Abstract
The FloTrac system is a system for cardiac output (CO) measurement that is less invasive than the pulmonary artery catheter (PAC). The purposes of this study were to (1) compare the level of agreement and trending abilities of CO values measured using the fourth version of the FloTrac system (CCO-FloTrac) and PAC-originated continuous thermodilution (CCO-PAC) and (2) analyze the inadequate CO-discriminating ability of the FloTrac system before and after cardiopulmonary bypass (CPB). Fifty patients were included. After exclusion, 32 patients undergoing cardiac surgery with CPB were analyzed. All patients were monitored with a PAC and radial artery catheter connected to the FloTrac system. CO was assessed at 10 timing points during the surgery. In the Bland–Altman analysis, the percentage errors (bias, the limits of agreement) of the CCO-FloTrac were 61.82% (0.16, − 2.15 to 2.47 L min) and 51.80% (0.48, − 1.97 to 2.94 L min) before and after CPB, respectively, compared with CCO-PAC. The concordance rates in the four-quadrant plot were 64.10 and 62.16% and the angular concordance rates (angular mean bias, the radial limits of agreement) in the polar-plot analysis were 30.00% (17.62°, − 70.69° to 105.93°) and 38.63% (− 10.04°, − 96.73° to 76.30°) before and after CPB, respectively. The area under the receiver operating characteristic curve for CCO-FloTrac was 0.56, 0.52, 0.52, and 0.72 for all, ≥ ± 5, ≥ ± 10, and ≥ ± 15% CO changes (ΔCO) of CCO-PAC before CPB, respectively, and 0.59, 0.55, 0.49, and 0.46 for all, ≥ ± 5, ≥ ± 10, and ≥ ± 15% ΔCO of CCO-PAC after CPB, respectively. When CO < 4 L/min was considered inadequate, the Cohen κ coefficient was 0.355 and 0.373 before and after CPB, respectively. The accuracy, trending ability, and inadequate CO-discriminating ability of the fourth version of the FloTrac system in CO monitoring are not statistically acceptable in cardiac surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Studies have revealed that the optimization of stroke volume or cardiac output (CO) during major surgeries is associated with improved prognoses [1,2,3]. For high-risk patients with coexisting conditions, such as heart failure and end-stage renal disease; extremely old patients; or patients with unstable preoperative vital signs, reliable intraoperative CO monitoring can be useful because therapeutic interventions may become highly complex [4]. Therefore, the continuous and precise monitoring of CO is essential during cardiac surgery. A pulmonary artery catheter (PAC) was introduced in 1970 for the direct measurement of right ventricular output [5]. However, the use of PACs has various risks, including arrhythmia, pulmonary infarction, infection, pulmonary embolus, and pulmonary artery rupture [6]. PACs also add considerable costs to patient care, and many clinical trials have failed to confirm that PAC monitoring improves patient outcomes [7,8,9,10,11,12]. These disadvantages associated with PACs have motivated researchers to develop for less-invasive CO measurement methods [13].
Funk et al. [13] revealed that among the available monitoring systems, transesophageal echocardiography and arterial pulse contour devices have the highest potential to replace PACs for CO measurement. Arterial pulse contour devices have been increasingly used in clinical practice because they can be conveniently applied without a learning curve. The FloTrac system (Edward Lifesciences, Irvine, CA, USA) is one such device that was first introduced in 2005 and which enables continuous CO measurement. The system is currently in its fourth version. Over the years, the performance of the FloTrac system has improved particularly in hypodynamic and normodynamic conditions, providing sufficiently accurate and precise CO measurement and trending ability for routine clinical use in the absence of large changes in the vascular tone [14]. Nevertheless, some studies have yielded statistically unacceptable levels of agreement for CO measurement using the fourth version of the FloTrac system (4th FloTrac) compared with the reference method [15,16,17,18,19,20], particularly for patients in critical condition.
