc) containing an antibiotic (Gentamicin 10 μg, Kanamycin 30 μg, Tetracyclin 30 μg, or Ampicillin 10 μg) was placed in the middle of the half plate containing the receiver strain. Plates then were incubated at 37 °C for 1 d. The inhibition zone was evaluated using NIH ImageJ followed by statistical analysis using GraphPad Prism 5. Two independent experiments consisting each of 3 technical replicates were performed with similar results.
To test the effects of volatiles applied as pure compounds on plants, fungi, or bacteria, similar experimental setups as described above were used. To assess plant growth, 5 seedlings were grown for 5 d on solid ½ MS on one side of two-compartment Petri dishes before treatment (Fig. S3). After 3 wk, biomass was measured and statistical analysis was performed using GraphPad Prism 5. Each treatment consisted of 5 technical replicates. Instead of using bacterial overnight cultures, defined quantities of compounds to be tested, or of solvent control dichloromethane, were mixed with a solution of dichloromethane and lanolin and applied on filter papers as described previously (Blom et al. 2011a) in the empty Petri dish compartment.
Construction of a Quorum-Sensing Mutant in B. ambifaria LMG 19182
See the supplementary material for a detailed protocol. Briefly, the bafI gene was amplified from genomic DNA extracted from B. ambifaria LMG 19182 by PCR, followed by cloning into pBluescript cloning vector. This vector then was transferred into E. coli Top10 competent cells for multiplication. A trimethoprim cassette was inserted into the gene, and the construct was cloned into pShaft2 suicidal vector (containing a chloramphenicol resistance cassette). This vector thereafter was transferred into E.coli pirλ competent cells for multiplication. Triparental mating was performed to introduce the plasmid into Burkholderia ambifaria. Transformants were selected on Pseudomonas Isolation Agar (Difco) containing trimethoprim. Those transformants showing resistance to trimethoprim but sensitivity to chloramphenicol were regarded as double cross-over mutants.
Analysis of AHL Production
In order to test for AHL production in Burkholderia ambifaria wild-type strains and B. ambifaria bafI mutant different sensor strains were used. A cross-streak method was used in which an AHL sensor strain (unable to produce AHLs but responding to them by producing GPF, violacein or bioluminescence) is streaked in a line on LB medium and orthogonal to it the strains to be tested. If AHLs are produced, they diffuse into the agar and activate the sensor. The plates were sealed with Parafilm M and incubated for 24 hr at 30 °C. The sensors used were Chromobacterium violaceum CV026, E. coli MT102 (pSB403), and Pseudomonas putida F117 harboring either plasmid pKR-C12 (highest sensitivity for AHLs with a C10-C14 acyl side chain) or pAS-C8 (highest sensitivity for AHLs with a C8 acyl side chain). The specificities of these biosensors allow the detection of a wide range of different AHL molecules.
Results
Burkholderia ambifaria Strains Produce Highly Bioactive Volatiles Independently of Their Origin
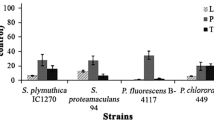
To test whether the origin of isolation of a given bacterial strain would influence its volatile-mediated effect on other organisms, three Burkholderia strains originating from i) maize roots, ii) pea rhizosphere, and iii) clinical environment were compared. Their volatile-mediated effects on three types of target organisms, namely plants, fungi, and bacteria were quantified. Strong effects were observed for all three organisms (Figs. 1, 2, and 3), yet no significant difference could be found between the different strains. When grown in the presence of B. ambifaria volatiles, Arabidopsis plants produced more biomass and more secondary roots, while the growth of the main root was reduced (Fig. 1). The growth of phytopathogenic fungi was significantly decreased upon exposure to B. ambifaria volatiles, whereby growth inhibition was much higher for Alternaria alternata than for Rhizoctonia solani (Fig. 2). For volatile-mediated effects of B. ambifaria on other bacteria, antibiotic resistance was used as a proxy. Exposing E. coli to the volatiles of the three B. ambifaria strains led to enhanced antibiotic resistance against the aminoglycosides gentamicin and kanamycin but not against ampicillin or tetracycline (Fig. 3). In all the above-mentioned bioassays, no significant difference could be observed among the different B. ambifaria strains, suggesting that whether a strain originates from the clinical or the plant environment does not affect its volatile emission.
