Abstract
Many volatile compounds secreted by bacteria play an important role in the interactions of microorganisms, can inhibit the growth of phytopathogenic bacteria and fungi, can suppress or stimulate plant growth and serve as infochemicals presenting a new type of interspecies communication. In this work, we investigated the effect of total pools of volatile substances and individual volatile organic compounds (VOCs) synthesized by the rhizosphere bacteria Pseudomonas chlororaphis 449 and Serratia plymuthica IC1270, the soil-borne strain P. fluorescens B-4117 and the spoiled meat isolate S. proteamaculans 94 on Arabidopsis thaliana plants. We showed that total gas mixtures secreted by these strains during their growth on Luria-Bertani agar inhibited A. thaliana growth. Hydrogen cyanide synthesis was unnecessary for the growth suppression. A decrease in the inhibition level was observed for the strain P. chlororaphis 449 with a mutation in the gacS gene, while inactivation of the rpoS gene had no effect. Individual VOCs synthesized by these bacteria (1-indecene, ketones 2-nonanone, 2-heptanone, 2-undecanone, and dimethyl disulfide) inhibited the growth of plants or killed them. Older A. thaliana seedlings were more resistant to VOCs than younger seedlings. The results indicated that the ability of some volatiles emitted by the rhizosphere and soil bacteria to inhibit plant growth should be considered when assessing the potential of such bacteria for the biocontrol of plant diseases.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria and other microorganisms secrete a large amount of various volatile substances (VSs), including volatile organic compounds (VOCs). VOCs are low molecular weight lipophilic compounds with a low boiling point and high vapor pressure. VOCs can readily evaporate and diffuse in gases and liquids (Kai et al. 2009; Effmert et al. 2012; Audrain et al. 2015a, b; Schmidt et al. 2015; Tyc et al. 2017; Veselova et al. 2019). The pool of all VSs synthesized by a bacterium or another organism is called the “volatilome” (Bailly and Weisskopf 2017). A database of identified VOCs (mVOC 2.0 database) has been published (http://bioinformatics.charite.de/mvoc/); it includes more than 2000 compounds secreted by almost 1000 species of bacteria and fungi (Lemfack et al. 2018). Bacterial VOCs belong to various chemical classes including alkenes, alcohols, ketones, terpenoids, benzoids, pyrazines, and sulfur-containing compounds (Lemfack et al. 2014, 2018; Schmidt et al. 2015; Avalos et al. 2018). However, at present VOCs of only a limited number of species and strains of bacteria and fungi have been identified. Many VOCs have not been identified due to the lack of appropriate markers in databases. In addition, the spectrum and the amounts of VOCs emitted by microorganisms depend on the conditions of their growth, including medium composition and pH (Blom et al. 2011a; Baily and Weisskopf 2012). VOCs are involved in a new type of microorganism communication playing the role of “infochemicals” that can transmit information over long distances (Effmert et al. 2012; Audrain et al. 2015a, b; Schmidt et al. 2015; Schulz-Bohm et al. 2017).
Plant growth-promoting rhizobacteria are able to produce a wide range of VOCs involved in plant-microbe interactions, such as stimulating plant growth and inducing plant systemic resistance to pathogens, in addition to playing a role in the biocontrol of plant diseases by direct antagonism with phytopathogenic bacteria and fungi (Ryu et al. 2004; Kai et al. 2009; Avalos et al. 2018; Fincheira and Quizos 2018). Another aspect of VOC involvement in bacterial communication is their influence on the functioning of quorum sensing regulatory systems (Schulz et al. 2010; Chernin et al. 2011; Ahmad et al. 2014; Schmidt et al. 2015; Helman and Chernin 2015) which are widespread among plant-associated bacteria and participate in the plant growth-promoting and biocontrol effects of the bacteria (Chernin 2011; Chernin et al. 2011). Much attention is currently being paid to the possibility of using VOCs in agriculture as a new alternative strategy using ecologically pure pesticides that are easily removed from the environment and therefore do not pollute the ecosystem (Audrain et al. 2015a; Tyc et al. 2017).