However, whether a lack of statistical agreement signifies clinical inapplicability remains debatable. For example, the CO values of 6.1 and 6.3 L min might not be statistically correlated; however, anesthesiologists may consider them to be adequate and acceptable with the same clinical management. As long as a monitor can discriminate inadequate CO, it is clinically applicable.
To date, no study has investigated the influence of cardiopulmonary bypass (CPB) on the measurement ability of the 4th FloTrac. Therefore, the present study (1) compared the level of agreement and trending abilities of CO values measured with the 4th FloTrac (CCO-FloTrac) and PAC-originated continuous thermodilution (CCO-PAC) and (2) analyzed the inadequate CO-discriminating ability of the 4th FloTrac before and after CPB.
2 Materials and methods
2.1 Patients
The present study was approved by the Institutional Review Board of Chang Gung Medical Foundation in Taiwan (registration number: 104-7177B). Patients who were aged ≥ 18 years, were undergoing planned elective cardiac surgery, and provided signed informed consent were included in the present study. Any patients with cardiac arrhythmia or an intra-cardiac shunt were excluded.
In total, 50 patients undergoing cardiac surgery were included in our study. The anesthetic induction drug and dosage varied according to the clinical condition. General anesthesia was maintained with sevoflurane (0.5–2.5%), fentanyl (0.5–2 μg/kg according to the clinical condition), and cisatracurium (2–4 mg/30 min). All patients were mechanically ventilated with a tidal volume of 8–10 mL/kg body weight at a frequency of 8–14 respiratory rate per minute to maintain end-tidal CO2 concentrations of 35–45 mmHg.
2.2 Data collection
An arterial pressure catheter was inserted into the radial artery and connected to the FloTrac/EV1000 system (version 4, Edwards Lifesciences, Irvine, CA, USA) to measure CCO-FloTrac. The PAC was inserted into the internal jugular vein, and its tip position was confirmed by pressure waves and transesophageal echocardiography. The PAC was connected to a Vigilance II Monitor (Edwards Lifesciences, USA) or an Abbott Q2 Plus CCO/ SvO2 Computer (Abbott Laboratories, IL, USA) to measure CCO-PAC.
As shown in Fig. 1, we recorded CCO-FloTrac and CCO-PAC at 10 timing points: (1) immediately after PAC placement and calibration, (2) immediately after the incision, (3) during sternotomy, (4) 5 min after sternotomy, (5) 10 min after sternotomy, (6) after the completion of protamine infusion, (7) 5 min after the completion of protamine infusion, (8) 15 min after the completion of protamine infusion, (9) at sternal closure, and (10) at the end of surgery. These 10 timing points were designated T1–T10, respectively.
A timeline indicating the 10 timing points throughout the surgery. Timing points: T1 immediately after PAC placement and calibration, T2 immediately after the incision, T3 during sternotomy, T4 5 min after sternotomy, T5 10 min after sternotomy, T6 after the completion of protamine infusion, T7 5 min after the completion of protamine infusion, T8 15 min after the completion of protamine infusion, T9 at sternal closure, T10 at the end of surgery
2.3 Statistical analyses
Statistical analyses were performed using SPSS (version 22.0, SPSS Inc., Chicago, IL, USA) and R 3.2.0 for Windows (Free Software Foundation Inc., Boston, MA, USA).
The level of agreement and bias between the CCO-FloTrac and CCO-PAC were evaluated by the Bland–Altman analysis corrected for repeated measures [21]. The percentage errors were calculated as 1.96 times the standard deviation of the bias divided by the mean CO of the reference method (CCO-PAC). A percentage error of < 30% was considered acceptable [22]. In addition, the Spearman correlation coefficient was used to evaluate the correlation between values.