Phenotypical response to bacterial volatile emission. a A stainless steel ring secludes the bacterial compartment from plants. Bar = 1 cm. b Plant parameters after constant exposure to the bacterial VOCs. Results shown are expressed as means ± s.e.m and are representative of biological duplicates. Letters indicate statistical significance according to one-way ANOVA followed by Tukey’s post-hoc test (N = 45 P < 0.001)
The volatile organic compounds emitted by Burkholderia ambifaria inhibit fungal growth independently from strain origin. Results shown are expressed as means ± s.e.m and are representative of biological triplicates. Letters indicate statistical significance according to one-way ANOVA followed by Tukey’s post-hoc test (N = 3 P < 0.001)
The volatile organic compounds emitted by Burkholderia ambifaria promote antibiotic resistance in E. coli independently from strain origin. a overview of the experimental setup used in this study. The VOCs emitting and receiving bacterial strains were plated in separate compartments of the Petri dish. b Resistance to antibiotics was determined as the area clear of bacterial growth surrounding the antibiotic disc. Results shown are expressed as means ± s.e.m and are representative of biological duplicates. Letters indicate statistical significance according to one-way ANOVA followed by Tukey’s post-hoc test (N = 3 P < 0.001)
Burkholderia ambifaria Strains from Different Origins Emit Similar Volatile Profiles
The volatiles released by the three strains were investigated by analyzing the compounds released into headspace by the use of CLSA and GC/MS to identify the compounds responsible for the observed bioactivity of B. ambifaria. The three B. ambifaria strains isolated from different environments showed similar volatile profiles (see Fig. 4 and Table 1), dominated by the same compound classes, sulfur compounds, ketones, and aromatic compounds. Overall, 40 different compounds were identified (see Fig. S1). The most abundant compound in all strains was dimethyl disulfide (1). Beside compound 1, several other sulfur-containing compounds that included dimethyl trisulfide (11), the methylthioketones 4-methylthio-2-butanone (13) and 1-methylthio-3-pentanone (20), as well as S-methyl methanethiosulphonate (17) and methyl methylthiomethyl disulfide (22) were present. These compounds are responsible for the characteristic smell of B. ambifaria. Furthermore, a plethora of ketones was produced by the strains. Several methylketones like 2-nonanone (21) and 2-undecanone (28), produced by all of the strains, were identified. In contrast, 2-heptanone (7), 2-dodecanone (31), and 2-pentadecanone (38) were found only in the rhizosphere strain (B. ambifaria LMG 19182). All strains produced longer mono-unsaturated methylketones such as tetradec-8-en-2-one (36). The double bond position was determined by addition of dimethyl disulfide and GC/MS analysis as described in Dickschat et al. (2005a). Further unsaturated methylketones with a chain length between C12 and C15 were compounds 30, 34, 37, and 39, but their concentration was too low to allow determination of the double bond position. Their identification was determined by their characteristic mass spectra showing typical ions of methyl ketones, m/z 43 and 58, together with other indicative spectral features. In addition, ethyl- and propylketones occurred as well: 3-hexanone (3), 3-decanone (36), 4-heptanone (5), and 4-octanone (12), accompanied by the corresponding alcohol of 5, 4-heptanol (6), in the clinical strain (B. ambifaria LMG 19467). All strains also produced ω-1 methyl-branched ketones. 4-Methyl-2-pentanone (2) was produced by all strains, in contrast to 5-methyl-3-hexanone (4), which was found only in the clinical strain. A lactone found in all strains was 3-methyl-4-butanolide (10). Another highly represented group of compounds were the nitrogen-containing alkylpyrazines 8, 14, 15, and 19, which represent one major group of bacterial volatiles (Schulz and Dickschat 2007). Ketones occurred also as a key structural component of several released aromatic compounds. All strains produced acetophenone (18), 1-phenyl-1,2-propanedione (25), and 2-acetylfuran (9). In the clinical strain (B. ambifaria LMG 19467), the nitrogen-containing aromatic ketones o-aminoacetophenone (29) and o-aminopropiophenone (32) occurred. This strain produced more aromatic compounds such as 1-phenylethanol (16), 1-phenylpropanol (23), and 1-phenyl-1-propanone (24). The terpenes isobornyl acetate (27), geranylacetone (33), and farnesylacetone (40) also were detected; however, as these compounds have been repeatedly detected in non-inoculated culture media, they are likely artefacts. The amount of volatiles produced can be estimated roughly by the detection limit of our system. Depending on the compound, between 0.1 and 1 ng present in the extracts can be detected. The amount of the compounds produced, therefore, was in the range between 0.1 and 100 ng with the exception of dimethyl disulfide, which was considerably more abundant. Nevertheless, it is important to remember that the recovery rate of the system for the compounds is unknown, because different adsorption coefficients on the adsorbent, glass, or medium exist. Moreover, the production rates of the compounds under natural conditions also is unknown.