The purpose of this work was to study the effect of the total pools of volatiles and individual VOCs emitted by the strains Pseudomonas chlororaphis 449 and Serratia plymuthica IC1270 isolated from the rhizosphere and P. fluorescens B-4117 isolated from soil on Arabidopsis thaliana growth. The effect of S. proteamaculans 94, a thermotolerant isolate from spoiled meat was also investigated. Strains of this species are known to live both in the rhizosphere of plants and in the soil (Berg et al. 2002; Sánchez et al. 2009). All the strains mentioned above were shown to inhibit phytopathogenic fungi in vitro, and at least the first three strains mentioned could protect plants against some bacterial and fungal diseases (Chernin et al. 1995; Khmel et al. 1998; Ovadis et al. 2004; Dandurishvili et al. 2011). The total gas mixtures and individual VOCs secreted by these strains suppressed the growth of Agrobacterium and cyanobacteria strains and of mycelium of phytopathogenic fungi, killed the nematodes Сaenorhabditis elegans and inhibited their development, and also killed Drosophila melanogaster (Dandurishvili et al. 2011; Popova et al. 2014; Plyuta et al. 2016). VOCs emitted by the strains were identified using gas chromatography–mass spectrometry analysis. The major VOCs were alkene 1-undecene, ketones 2-nonanone and 2-undecanone (2-heptanone synthesized in small quantities was used for comparison with other ketones) for the P. chlororaphis 449 strain; 1-undecene for the P. fluorescens B-4117 strain and a sulfiding agent dimethyl disulfide (DMDS) for the strains S. plymuthica IC1270 and S. proteamaculans 94 (Dandurishvili et al. 2011; Popova et al. 2014).
Here, we showed that the total pools of VSs emitted by the studied Pseudomonas and Serratia strains were able to modulate the growth of A. thaliana plants. A decrease in plant growth inhibition due to VS action was observed when the strain P. chlororaphis 449 had a mutation in the gacS gene, which encodes the sensor kinase GacS. At the same time, inactivation of the rpoS gene encoding the σS subunit of RNA polymerase did not affect plant growth. The effect of individual VOCs synthesized by the tested bacterial strains (ketones, 1-undecene and DMDS) on plants was also investigated.
Materials and methods
Organisms, growth conditions, chemicals
The bacterial strains used in this work are listed in Table 1. The Pseudomonas and Serratia strains were grown in liquid Luria-Bertani broth (LB), g/l: Bacto Tryptone—10; Yeast Extract—Pronadisa, Hispanlab, S.A., Madrid, Spain—5; NaCl—Reachim, Russia—10; or on LA: LB supplemented with 1.5% Difco agar. Bacteria were grown at 28 °C, or 24 °C (when cocultivated with plants).
The seeds of A. thaliana ecotype Columbia (accession CS70000; Col-0) were obtained from the ABRC Stock Center (https://abrc.osu.edu/stocks/number/CS70000). The plants were grown on agarized Murashige and Skoog (MS) Basal Medium plant cell culture with sucrose and agar (Sigma-Aldrich).
The following VOCs purchased from Sigma-Aldrich Chimie GmbH (Steinheim, Germany) were used: > 99% pure dimethyl disulfide (DMDS), > 99% pure 2-heptanone, > 99% pure 2-nonanone, 99% pure 2-undecanone and 98% pure 1-undecene (Fig. 1).
Effect of total bacterial volatiles or individual VOCs on the growth of A. thaliana seedlings
Seeds of A. thaliana placed on filter paper in a Petri dish (92 × 16 mm) were sterilized with a solution of 5% H2O2 in 70% C2H5OH for 2 min. The seeds were then dried and transferred by a needle to a Petri dish with MS medium. The plates were incubated for 2 days at 4 °C. Then, the Petri dishes were removed from the refrigerator and incubated in a climate chamber in a 12 h light/12 h dark cycle at 24 °C. After 6 days, two cotyledonous leaves appeared on the A. thaliana seedlings.
The effect of VS-producing bacterial strains on A. thaliana growth was tested using two-compartment plastic Petri dishes (92 × 16 mm). First, the studied bacteria were grown for 17–20 h in LB at 28 °C with aeration. Then, 20 µl of the cultures (~ 5 × 107 cells) was dropped onto a solidified nutrient media LA in one of the two compartments and distributed by a microbiological loop on the surface of the medium, while another compartment was filled with solidified MS medium onto which 6-day-old A. thaliana seedlings with two cotyledonous leaves were transferred from the climate chamber. The dishes were tightly closed with 4 layers of Parafilm M and incubated in a growth chamber at 24 °C in a 12 h light/12 h dark cycle for additional 2 weeks. In this system, only the volatiles emitted by the VS-producing bacterial strains and not the bacterial cells themselves could reach the target plants.
In experiments with individual VOCs, the compounds under study were placed on strips of sterile filter paper in one compartment of a dish, whereas plant seedlings were placed in the other compartment filled with solidified MS medium and treated as described above. Finally, the plants were removed from the dishes, dried with sterile filter paper and weighed on laboratory scales.