The trending ability of the CCO-FloTrac was assessed with a four-quadrant plot and a polar-plot analysis. For the four-quadrant plot, the concordance rate was defined as the proportion of the number of paired CO changes (ΔCO) with the same direction of changes in both methods, which were present in the upper-right and lower-left quadrants. The central-zone data with ΔCO < ± 0.5 L min were considered statistical noise and excluded. After exclusion, a concordance rate of >90% was considered acceptable [23]. The polar-plot method, as described by Critchley et al. [24], was methodologically derived from the four-quadrant plot method and is a more advanced statistical method for evaluating the trending ability of a CO monitor. This analysis required a data rotation of the four-quadrant plot by 45° in the clockwise direction. The angular concordance rate (the percentage of points within a ± 30° radial zone), angular bias (the average angle from the horizontal axis), and radial limits of agreement (the radial zone containing 95% of the total number of data points) were calculated. The trending ability between the reference and tested methods was considered to be excellent when the following limits were satisfied: (1) angular concordance rat ≥ 95%, (2) mean angular bias within ± 5°, and (3) the radial limits of agreement within ± 30°.
The areas under the receiver operating characteristic curve (AUCs) were calculated to compare the detection ability of the FloTrac system before CPB, after CPB, and at different extents of ΔCO (± 5, ± 10, and ± 15%) of CCO-PAC [25]. The AUCs of 0.9–1, 0.75–0.9, and 0.5–0.75 represented excellent, good, and poor abilities, respectively; an AUC < 0.5 indicated a failed diagnostic value.
To investigate the inadequate CO-discriminating ability of the 4th FloTrac, the Cohen κ coefficient was applied to evaluate the interrater agreement between CCO-FloTrac and CCO-PAC. CO was defined as adequate when the value was within the normal range (4–8 L min), whereas a CO of <4 L/min was considered inadequate. The extent of agreement among the κ values was interpreted according to the terminology by Landis and Koch [26]; specifically, κ > 0.8 indicated excellent agreement, 0.6–0.8 indicated good agreement, 0.4–0.6 indicated moderate agreement, and < 0.4 indicated poor agreement.
For all statistical tests, p < 0.05 was considered statistically significant.
3 Results
Fifty patients were enrolled. Eighteen of them were excluded due to a new onset cardiac arrhythmia or an intra-cardiac shunt newly found by trans-esophageal echocardiography during the surgeries. A total of 32 patients underwent final analysis; 139 and 130 paired CO measurements were collected before and after CPB. Table 1 presents a list of patient characteristics.
Figure 2 shows the course of measurement of the CCO-FloTrac and CCO-PAC. Across both systems, CO was higher after CPB (p < 0.001). The mean CO values measured by the two methods were closest at T1, at which point the Spearman correlation coefficient was 0.452 (p = 0.018). At other timing points, the mean value of the CCO-FloTrac was higher than that of CCO-PAC by approximately 0.3–0.8 L min. Notably, three intervals revealed different trending directions between the two methods. First, at T1 and T2, CCO-PAC and the CCO-FloTrac trended in opposite directions. Second, at T6 and T7, CCO-PAC was unchanged while the CCO-FloTrac increased. Finally, at T7 and T8, CCO-PAC increased while the CCO-FloTrac values remained at the same mean value of CO.
Course of the CCO-FloTrac (dashed line) and CCO-PAC (continuous line) values. The timing points are indicated in Fig. 1
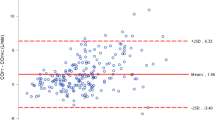
Figure 3 shows the Bland–Altman plot. The total calculated paired CO measurements, mean biases, standard deviations, limits of agreement, and percentage errors were 139 and 130, 0.16 and 0.48 L min, 1.18 and 1.25 L min, − 2.15 to 2.47 and − 1.97 to 2.94 L min, and 61.82 and 51.80% before and after CPB, respectively.
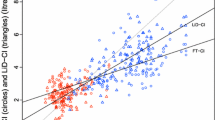
Figures 4 and 5 present a comparison of the trending abilities of the CCO-FloTrac and CCO-PAC. Figure 4 reveals the four-quadrant plot, and shows that the concordance rates were 64.10 and 62.16% before and after CPB, respectively. The results of the polar-plot analysis are depicted in Fig. 5. The angular concordance rates, angular mean biases, and radial limits of agreement were 30.00 and 38.63%, 17.62° and − 10.04°, − 70.69° to 105.93° and − 96.73° to 76.30° before and after CPB, respectively.