Total ion chromatograms of the headspace extracts from strains originating from a the roots (Burkholderia ambifaria LMG17828), b the rhizosphere (B. ambifaria LMG 19182) and c the clinical environment (B. ambifaria LMG 19467). Numbers refer to Table 1. Artefacts occurring in the medium are marked with an asterisk
Production of Bioactive Volatiles is not Regulated by QS in the Rhizosphere Isolate B. ambifaria LMG 19182
To investigate whether emission of bioactive volatiles could be regulated by QS in B. ambifaria, an antibiotic resistance cassette was introduced into the acyl homoserine lactone (AHL) synthase gene bafI, which resulted in loss of AHL production (Fig. S2). The bafI mutant was tested for volatile-mediated effects on plants, fungi, and bacteria. No significant difference was observed between the AHL-deficient mutant and its corresponding wild-type strain when tested for effects on fungi and bacteria (Fig. 5). However, slight changes in the induced plant phenotypes were observed, with a significantly longer main root growth, and a tendency towards more secondary roots and higher biomass for the bafI mutant relative to the wild-type (Fig. 5). However, the volatile profiles from both strains were very similar (Fig. 5d), suggesting that quorum-sensing plays, if at all, only a minor role in the production of volatiles.
Impact of the VOCs emitted by the rhizosphere strain (Burkholderia ambifaria LMG 19182) and its bafI mutant on a Arabidopsis growth, b fungal growth, and c E. coli antibiotic resistance. d Total ion chromatograms of the headspace extracts from the rhizosphere strain (B. ambifaria LMG 19182) and its bafl mutant. Results shown are expressed as means ± s.e.m and are representative of biological duplicates. Letters indicate statistical significance according to one-way ANOVA followed by Tukey’s post-hoc test (a, N = 45 P < 0.001; b, N = 3 P < 0.001; c, N = 3 P < 0.001)
Exposure of Target Organisms to Single Volatiles Partially Mimics the Effects Caused by the Complex Blend
In order to identify the active molecules within the complex blends of emitted volatiles, a subset of the identified compounds (Table 1) was tested in three concentrations on the three different target organisms. Although none of the compounds used as pure substance could elicit the same effects caused by the complex blends, some of them showed significant positive or negative effects on plant, fungal growth or antibiotic resistance (Table 2). Plants produced more biomass when exposed to all three concentrations of dimethyl disulfide (1) and acetophenone (18), as well as to given concentrations of 2,5-dimethylpyrazine (8), 3-hexanone (3), and o-aminoacetophenone (29). In contrast, the thioketones 1-methylthio-3-pentanone (20) and 4-methylthio-2-butanone (13) induced reduction of biomass in all concentrations. High concentrations of 4-methyl-2-pentanone (2) and 4-octanone (12) also were deleterious to the plants. Notably, none of the growth-inhibiting compounds induced necrosis or chlorosis, suggesting absence of toxicity even for growth-inhibiting volatiles. Fungi reacted differentially to exposure to pure volatiles. Alternaria alternata, which showed the strongest growth inhibition upon exposure to the volatile blends from any of the B. ambifaria strains (Fig. 2), was less susceptible to pure compounds than R. solani. High concentrations of dimethyl trisulfide (11), 2-nonanone (21), 1-phenyl-1,2-propanedione (25), and of 2-undecanone (28) reduced the growth of both fungi, while R. solani was additionally inhibited by dimethyl disulfide (1), 4-octanone (12), S-methyl methanthiosulphonate (17), acetophenone (18), and phenylpropan-1-one (24). None of the volatiles tested exhibited any activity on the growth of Fusarium solani, suggesting specificity of the fungal response to bacterial volatiles. Resistance to kanamycin and/or gentamicin was significantly increased upon exposure to 1-methylthio-3-pentanone (20), as well as to o-aminoacetophenone (29). Interestingly, dimethyl disulfide (1) increased susceptibility to ampicillin in E. coli.
Discussion
Over the last decade, bacterial volatile emissions and their possible practical applications have received increasing attention. For instance, the possibility or using the smell of bacteria for diagnostic purposes with so-called electronic noses seems promising (Chambers et al. 2012; Pavlou and Turner 2000). In a study comparing a Mycobacterium tuberculosis strain with various other Mycobacterium sp. strains, the pathogen could be discriminated clearly from its close relatives by different volatile emission profiles (Nawrath et al. 2012). Other studies have investigated volatile production by closely or distantly related bacterial species, and, as expected, more closely related bacteria produced more similar blends of volatiles than distantly related ones (Kai et al. 2008; Ryu et al. 2003). However, whether strains of one species with a different lifestyle history would emit the same volatile bouquets or whether differentiation according to their environmental niches would be feasible has not been investigated. This question was assessed in the present study by using B. ambifaria as a model species. It was shown to act as a plant-growth promoting rhizobacterium in maize, to be a potent biocontrol agent against fungi (Coenye et al. 2001), and it can also infect patients suffering from cystic fibrosis (Ciccillo et al. 2002; Coenye et al. 2001).