To determine the effects of VOCs on older plants, the experiments were conducted as mentioned above, except that after 6 days of growth at 24 °C plants were placed into one part of a two-compartment Petri dish filled with MS medium and left for 2 weeks in a growth chamber. Then individual VOCs on sterile filter paper strips were placed into the other compartment of the dish, and the dish was tightly closed with Parafilm M and incubated for additional 2 weeks as indicated above. All experiments were repeated at least three times, with three plates and 5 plants on a dish in each experiment.
HCN assay
The semiquantitative analysis of cyanide production was performed with an Aquaquant-14417.0001 Test system (Merck). Cultures of the tested strains were grown for 48 h with aeration at 28 °C in LB containing 2 g/l NaCl as described earlier (Popova et al. 2014). Each strain was tested twice for HCN production.
Statistical analysis
The statistical analysis of experiments was carried out using the Microsoft Excel descriptive statistics program, and the standard error of the mean (SEM) was calculated. The differences among the data were significant at the level of p ≤ 0.05.
Results
Action of pools of volatiles emitted by Pseudomonas and Serratia strains on A. thaliana growth
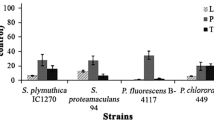
To study the effect of bacterial volatiles on the growth of A. thaliana seedlings, the bacteria were grown on LA medium in one compartment of a Petri dish, while plants were placed in the other compartment filled with MS medium as described above. After two weeks of incubation, the effect of VSs became clearly visible as compared to the control (growth of seedlings without bacteria). The total pools of volatiles secreted by the selected bacteria strongly suppressed the growth of A. thaliana compared to the control (Fig. 2). When the plants were transferred to fresh medium after treatment, the growth of seedlings was not restored which supported the lethal effect of VSs. If the Petri dishes were not wrapped with parafilm, the effect was weaker probably due to at least partial evaporation of VSs. Under the action of volatiles, the seedlings lost their green color, which might indicate a disruption of photosynthesis and/or the death of the plants.
Effect of total VSs emitted by Pseudomonas and Serratia strains on A. thaliana growth. A. thaliana plants were grown on MS medium in one compartment of a Petri dish, and bacteria were grown on LA medium in the other compartment of the dish (inoculum was 20 µl of overnight culture, ~ 5 × 107 cells, Figs. 2, 3 and 4). An average mass of the plant grown without bacteria (control, 60–70 mg, Figs. 2, 3 and 4) was taken as 100%. Values are expressed as mean in percent of the control ± SEM (error bars). The results marked with asterisk (*) are significantly different from the non-treated control according to a Student’s t-test (p ≤ 0.05)
Effect of inactivation of the gacS and rpoS genes on HCN synthesis and inhibition of A. thaliana plant growth by a pool of VSs emitted by the strain P. chlororaphis 449
The production of secondary metabolites by many bacteria, including Pseudomonas and Serratia, is regulated by the GacA/GacS two-component regulatory system (Heeb and Haas 2001; Ovadis et al. 2004; Cheng et al. 2013). This system consists of a membrane-bound sensor kinase GacS and a transcriptional response regulator GacA. Mutations in the gene encoding one of these proteins result in the loss of antimicrobial production, including production of volatile hydrogen cyanide (HCN) (Heeb and Haas 2001). HCN has a toxic effect on microorganisms and plants and is an important factor in the biological control of plant diseases (Voisard et al. 1989; Corbell and Loper 1995; Blumer and Haas 2000). It was suggested that bacterial cyanogenesis might be a key factor responsible for the plant-killing effects of bacterial volatiles (Blom et al. 2011b). Mutations in the gacA/gacS genes can also affect VOC synthesis (Han et al. 2006; Cheng et al. 2016; Ossowicki et al. 2017). Apart from the GacA/GacS system, the σS subunit of RNA polymerase, encoded by the rpoS gene, is involved in the regulation of metabolic processes during the transition of cells to the stationary growth phase, when secondary metabolites are synthesized (Hengge-Aronis 1999).
Using previously obtained mutants of the strain P. chlororaphis 449 with the inactivated gacS and rpoS genes (Veselova et al. 2009; Lipasova et al. 2009), we studied the effect of inactivation of these genes on HCN synthesis and inhibition of A. thaliana plant growth by a pool of VSs emitted by the mutants (Table 2; Fig. 3). The results presented in Table 2 showed that the wild-type P. chlororaphis strain 449 synthesized a detectable amount of HCN, while gacS and rpoS mutants virtually did not produce this compound. Nevertheless, the total pools of volatiles of all three strains inhibited the growth of A. thaliana, but the inhibition by the VSs of the gacS mutant was lower than that by VSs of the wild-type P. chlororaphis 449 strain or the rpoS mutant (Fig. 4). Thus, the HCN synthesis did not appear to be important for the inhibitory action of total VSs emitted by gacS and rpoS mutants of the P. chlororaphis 449 strain on Arabidopsis plant growth.