Four-quadrant plot for evaluating the agreement in ΔCO direction between the CCO-FloTrac and CCO-PAC. The central-zone data with ΔCO < ± 0.5 L min were considered statistical noise and excluded. The upper-right and lower-left quadrants are the areas with the same ΔCO direction measured by the two methods. The dashed line represents the line x = y
Table 2 shows the AUCs. The AUCs of the CCO-FloTrac were 0.56, 0.52, 0.52, and 0.72 for all, ± 5, ± 10, and ± 15% ΔCO of CCO-PAC before CPB, respectively, and 0.59, 0.55, 0.49, and 0.46 for all, ± 5, ± 10, and ± 15% ΔCO of CCO-PAC after CPB, respectively.
Table 3 shows the Cohen κ coefficient results. A CO of < 4 L/min was considered inadequate. The Cohen κ coefficients were 0.355 and 0.373 before and after CPB, respectively (both p < 0.001), and at T1, it was 0.308 (p = 0.100).
4 Discussion
Our study reveals that the 4th FloTrac cannot replace the PAC for CO monitoring in cardiac surgery. Indeed, CPB may reduce the effectiveness of the 4th FloTrac in CO monitoring. With a precision error exceeding the Critchley criteria [22] in the Bland–Altman analysis (Fig. 3), this system lacks the required accuracy for CO measurement. The four-quadrant and polar-plot analyses (Figs. 4, 5) also indicate that the trending ability of this system for CO measurement is unreliable, and the AUCs show that the FloTrac system at different ΔCO extents has a poor detection ability (Table 2). Furthermore, the inadequate CO-discriminating ability of the system was insufficient according to the Cohen κ coefficient analysis (Table 3). Although Fig. 2 shows the closest mean CO values between the two methods at T1 (immediately after PAC placement), the correlation (Spearman correlation coefficient = 0.452, p = 0.018) and inadequate CO-discriminating ability (Cohen κ coefficient = 0.308, p = 0.100) of the system are not statistically acceptable, indicating that even without surgical influences, the 4th FloTrac is unsuitable for CO monitoring in patients with cardiovascular diseases under anesthesia. On the basis of these results, we do not recommend the use of the 4th FloTrac for CO monitoring in cardiac surgery.
Schlöglhofer et al. [27] reviewed the first, second, and third versions of the FloTrac system and concluded that all of them had a large percentage error under both hemodynamically stable and unstable conditions. Several other studies have similarly indicated that the CO calculated using the third version of the FloTrac system may grossly deviate from that calculated using the PAC under hemodynamically unstable conditions [17, 19, 20, 28], particularly in patients with low systemic vascular resistance, high doses of vasopressor therapy, or low CO [29,30,31,32,33,34,35]. Edward Lifesciences improved the software package and released its fourth version in May 2014.
In the 4th FloTrac, CO = PR × SD × K4 × Kfast [36], where PR, SD, and K4 and Kfast are the pulse rate, standard deviation of the arterial pressure, and autocalibration factors as the proprietary algorithm of Edwards, respectively. K4 contains the multivariate polynomial equations of waveform variables, such as skewness and kurtosis. Kfast is the newly added component in the 4th FloTrac, and is inversely proportional to pressure. After analyzing the vascular tone based on waveform morphology and patient characteristics (age, sex, body height, and body weight), K4 and Kfast are computed. PR, SD, and Kfast are calculated every 20 s, and K4 is averaged every minute. Notably, the accuracy of the 4th FloTrac in CO monitoring can be interfered with by: (1) sudden ΔCO; factors influencing (2) pulse rate, (3) blood pressure, or (4) arterial waveform; or (5) patient characteristics other than age, sex, body height, and body weight. For example, the anatomic distance between the left ventricle and the peripheral artery may lead to a time lag for the 4th FloTrac in CO measurement. This lag may be increased by the occurrence of sudden, unstable ΔCO, longer heart-to-arm length, and impaired vascular quality. Furthermore, the inability of the peripheral arteries to represent aortic pressure (particularly systolic pressure) [37,38,39,40,41] may also lead to imprecise CO monitoring by the FloTrac system.