When plants, fungi, or bacteria were exposed to the volatiles of either of three strains, effects were strong, as shown in Figs. 1, 2, and 3. However, no influence of the strain’s origin was observed. Plant biomass was increased about 3-fold, while lateral root numbers were significantly higher and main root significantly shorter (Fig. 1). These alterations in root architecture caused by exposure to bacterial volatiles corroborate earlier findings (Bailly and Weisskopf 2012; Gutierrez-Luna et al. 2010), and the extent of biomass promotion is in the range of what has been reported for other Burkholderia species (Blom et al. 2011a). While production of diffusible molecules with potent antifungal properties such as pyrrolnitrin, burkholdines, occidiofungin, or 4-hydroxy-2-alkylquinoline is well-described for Burkholderia ambifaria (Lu et al. 2009; Schmidt et al. 2009; Tawfik et al. 2010), the contribution of volatile compounds to the overall antifungal activity of the organism is largely unknown. When the phytopathogenic fungus A. alternata was exposed to the volatiles of any of the three B. ambifaria strains, growth was almost completely inhibited (Fig. 2). In contrast, R. solani was much less inhibited, possibly due to its fast growth, allowing less time for the accumulation of bacterial volatiles in the headspace of the Petri dish. Finally, when E. coli was exposed to the volatiles of B. ambifaria, antibiotic resistance to gentamicin and kanamycin but not to ampicillin was increased (Fig. 3). Recent studies have reported volatile-mediated increases in antibiotic resistance in bacteria, e.g., through indole emission (Lee et al. 2010), ammonia (Bernier et al. 2011), or hydrogen sulfide (Shatalin et al. 2011), suggesting that this might be a more widespread phenomenon than previously thought. However, the ecological significance of volatile-mediated induced resistance remains to be elucidated. While hydrogen sulfide seemed to trigger broad-range resistance to all kinds of tested antibiotics, probably due to alleviation of oxidative stress, ammonia was shown to specifically induce increased resistance to ampicillin and tetracycline, but not to kanamycin or gentamicin.
We showed that volatiles from any of the B. ambifaria strains tested induced strong phenotypic changes in plants, fungi, and bacteria. This is not surprising given that the volatile profiles of the three closely related strains were similar. We identified components from every substance class in every strain, although some differences were observed. The most interesting substance classes are the sulfur-containing compounds and the different ketones. Most of the volatile compounds produced by B. ambifaria are commonly produced by many unrelated bacteria (Citron et al. 2012; Schulz and Dickschat 2007). The quantitatively most abundant volatile dimethyl disulfide is produced by a large variety of soil and marine bacteria (Schulz and Dickschat 2007). It is an indicator of methyl sulfide production, a compound not detectable by the methods used. Methyl sulfide can dimerize oxidatively in air to form dimethyl disulfide (1) (Chin and Lindsay 1994) or on charcoal, which also leads to formation of the related compounds dimethyl trisulfide (11), S-methyl methanethiosulphonate (17), and methyl methylthiomethyl disulfide (22) (Bashkova et al. 2002). Nevertheless, the frequent detection of dimethyl disulfide also with adsorbents other than charcoal excludes that its occurrence is an analytical artefact (Schulz and Dickschat 2007). The other group of sulfur compounds detected were the β-methylthioketones 4-methylthio-2-butanone (13) and 1-methylthio-3-pentanone (20), likely formed from methionine or by addition of methyl sulfide to α,β-unsaturated ketones. These compounds are well-known natural flavor components, occurring in many food products (Anadon et al. 2010). Ketone 13 has been found in marine bacteria (Dickschat et al. 2005a, b), but to the best of our knowledge, 1-methylthio-3-pentanone (20) has not been reported previously to be produced by a bacterium.
The largest group of compounds detected were ketones, including aliphatic and aromatic ketones, as well as the already mentioned sulfur-containing compounds 13 and 20. This is reminiscent of Cytophaga bacteria that also predominately produce ketones (Dickschat et al. 2005a). A group of small, very volatile ketones was dominated by 4-methyl-2-pentanone (2), known from different bacteria (Schulz and Dickschat 2007). 4-Octanone (12) has been detected previously in Pseudomonas strains (Pittard et al. 1982), but 4-heptanone (7) has not been reported before from bacteria. Longer aliphatic methyl ketones ranging from C12 to C16 also have been detected (30, 34, 36, 37, and 39), sometimes with an additional double bond. They are especially abundant in the rhizosphere strain B. ambifaria LMG 19182. Identical or similar compounds have been identified from artic bacteria of the cytophaga-flavobacterium-bacteroids group (Dickschat et al. 2005a). Aromatic ketones included acetophenone (18), a widespread bacterial volatile and propiophenone (24), as well as their reduction products, the alcohols 16 and 23. o-Aminoacetophenone (29) is a characteristic compound of many Pseudomonas aeruginosa strains (Scott-Thomas et al. 2010), but also occurs in other bacteria (Citron et al. 2012; Schulz and Dickschat 2007). The homolog o-aminopropiophenone (32) has not been described as a bacterial volatile before. Although isobornyl acetate (27), geranylacetone (33) and farnesylacetone (40) were not detected in the non-inoculated medium controls, they have been repeatedly found in different laboratories as components of the medium. Moreover, the absence of other terpenes in the bacterial bouquets suggests that these three compounds originated from the medium rather than from the bacteria. The pyrazine 2,5-dimethylpyrazine (8) also can be formed in the medium, but its relatively high concentration and its absence in the control point to bacterial formation in this case. Bacteria can produce this compound in large amounts, e.g., Corynebacterium glutamicum (Dickschat et al. 2010).