Effect of VSs of P. chlororaphis 449 and its gacS and rpoS mutants on the growth of A. thaliana seedlings. Bacteria were grown on LA medium in one compartement of the Petri dish, A. thaliana plants were grown on MS medium in the other compartment of the dish. The bar heights correspond to the average mass of the plant in percent of the control value ± SEM (error bars). The results marked with asterisk (*) are significantly different from the non-treated control according to a Student’s t-test (p ≤ 0.05)
The action of VOCs DMDS, 2-nonanone, 2-heptanone, 2-undecanone and 1-undecene on the growth of A. thaliana
A. thaliana seedlings were grown on MS medium in one compartment of the Petri dish. VOCs were added on the stripes of sterile filter paper and placed in the other compartment of the dish. The bar heights correspond to the average mass of the plant in percent of the control value ± SEM (error bars). The results marked with asterisk (*) are significantly different from the non-treated control according to a Student’s t-test (p ≤ 0.05)
Effect of individual VOCs emitted by the Pseudomonas and Serratia strains on A. thaliana growth
We also studied the action of the major individual VOCs produced by the tested strains of Pseudomonas and Serratia (Dandurishvili et al. 2011; Popova et al. 2014) on the growth of A. thaliana seedlings (Fig. 4). The data obtained revealed the difference in the action of various VOCs and the dependence of the growth inhibition on the amount of the volatile compound added. 2-Nonanone and DMDS inhibited plant growth starting at low doses of 5 and 10 µmol, respectively, while the action of 2-heptanone was significant only starting at a dose of 25 µmol. In the case of 2-undecanone, strong inhibition was observed at a dose of 5 µmol, whereas 1-undecene significantly inhibited growth only at doses over 10 µmol.
Ketones and DMDS had a plant-killing effect. None of the tested VOCs stimulated A. thaliana growth. Plants whose growth was strongly inhibited by two weeks of action of VOCs did not resume their growth when transferred into fresh medium (the dishes were not wrapped with parafilm). Older A. thaliana seedlings were more resistant to VOCs than younger seedlings. This was tested in experiments in which VOCs were added not immediately after the appearance of two cotyledonous leaves (6-day-old seedlings) but after A. thaliana had grown for another two weeks (Fig. 5).
The action of VOCs (added after 20 days of the growth of A. thaliana seedlings) on the growth of A. thaliana plants. A. thaliana plants were grown in one compartment of the Petri dish. Stripes of sterile filter paper with different VOCs were added to another compartments of a Petri dishes after 20 days of the A. thaliana growth. The Petri dishes was further incubated with VOCs for two weeks. An average control mass (160–180 mg) of the plant grown without addition of VOCs was taken as 100%. The bar heights correspond to the average mass of the plant in percent of the control value ± SEM (error bars). The results marked with asterisk (*) are significantly different from the non-treated control according to a Student’s t-test (p ≤ 0.05)
Discussion
The purpose of this work was to study the effect of volatile compounds produced by the rhizosphere bacteria P. chlororaphis 449 and S. plymuthica IC1270, the soil-borne strain P. fluorescens B-4117 and S. proteamaculans strain 94 isolated from spoiled meat on the growth of the model plant A. thaliana. Earlier, we showed that volatiles emitted by these bacterial strains, including the main headspace organic compounds ketones (2-nonanone, 2-undecanone), alkene 1-undecene, and the sulfiding agent DMDS, inhibited the growth of the strains A. tumefaciens and Synechococcus sp., as well as the growth of various fungal mycelia. In addition, these volatiles had a suppressive effect on nematodes and flies (D. melanogaster) (Popova et al. 2014). The effect of VOCs on the formation of biofilms and the survival of the examined bacterial strains in mature biofilms was also demonstrated (Plyuta et al. 2016). We showed here that the total pools of VSs of the Pseudomonas and Serratia strains studied and the individual ketones and DMDS had a strong action on A. thaliana. 2-Undecanone that acted weakly against agrobacteria had a strong effect on the plants. Interestingly, 1-undecene, a major VOC synthesized by P. fluorescens B-4117 and P. chlororaphis 449, did not significantly affect the growth of A. tumefaciens C58, the cyanobacterium Synechococcus sp. PCC 7942, or the fungus Rhizoctonia solani, but killed Drosophila flies at doses of 25 and 100 µmol and inhibited the growth of A. thaliana. Thus, there is a difference in the sensitivity of various organisms to different VOCs. The data obtained confirmed the role of bacterial volatiles as important compounds involved in interactions between organisms (Effmert et al. 2012; Piechulla and Degenhardt 2014; Schmidt et al. 2015; Tyc et al. 2017; Sharifi and Ryu 2018). Although individual VOCs of four tested strains of Pseudomonas and Serratia do participate in the suppression of A. thaliana growth by volatiles, the suppression is more likely a cooperative effect of a combination of volatiles produced by the bacteria.