Studies have obtained controversial results with the 4th FloTrac. Ji et al. [42] compared the effects of phenylephrine administration on ΔCO measured using the third and fourth versions of the FloTrac system in elective surgery, and used the Nexfin algorithm (Edwards BMEYE B.V, Amsterdam, Netherlands) as the reference method. They concluded that the 4th FloTrac significantly improved the system’s ability to accurately detect ΔCO induced by phenylephrine. However, Hattori et al. [16] reached a different conclusion. They investigated patients who underwent cardiac resynchronization therapy (CRT) and lead implantation surgery, and used three-dimensional echocardiography (3DE) as the reference method. Their results demonstrated that CO values measured using the 4th FloTrac had a significantly limited agreement with those measured through 3DE, and the tracking ability of the 4th FloTrac was below acceptable limits both after phenylephrine administration and CRT. Elsewhere, Shih et al. [43] revealed that the 4th FloTrac does not have reliable CO measurement and trending abilities in liver transplantation compared with the CCO-PAC. Suehiro et al. [36] used bolus thermodilution through the PAC as a reference method. A clinically irreplaceable discrepancy was observed between the two methods in CO measurements, but they also determined that the 4th FloTrac had a nearly clinically acceptable tracking ability (a concordance rate of 87.0% in the four-quadrant analysis and an angular concordance rate of 83.0% in the polar-plot analysis) after phenylephrine administration in patients who underwent cardiac surgery. Cho et al. [44] evaluated the performance of the 4th FloTrac in off-pump coronary artery bypass surgery, and yielded a percentage error of 33.8% in the Bland–Altman analysis and a concordance rate of 79.5% in the four-quadrant analysis compared with bolus thermodilution through the PAC. These results highlighted the limited use of the 4th FloTrac in off-pump coronary artery bypass surgery.
Based on the results of the present study, the clinical use of the 4th FloTrac for CO monitoring is not recommended either before or after CPB. CO values are typically higher after CPB (Figs. 2, 3) because the patient’s cardiac function improves after surgical interventions and the use of inotropic agents. (In our study, all patients were given dobutamine infusion. Some patients needed additional inotropics such as epinephrine infusion for cardiac support. The dosage was adjusted according to clinical condition.) However, a central-to-radial arterial pressure gradient may occur after CPB, varying from 10 min after the discontinuation of CPB [45] to sternal closure [46] and with an incidence ranging from 10 to 72% [47] The central-to-radial arterial pressure gradient may interfere with CO measurement by the FloTrac system. During and after CPB, factors such as hypoperfusion, acidosis, electrolyte imbalance, hemodilution, coagulopathy, surgical blood loss, hypothermia, prolonged drug clearance, and altered drug potency [48,49,50,51,52] may affect the vascular tone and CO. Furthermore, CPB and surgical stress induce a profound systemic inflammatory response [53, 54], which also affects the vascular tone. All of the aforementioned factors can change the arterial waveform morphology and disturb the CO monitoring ability of the FloTrac system. This observation might explain the unimproved statistical outcomes of the 4th FloTrac for CO monitoring after CPB.
The present study has some limitations. First, we excluded patients with arrhythmia. Although many patients with various arrhythmias require CO monitoring, the present results may not be applicable to them. Second, the study patients were not restricted to a single surgical method. Different surgical processes and multiple valve conditions may have influenced our results. Third, to ensure a convenient clinical practice, we used PAC-originated continuous thermodilution as the CO measurement reference method. Although some studies showed an unacceptable precision between continuous thermodilution and bolus thermodilution technique, the current gold standard for CO measurement, after hypothermic CPB [55, 56], other studies indicated that these two methods are interchangeable [57, 58].