A striking feature of the volatile bouquet of B. ambifaria was the occurrence of homologous pairs of compounds. Certain ketones as 4-methyl-2-pentanone (2), 4-heptanone (5), 4-methylthio-2-butanone (13), acetophenone (18), 1-phenylethanol (16), and o-aminoacetophenone (29) were accompanied by compounds with an additional carbon atom in the chain. These elongated compounds occurred only in the clinical isolate LMG 19467. Their formation may be explained by a common biosynthetic pathway shown in Fig. 6. This model is based on known biosynthetic pathways with a postulated additional final reaction. Independently of the origin of the precursor from the amino acid pool of fatty acid biosynthesis, an enzyme-bound intermediate may be elongated either by a C2 (acetate or malonate) or a C3-unit (propionate or methylmalonate). Final reductive decarboxylation leads to the volatiles. Whether the other strains also are able to produce these compounds is unclear because the overall production rate was relatively low compared e.g., to streptomycetes. Therefore, minor components might be below the detection limit of our method. The overall biosynthetic potential of all three strains was similar. Nevertheless, differences as those discussed above were detected. The strain isolated from maize roots (LMG 17828) produced the least diverse volatile bouquet, while the strain originating form pea rhizosphere (LMG 19182) preferentially produced longer ketones. The clinical strain (LMG 19467) produced the most diverse array of compounds. Interestingly, a similar trend has been observed in Mycobacterium strains, where the volatile profiles from virulent strains are more diverse than those from avirulent strains (Nawrath et al. 2012).
Proposed biosynthetic pathway to homolog pairs of compounds. The final step includes C2 (acetyl) or C3 (propionyl) elongation. The biosynthesis is assumed to be performed on an enzyme bound educt (SEnz, e. g. acyl carrier protein), but it could work also independently of such a protein. Elongation may proceed with malonate and methylmalonate instead of acetate or propionate as indicated in the figure
In Gram-negative bacteria, many of the properties important for the interaction with other organisms (virulence of pathogenic bacteria, rhizosphere competence in plant-growth promoting rhizobacteria) are controlled by quorum-sensing (QS) (Whitehead et al. 2001). So far, little is known about the role of QS in regulating production of volatile compounds, with the exception of hydrogen cyanide, which has been shown to be under QS regulation in Pseudomonas and Chromobacterium species (Blom et al. 2011b; Pessi and Haas 2000; Throup et al. 1995). One study reported that the volatile-mediated antifungal activity of Serratia plymuthica HRO-C48 was increased in a QS-impaired strain, suggesting that in this particular case, QS might negatively regulate the production of antifungal volatiles, or positively regulate the production of fungi-stimulating compounds (Müller et al. 2009). To investigate whether production of bioactive volatiles are controlled by QS in B. ambifaria, we constructed a bafI mutant of the rhizosphere strain B. ambifaria LMG 19182. In this mutant, AHL synthesis was abolished (Fig. S2), yet no significant difference could be observed between wild-type and QS mutant in the various bioassays, involving volatile activity on plants, fungi, or bacteria (Fig. 5a–c). This is not surprising as the volatile profiles of the two strains were very similar (Fig. 5d) This suggests that volatile production is constitutive in B. ambifaria and is not regulated by QS, unlike other important traits pertaining to the interactions with higher organisms, such as the production of diffusible antifungal compounds (Zhou et al. 2003).