The role of global regulatory systems in the observed effect of the total pool of volatiles on the growth of A. thaliana was studied here using as a model strain of the rhizosphere P. chlororaphis 449 and its previously obtained mutants, that had mutations in the global regulatory genes rpoS and gacS that encode the sigma S subunit of RNA polymerase and the sensor kinase of the GacA/GacS regulatory system, respectively. The rpoS mutation did not change the effect of VSs on A. thaliana, while the mutation in the gacS gene reduced it. Unfortunately, we were not able to access the effect of the gacS mutation on the synthesis of individual volatile compounds emitted by this strain. The participation of the GacA/GacS two-component regulatory system in the synthesis of VOCs was shown earlier for Pseudomonas strains. Han et al. (2006) determined that GacS-dependent production of 2R, 3R-butanediol of P. chlororaphis 06 was a major determinant of induced systemic resistance against Erwinia carotovora in tobacco plants. A significant decrease in the amounts of synthesized VOCs, including three acyclic alkenes, was determined in a gacS mutant of the P. fluorescens SBW25 strain (Cheng et al. 2016). An important role of the GacA/GacS system in the production of volatiles was shown for P. donghuensis P482; the synthesis of DMDS, S-methyl thioacetate, methyl thiocyanate, dimethyl trisulfide, 1-undecane and HCN depended on the GacA/GacS system. A gacA mutant entirely lost the ability to inhibit microbial plant pathogen activity through its volatiles (Ossowicki et al. 2017).
It is known that many bacterial strains, such as strains of Pseudomonas and Chromobacterium that inhibit the growth of and kill A. thaliana, synthesize cyanide (CN- or HCN) (Rudrappa et al. 2008; Blom et al. 2011b) suggested that HCN might be responsible for killing A. thaliana. However, the data presented here showed a rpoS mutant of P. chlororaphis 449 had a strong inhibition effect on A. thaliana growth although the mutant practically did not synthesize HCN. Similarly, the gacS mutant with the inactivated sensor kinase gene did not produce HCN but still inhibited the growth of A. thaliana. Last, the P. fluorescens strain B-4117 and two tested Serratia strains virtually did not produce HCN (Popova et al. 2014) but still demonstrated a strong effect on A. thaliana growth. Altogether, the data showed that for the strains studied in this work, HCN was not a principal plant growth inhibitor. The same conclusion was made by us earlier for the VS effect of several P. chlororaphis, P. fluorescens and Serratia strains on Agrobacterium tumefaciens, Synechococcus, and fungi (Popova et al. 2014).
We also investigated the effect of the predominate individual VOCs secreted by the studied strains (ketones, DMDS, and 1-undecene) on the growth of A. thaliana seedlings. Little is known about the effects of various pure VOCs on plants and the mechanisms of their action. The effects of some VOCs that stimulate plant growth have been described (rev. in Fincheira a. Quiroz 2018), for example, 2,3-butanediol (Ryu et al. 2003), acetophenone, 3-hexanone (Groenhagen et al. 2013), and DMDS (Meldau et al. 2013; Groenhagen et al. 2013). Meldau et al. (2013) concluded that the sulfiding agent DMDS produced by the naturally associated bacterium Bacillus sp. B55 promotes Nicotiana attenuate growth by enhancing sulfur nutrition. From the VOCs tested in our work, the ketones 2-nonanone and 2-undecanone reduced the growth of A. thaliana (up to approximately 2–3 times depending on the dose of compound), and 2-heptanone slightly increased the growth (by 1.3−1.4 times) (Groenhagen et al. 2013). In our experiments, all studied compounds caused significant inhibition of plant growth. Apparently, the effects of individual VOCs on plants may depend on many factors, including the species of plants, the conditions of their growth, and the conditions of the experiments. Further research into these effects is required and is important both for elucidating the mechanisms of action of these substances and for understanding the regularities of the influence of total pools of bacterial volatiles and the functional role of individual VOCs secreted by bacteria on plants.
Conclusions
The production of volatiles, including VOCs emitted by bacteria, constitutes an important mechanism for the interaction of plants with bacteria and might be a significant factor in the biological control of plant diseases. Volatiles of bacteria-antagonists of phytopathogenic microorganisms suppress the growth and kill pathogenic fungi and bacteria. It was shown that individual VOCs (e.g., DMDS) can be used for preplanting fumigation of soils (Audrain et al. 2015a; Schulz-Bohm et al. 2017; Tyc et al. 2017; Bailly and Weiskopf 2017). A plant-growth stimulating effect was previously described as a strain- and volatile-specific phenomenon (Sharifi and Ryu 2018).
The data obtained in this work using A. thaliana as a model plant and P. fluorescens, P. chlororaphis, S. plymuthica, and S. proteamaculans strains previously described as antagonists of phytopathogenic microorganisms confirmed the ability of bacterial volatiles to serve as important mediators of plant-bacteria interactions. However, we found that the individual volatiles, including the main VOCs (ketones 2-nonanone, 2-undecanone, DMDS and 1-undecene) emitted by the tested bacterial strains, demonstrated plant growth suppression but not stimulation effects. Even though the observed inhibitory effect was yet detected only in vitro and not in real greenhouse and field conditions, these results need to be taken into consideration when deciding whether strains producing these specific volatiles can indeed be efficient as biocontrol agents. On the other hand, the ability of VOCs to suppress seed germination (Lee et al. 2014 and our preliminary data) and plant growth may be useful in preplanting fumigation of soils to kill not only phytopathogenic fungi and bacteria but also weeds; subsequent evaporation of VOCs will make the soil suitable for sowing useful plants. The possibility and perspective of using volatiles emitted by microorganisms require serious study under natural conditions.
References
Ahmad A, Viljoen AM, Chenia HY (2014) The impact of plant volatiles on bacterial quorum sensing. Lett Appl Microbiol 60:8–19. https://doi.org/10.1111/lam.12343
Audrain B, Farag MA, Ryu C-M, Ghigo J-M (2015) Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol Rev 39:222–233. https://doi.org/10.1093/femsre/fuu013
Audrain B, Letoffe S, Ghigo JM (2015b) Airborne bacterial interactions: functions out of thin air? Front Microbiol 6:1476. https://doi.org/10.3389/fmicb.2015.01476
Avalos M, van Wezel GP, Raaijmakers JM, Garbeva P (2018) Healthy scents: microbial volatiles as new frontier in antibiotic research? Curr Opin Microbiol 45:84–91. https://doi.org/10.1016/j.mib.2018.02.011
Bailly A, Weisskopf L (2012) The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal Behav 7:79–85. https://doi.org/10.4161/psb.7.1.18418
Bailly A, Weisskopf L (2017) Mining the volatilomes of plant-associated microbiota for new biocontrol solutions. Front Microbiol 8:1638. https://doi.org/10.3389/fmicb.2017.01638
Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K (2002) Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol 68:3328–3338. https://doi.org/10.1128/AEM.68.7.3328-3338.2002
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058. https://doi.org/10.1111/j.1462-2920.2011.02582.x
Blom D, Fabbri C, Eberl L, Weisskopf L (2011) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77:1000–1008. https://doi.org/10.1128/AEM.01968-10
Blumer C, Haas D (2000) Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol 173:170–177. https://doi.org/10.1007/s002039900127
Cheng X, Cordovez V, Etalo DW, van der Voort M, Raaijmakers JM (2016) Role of the GacS sensor kinase in the regulation of volatile production by plant growth-promoting Pseudomonas fluorescens SBW25. Front Plant Sci. 7:1706. https://doi.org/10.3389/fpls.2016.01706
Cheng X, De Bruijn I, Van Der Voort M, Loper JE, Raaijmakers JM (2013) The Gac regulon of Pseudomonas fluorescens SBW25. Environ Microbiol Rep 5:608–619. https://doi.org/10.1111/1758-2229.12061
Chernin LS, Ismailov Z, Haran S, Chet I (1995) Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogens. Appl Environ Microbiol 61:1720–1726. https://aem.asm.org/content/61/5/1720