When exposing plants, fungi, and bacteria to selected pure volatiles, changes in growth and antibiotic resistance were less marked than when the organisms faced the complex blends, suggesting synergistic effects and/or the need for slow and continuous exposure to the volatiles. Nevertheless, some significant effects were observed, e.g., for dimethyl disulfide (1), the most abundant volatile identified in the bouquet of the three B. ambifaria strains. Dimethyl disulfide induced strong growth promotion in Arabidopsis, whereas fungi reacted variably, with inhibition of R. solani but no effect on A. alternata or F. solani. E. coli was more resistant to gentamicin but less to ampicillin. This sulfur compound previously has been shown to possess antifungal properties (Wang et al. 2013). Moreover, it has been reported to affect plant growth in a concentration-dependent manner: treatment with much higher amounts than described in this study was deleterious to Arabidopsis and tomato, while low dimethyl disulfide amounts promoted tomato growth in field experiments (Faruk et al. 2012; Kai et al. 2010). While the broad antifungal properties of disulfides have been investigated in detail (Baerlocher et al. 1999), S-methyl methanethiosulphonate (17), along with other thiosulphonate fungitoxins, has been shown to be selectively efficient against Aspergillus niger (Baerlocher et al. 2000). Similarly, we report here growth inhibition of R. solani but not of A. alternata upon exposure to 4-octanone (12), S-methyl methanethiosulphonate (17), acetophenone (18), or 1-phenylpropanone (24). 2- Undecanone (28) was the only volatile that reduced the growth of both fungi at intermediate concentrations. This compound previously was reported to modulate the growth of R. solani in a concentration-dependent manner (Weise et al. 2012). Both 4-(methylthio)-2-butanone (13) and 1-(methylthio)-3-pentanone (20) drastically reduced plant growth but mildly impacted fungal growth. Although these compounds were not reported to hold such properties, the acidic form 3-methylthiopropanoic acid produced by Enterobacter intermedium and Xanthomonas campestris was described as fungicidal and phytotoxic (Kim et al. 2003; Noda et al. 1980). Acetophenone (18) and, to a lesser extent, its reduced form 1-phenylethanol (16) promoted plant growth, while 1-phenylpropanone (24) treatment was deleterious. To our knowledge, these substances have not yet been investigated for their impact on plant development. However, acetophenone and acetophenone-based phytoanticipins and phytoalexins (such as acetosyringone) are well-known plant defense compounds (Curir et al. 2000; Grayer and Kokubun 2001). In our study, both acetophenone (18) and 1-phenylpropanone (24) showed antifungal activity against R. solani when applied in the highest amounts. Finally, 4-methyl-2-pentanone (2), best known under the name methyl isobutyl ketone (MIBK), is a common industrial solvent recognized for its toxicity towards aquatic organisms and birds, but chronic or acute effects on plants or terrestrial animals have so far not been reported. In our hands, however, this compound greatly reduced Arabidopsis biomass, while it promoted R. solani growth.
The main aim of this study was to determine whether volatile emission of a bacterial strain would be influenced by its origin of isolation, reflecting its previous lifestyle. Our results indicate that this is not the case in Burkholderia ambifaria. The strains isolated from the roots, the rhizosphere or the clinical environment produced similar blends of volatiles, which resulted in similar, highly significant effects on plant growth, fungal growth, or bacterial antibiotic resistance. In contrast to many other traits relevant to interactions of bacteria with higher organisms, emission of volatiles seems to be a constitutive phenomenon in B. ambifaria, as indicated by similar volatile-mediated activity between the wild-type and its AHL-deficient mutant. Finally, exposure of plants, fungi, and bacteria to single volatiles only partially mimicked the effects of the strains’ complex blends, suggesting that compounds might act synergistically within the mixture, or that the natural dynamics of volatile emission when biologically produced is necessary to cause changes in growth and antibiotic resistance in target organisms.
References
Anadon A, Binderup M-L, Bursch W, Castle L, Crebelli R, Engel K-H, Franz R, Gontard N, Haertle T, Husoy T, Jany K-D, Leclercq C, Lhuguenot JC, Mennes W, Milana MR, Pfaff K, Svensson K, Toldra F, Waring R, Wolfle D, Sundh UB, Beltoft V, Carere A, Frandsen H, Gurtler R, Hill F, Larsen JC, Lund P, Mulder G, Norby K, Pascal G, Pratt I, Speijers G, Wallin H, Nielsen KR (2010) Flavouring group evaluation 8, Revision 2 (FGE.08rev2): aliphatic and alicyclic mono-, di-, tri-, and polysulphides with or without additional oxygenated functional groups from chemical groups 20 and 30. EFSA J 8:1408–1532