Chernin L (2011) Quorum-sensing signals as mediators of PGPRs’ beneficial traits. In: Maheshwari DK (ed) Bacteria in Agrobiology: Plant Nutrient Management. Springer-Verlag, Berlin Heidelberg, pp 209–236
Chernin L, Toklikishvili N, Ovadis M, Kim S, Ben-Ari J, Khmel I, Vainstein A (2011) Quorum-sensing quenching by rhizobacterial volatiles. Environ Microbiol Rep 3:698–704. https://doi.org/10.1111/j.1758-2229.2011.00284.x
Corbell NA, Loper JE (1995) A global regulator of second metabolite production in Pseudomonas fluorescens Pf-5. J Bacteriol 177:6230–6236. https://doi.org/10.1128/jb.177.21.6230-6236.1995
Dandurishvili N, Toklikishvili N, Ovadis M, Eliashvili P, Giorgobiani N, Keshelava R, Tediashvili M, Vainstein A, Khmel I, Szegedi E, Chernin L (2011) Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J Appl Microbiol 110:341–352. https://doi.org/10.1111/j.1365-2672.2010.04891.x
Demidyuk IV, Kalashnikov AE, Gromova TY, Gasanov EV, Safina DR, Zabolotskaya MV, Rudenskaya GN, Kostrov SV (2006) Cloning, sequencing, expression, and characterization of protealysin, a novel neutral proteinase from Serratia proteamaculans representing a new group of thermolysin-like proteases with short N-terminal region of precursor. Protein Expr Purif 47:551–561. https://doi.org/10.1016/j.pep.2005.12.005
Effmert U, Kalderas J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38:665–703. https://doi.org/10.1007/s10886-012-0135-5
Fincheira P, Quiroz A. (2018) Microbial volatiles as plant growth inducers. Microbiol Res 208:63–75. https://doi.org/10.1016/j.micres.2018.01.002
Groenhagen U, Baumgartner R, Bailly A, Gardiner A, Eberl L, Schulz S, Weisskopf L (2013) Production of bioactive volatiles by different Burkholderia ambifaria strains. J Chem Ecol 39:892–906. https://doi.org/10.1007/s10886-013-0315-y
Han SH, Lee SJ, Moon JH, Park KH, Yang KY, Cho BH, Kim KY, Kim YW, Lee MC, Anderson AJ, Kim YC (2006) GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant-Microbe Interact 19:924–930. https://doi.org/10.1094/MPMI-19-0924
Heeb S, Haas D (2001) Regulatory roles of the GacS/GacA twocomponent system in plant-associated and other Gram-negative bacteria. Mol Plant-Microbe Interact 14:1351–1363. https://doi.org/10.1094/MPMI.2001.14.12.1351
Helman Y, Chernin L (2015) Silencing the mob: disrupting quorum sensing as a means to fight plant disease. Molecular Plant Pathology 16:316–329. https://doi.org/10.1111/mpp.12180
Hengge-Aronis R (1999) Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr Opin Microbiol 2:148–152. https://doi.org/10.1016/S1369-5274(99)80026-5
Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81:1001–1012. https://doi.org/10.1007/s00253-008-1760-3
Khmel IA, Sorokina TA, Lemanova NB, Lipasova VA, Metlitski OZ, Burdeinaya TV, Chernin LS (1998) Biological control of crown gall in grapevine and raspberry by two Pseudomonas strains with a wide spectrum of antagonistic activity. Biocontrol Sci Tech 8:45–57. https://doi.org/10.1080/09583159830423
Lee S, Hung R, Schink A, Mauro J, Bennett JW (2014) Arabidopsis thaliana for testing thephytotoxicity of volatile organic compounds. Plant Growth Regul 74:177–186. https://doi.org/10.1007/s10725-014-9909-9
Lemfack MC, Gohlke BO, Toguem SMT, Preissner S, Piechulla B, Preissner R (2018) mVOC 2.0: a database of microbial volatiles. Nucl Acids Res 46(D1):D1261–D1265. https://doi.org/10.1093/nar/gkx1016
Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B (2014) mVOC: a database of microbial volatiles. Nucl Acids Res 42(Database issue):D744–D748. https://doi.org/10.1093/nar/gkt1250
Lipasova VA, Atamova EE, Khmel IA (2009) Synthesis of N-acyl homoserine lactones and phenazines, some enzymatic activities, and antifungal activity of Pseudomonas chlororaphis 449 carrying an inactivated rpoS gene. Molec Genet Microbiol Virol 24:7–11
Meldau DG, Meldau S, Hoang LH, Underberg S, Wünsche H, Ian T. Baldwin IT (2013) Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. The Plant Cell 25:2731–2747. https://doi.org/10.1105/tpc.113.114744
Ossowicki A, Jafra S, Garbeva P (2017) The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 12(3):e0174362. https://doi.org/10.1371/journal.pone.0174362
Ovadis M, Liu X, Gavriel S, Ismailov Z, Chet I, Chernin L (2004) The global regulator genes from biocontrol strain Serratia plymuthica IC1270: cloning, sequencing, and functional studies. J Bacteriol 186:4986–4993. https://doi.org/10.1128/JB.186.15.4986-4993.2004