Baerlocher FJ, Langler RF, Fredriksen MU, Georges NM, Witherell RD (1999) Structure-activity relationships for selected sulfur-rich antifungal compounds. Aust J Chem 52:167–172
Baerlocher FJ, Baerlocher MO, Langler RF, MacQuarrie SL, Marchand ME (2000) New and more potent antifungal disulfides. Aust J Chem 53:1–5
Bailly A, Weisskopf L (2012) The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal Behav 7:79–85
Bashkova S, Bagreev A, Bandosz TJ (2002) Effect of surface characteristics on adsorption of methyl mercaptan on activated carbons. Ind Eng Chem Res 41:4346–4352
Bernier SP, Letoffe S, Delepierre M, Ghigo JM (2011) Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol Microbiol 81:705–716
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011a) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058
Blom D, Fabbri C, Eberl L, Weisskopf L (2011b) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77:1000–1008
Campos VP, de Pinho RSC, Freire ES (2010) Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Cienc Agrotec 34:525–535
Chambers ST, Scott-Thomas A, Epton M (2012) Developments in novel breath tests for bacterial and fungal pulmonary infection. Curr Opin Pulm Med 18:228–232
Chernin L, Toklikishvili N, Ovadis M, Kim S, Ben-Ari J, Khmel I, Vainstein A (2011) Quorum-sensing quenching by rhizobacterial volatiles. Environ Microbiol Rep 3:698–704
Chin HW, Lindsay RC (1994) Ascorbate and transition-metal mediation of methanethiol oxidation to dimethyl disulfide and dimethyl trisulfide. Food Chem 49:387–392
Ciccillo F, Fiore A, Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L (2002) Effects of two different application methods of Burkholderia ambifaria MCI 7 on plant growth and rhizospheric bacterial diversity. Environ Microbiol 4:238–245
Citron CA, Rabe P, Dickschat JS (2012) The scent of bacteria: headspace analysis for the discovery of natural products. J Nat Prod 75:1765–1776
Coenye T, Mahenthiralingam E, Henry D, LiPuma JJ, Laevens S, Gillis M, Speert DP, Vandamme P (2001) Burkholderia ambifaria sp nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. Int J Syst Evol Microbiol 51:1481–1490
Curir P, Danieli B, Dolci M, Pasini C, Guglieri L, Sacco M (2000) Reductive detoxification of the acetophenone skeleton of the carnation phytoanticipin by Fusarium oxysporum f.sp dianthi. Plant Pathol 49:742–747
Dickschat JS, Helmke E, Schulz S (2005a) Volatile organic compounds from arctic bacteria of the Cytophaga-Flavobacterium-Bacteroides group: a retrobiosynthetic approach in chemotaxonomic investigations. Chem Biodivers 2:318–353
Dickschat JS, Wagner-Dobler I, Schulz S (2005b) The chafer pheromone buibuilactone and ant pyrazines are also produced by marine bacteria. J Chem Ecol 31:925–947
Dickschat JS, Wickel S, Bolten CJ, Nawrath T, Schulz S, Wittmann C (2010) Pyrazine biosynthesis in Corynebacterium glutamicum. Eur J Org Chem 2010:2687–2695
Effmert U, Kalderas J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38:665–703
Faruk M, Rahman M, Mustafa M, Coosemans IJ (2012) Dimethyl disulfide- A potential biopesticide against root-knot nematode of tomato (Lycopersicon Esculentum L.). Bangladesh J Agric Res 36:685–695
Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Grayer RJ, Kokubun T (2001) Plant-fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 56:253–263
Gutierrez-Luna FM, Lopez-Bucio J, Altamirano-Hernandez J, Valencia-Cantero E, de la Cruz HR, Macias-Rodriguez L (2010) Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 51:75–83
Han SH, Lee SJ, Moon JH, Park KH, Yang KY, Cho BH, Kim KY, Kim YW, Lee MC, Anderson AJ, Kim YC (2006) GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant Microbe Interact 19:924–930
Ishibashi H, Takamuro I, Mizukami Y-I, Irie M, Ikeda M (1989) A convenient general access to α-sulfenylated acetophenones and alkanones. Synth Commun 19:443–452
Kai M, Piechulla B (2010) Impact of volatiles of the rhizobacteria Serratia odorifera on the moss Physcomitrella patens. Plant Signal Behav 5
Kai M, Vespermann A, Piechulla B (2008) The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav 3:482–484
Kai M, Crespo E, Cristescu SM, Harren FJM, Francke W, Piechulla B (2010) Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl Microbiol Biotechnol 88:965–976
Kim YC, Kim HJ, Park KH, Cho JY, Kim KY, Cho BK (2003) 3-methylthiopropanoic acid produced by Enterobacter intermedium 60-2G inhibits fungal growth and weed seedling development. J Antibiot 56:177–180
Lee HH, Molla MN, Cantor CR, Collins JJ (2010) Bacterial charity work leads to population-wide resistance. Nature 467:82–85
Li QL, Ning P, Zheng L, Huang JB, Li GQ, Hsiang T (2010) Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol Technol 58:157–165
Lu SE, Novak J, Austin FW, Gu GY, Ellis D, Kirk M, Wilson-Stanford S, Tonelli M, Smith L (2009) Occidiofungin, a unique antifungal glycopeptide produced by a strain of Burkholderia contaminans. Biochemistry-Us 48:8312–8321