Piechulla B, Degenhardt J (2014) The emerging importance of microbial volatile organic compounds. Plant Cell Environ 37:811–812. https://doi.org/10.1111/pce.12254
Plyuta V, Lipasova V, Popova A, Koksharova O, Kuznetsov A, Szegedi E, Chernin L, Khmel I (2016) Influence of volatile organic compounds emitted by Pseudomonas and Serratia strains on Agrobacterium tumefaciens biofilms. APMIS 124:586–594. https://doi.org/10.1111/apm.12547
Popova AA, Koksharova OA, Lipasova VA, Zaitseva JV, Katkova-Zhukotskaya OA, Eremina SIu, Mironov AS, Chernin LS, Khmel IA (2014) Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed Res Int 2014:125704. https://doi.org/10.1155/2014/125704
Rudrappa T, Splaine RE, Biedrzycki ML, Bais HP (2008) Cyanogenic Pseudomonads influence multitrophic interactions in the rhizosphere. PLoS ONE 3:e2073. https://doi.org/10.1371/journal.pone.0002073
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932. https://doi.org/10.1073/pnas.0730845100
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. https://doi.org/10.1104/pp.103.026583
Sánchez LA, Gómez FF, Delgado OD (2009) Cold-adapted microorganisms as a source of new antimicrobials. Extremophiles 13:111–120. https://doi.org/10.1007/s00792-008-0203-5
Schmidt R, Cordovez V, de Boer W, Raaijmakers J, Garbeva P (2015) Volatile affairs in microbial interactions. ISME J 9:1–7. https://doi.org/10.1038/ismej.2015.42
Schulz S, Dickschat JS, Kunze B, Wagner-Dobler I, Diestel R, Sasse F (2010) Biological activity of volatiles from marine and terrestrial bacteria. Mar Drugs 8:2976–2987. https://doi.org/10.3390/md8122976
Schulz-Bohm K, Martín-Sánchez L, Garbeva P (2017) Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front Microbiol 8:2484. https://doi.org/10.3389/fmicb.2017.02484
Sharifi R, Ryu CM (2018) Revisiting bacterial volatile-mediated plant growth promotion: lessons from the past and objectives for the future. Ann Bot 122:349–358. https://doi.org/10.1093/aob/mcy108
Tyc O, Song C, Dickschat JS, Vos M, Garbeva P (2017) The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol 25:280–292. https://doi.org/10.1016/j.tim.2016.12.002
Veselova M, Lipasova V, Protsenko MA, Buza N, Khmel IA (2009) GacS-dependent regulation ofenzymic and antifungal activities and synthesis of N-acylhomoserine lactones in rhizospheric strain Pseudomonas chlororaphis 449. Folia Microbiol 54:401–408. https://doi.org/10.1007/s12223-009-0056-z
Veselova MA, Plyuta VA, Khmel IA (2019) Volatile compounds of bacterial origin: structure, biosynthesis, and biological activity. Microbiology 88:261–274. https://doi.org/10.1134/S002626171
Voisard C, Keel C, Haas D, Defago G (1989) Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J 8:351–358. https://doi.org/10.1002/j.1460-2075.1989.tb03384.x
Acknowledgements
This research was partially supported by the Russian Foundation for Basic Research Grant (No. 18-04-00375) and fund within the state assignment of NRC «Kurchatov Institute» - IMG for 2020–2021 (No. 121030200227-6). We are grateful to Dr. Boris Glotov for critical reading of the manuscript and helpful discussions.
Funding
This study was funded by the Russian Foundation for Basic Research (grant number 18-04-00375) and fund within the state assignment of NRC «Kurchatov Institute» - IMG for 2020–2021 (No.121030200227-6).
Author information
Authors and Affiliations
Contributions
Conceptualization: I.A.K.; Methodology: I.A.K.; E.V.K.; Formal analysis: V.A.P.; I.A.K.; Investigation: I.A.K.; A.S.C.; D.E.S.; Writing - original draft preparation: I.A.K.; L.S.C.; V.A.P.; Writing – review and editing: I.A.K.; V.A.P.; L.S.C.; O.A.K.; Visualization, V.A.P.; O.A.K.; Funding acquisition: I.A.K.; Resources: I.A.K.; Supervision: I.A.K.; V.A.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical standards for the preparation of this manuscript have been followed by all authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plyuta, V.A., Chernikova, A.S., Sidorova, D.E. et al. Modulation of Arabidopsis thaliana growth by volatile substances emitted by Pseudomonas and Serratia strains. World J Microbiol Biotechnol 37, 82 (2021). https://doi.org/10.1007/s11274-021-03047-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03047-w