Mahenthiralingam E, Urban TA, Goldberg JB (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156
Müller H, Westendorf C, Leitner E, Chernin L, Riedel K, Schmidt S, Eberl L, Berg G (2009) Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microbiol Ecol 67:468–478
Nawrath T, Mgode GF, Weetjens B, Kaufmann SHE, Schulz S (2012) The volatiles of pathogenic and nonpathogenic mycobacteria and related bacteria. Beilstein J Org Chem 8:290–299
Noda T, Sato Z, Kobayashi H, Iwasaki S, Okuda S (1980) Isolation and structural elucidation of phytotoxic substances [for rice plant, Oryza sativa] produced by Xanthomonas campestris pv. oryzae (Ishiyama) Dye. Ann Phytopathol Soc Jpn 46:663–666
Pavlou AK, Turner APF (2000) Sniffing out the truth: clinical diagnosis using the electronic nose. Clin Chem Lab Med 38:99–112
Pessi G, Haas D (2000) Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol 182:6940–6949
Pittard BT, Later DW, Lee ML, Freeman LR (1982) Identification of volatile organic-compounds produced by fluorescent pseudomonads on chicken breast muscle. Appl Environ Microbiol 43:1504–1506
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932
Schmidt S, Blom JF, Pernthaler J, Berg G, Baldwin A, Mahenthiralingam E, Eberl L (2009) Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ Microbiol 11:1422–1437
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Prod Rep 24:814–842
Schulz S, Fuhlendorff J, Reichenbach H (2004) Identification and synthesis of volatiles released by the myxobacterium Chondromyces crocatus. Tetrahedron 60:3863–3872
Scott-Thomas AJ, Syhre M, Pattemore PK, Epton M, Laing R, Pearson J, Chambers ST (2010) 2-Aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med 10
Shatalin K, Shatalina E, Mironov A, Nudler E (2011) H2S: a universal defense against antibiotics in bacteria. Science 334:986–990
Tawfik KA, Jeffs P, Bray B, Dubay G, Falkinham JO, Mesbah M, Youssef D, Khalifa S, Schmidt EW (2010) Burkholdines 1097 and 1229, potent antifungal peptides from Burkholderia ambifaria 2.2N. Org Lett 12:664–666
Throup J, Winson MK, Bainton NJ, Bycroft BW, Williams P, Stewart GSAB (1995) Signalling in bacteria beyond luminescence. In: Campbell AK, Kricka LJ, Standley PE (eds) Bioluminescence and chemiluminescence: fundamental and applied aspects. Wiley, Chichester, pp 89–92
Velazquez-Becerra C, Macias-Rodriguez LI, Lopez-Bucio J, Altamirano-Hernandez J, Flores-Cortez I, Valencia-Cantero E (2011) A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 339:329–340
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73:5639–5641
Voisard C, Keel C, Haas D, Defago G (1989) Cyanide production by Pseudomonas fluorescens helps suppress black root-rot of tobacco under gnotobiotic conditions. EMBO J 8:351–358
Wang C, Wang Z, Qiao X, Li Z, Li F, Chen M, Wang Y, Huang Y, Cui H (2013) Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol Lett 341:45–51
Weise T, Kai M, Gummesson A, Troeger A, von Reuss S, Piepenborn S, Kosterka F, Sklorz M, Zimmermann R, Francke W, Piechulla B (2012) Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85–10. Beilstein J Org Chem 8:579–596
Weisskopf L, Bailly A (2013) Plant growth modulation by bacterial volatiles: a focus on Burkholderia species. In: Bruijn Fd (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken, New Jersey
Whitehead NA, Barnard AML, Slater H, Simpson NJL, Salmond GPC (2001) Quorum-sensing in gram-negative bacteria. FEMS Microbiol Rev 25:365–404
Zhao LJ, Yang XN, Li XY, Mu W, Liu F (2011) Antifungal, insecticidal and herbicidal properties of volatile components from Paenibacillus polymyxa strain BMP-11. Agric Sci China 10:728–736
Zhou H, Yao F, Roberts DP, Lessie TG (2003) AHL-deficient mutants of Burkholderia ambifaria BC-F have decreased antifungal activity. Curr Microbiol 47:174–179
Zou CS, Li ZF, Yu DQ (2010) Bacillus megaterium Strain XTBG34 promotes plant growth by producing 2-pentylfuran. J Microbiol 48:460–466
Acknowledgments
The authors are grateful to Prof. Dr. Katharina Riedel for initiating collaboration between S.S. and L.W. and for helpful discussions. We thank Dr. Aurélien Carlier for his valuable advice regarding construction of the bafI mutant. This research was partly funded by a Plant Science Centre-Syngenta Fellowship to A.B. and by the Swiss National Science Foundation (project 31003A-130089).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ulrike Groenhagen and Rita Baumgartner equally contributing authors.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2256 kb)
Rights and permissions
About this article
Cite this article
Groenhagen, U., Baumgartner, R., Bailly, A. et al. Production of Bioactive Volatiles by Different Burkholderia ambifaria Strains. J Chem Ecol 39, 892–906 (2013). https://doi.org/10.1007/s10886-013-0315-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0315-y