5 Conclusion
The accuracy, trending ability, and inadequate CO-discriminating ability of the 4th FloTrac in CO monitoring are not statistically acceptable in cardiac surgery. Even without surgical stress, the application of the 4th FloTrac on an anesthetized critically ill patient is not ideal. Furthermore, ΔCO detection after CPB by this system is not improved. Therefore, the 4th FloTrac cannot replace the PAC for CO measurement in cardiac surgery.
References
Gan TJ, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97(4):820–6.
Wakeling HG, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95(5):634–42.
Noblett SE, et al. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93(9):1069–76.
Vincent JL, Fagnoul D. Do we need to monitor cardiac output during major surgery? Anesthesiology. 2012;117(6):1151–2.
Swan HJ, et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447–51.
Domino KB, et al. Injuries and liability related to central vascular catheters: a closed claims analysis. Anesthesiology. 2004;100(6):1411–8.
Sandham JD, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14.
Gore JM, et al. A community-wide assessment of the use of pulmonary artery catheters in patients with acute myocardial infarction. Chest. 1987;92(4):721–7.
Zion MM, et al. Use of pulmonary artery catheters in patients with acute myocardial infarction. Analysis of experience in 5,841 patients in the SPRINT Registry. SPRINT Study Group. Chest. 1990;98(6):1331–5.
Connors AF Jr, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889–97.
Heyland DK, et al. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24(3):517–24.
Ivanov RI, et al. Pulmonary artery catheterization: a narrative and systematic critique of randomized controlled trials and recommendations for the future. New Horiz. 1997;5(3):268–76.
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108(3):887–97.
Slagt C, Malagon I, Groeneveld AB. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth. 2014;112(4):626–37.
Nakasuji M, et al. Disagreement between fourth generation FloTrac and LiDCOrapid measurements of cardiac output and stroke volume variation during laparoscopic colectomy. J Clin Anesth. 2016;35:150–6.
Hattori K, et al. Accuracy and trending ability of the fourth-generation FloTrac/Vigileo system in patients with low cardiac index. J Cardiothorac Vasc Anesth. 2016;31:99–104
Asamoto M, et al. Reliability of cardiac output measurements using LiDCOrapid and FloTrac/Vigileo across broad ranges of cardiac output values. J Clin Monit Comput. 2016;31:709–716
Lee M, et al. Agreement in hemodynamic monitoring during orthotopic liver transplantation: a comparison of FloTrac/Vigileo at two monitoring sites with pulmonary artery catheter thermodilution. J Clin Monit Comput. 2016;31:343–351
Montenij LJ, et al. Accuracy, precision, and trending ability of uncalibrated arterial pressure waveform analysis of cardiac output in patients with impaired left ventricular function: a prospective, observational study. J Cardiothorac Vasc Anesth. 2016;30(1):115–21.
Tomasi R, et al. Comparison of an advanced minimally invasive cardiac output monitoring with a continuous invasive cardiac output monitoring during lung transplantation. J Clin Monit Comput. 2016;30(4):475–80.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15(2):85–91.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111(5):1180–92.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011;25(3):536–46.
Cook NR. Methods for evaluating novel biomarkers—a new paradigm. Int J Clin Pract. 2010;64(13):1723–7.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Schloglhofer T, Gilly H, Schima H. Semi-invasive measurement of cardiac output based on pulse contour: a review and analysis. Can J Anaesth. 2014;61(5):452–79.
Singh S, Taylor MA. Con: the FloTrac device should not be used to follow cardiac output in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2010;24(4):709–11.
Metzelder S, et al. Performance of cardiac output measurement derived from arterial pressure waveform analysis in patients requiring high-dose vasopressor therapy. Br J Anaesth. 2011;106(6):776–84.
Ganter MT, et al. Continuous cardiac output measurement by un-calibrated pulse wave analysis and pulmonary artery catheter in patients with septic shock. J Clin Monit Comput. 2016;30(1):13–22.
Marque S, et al. Cardiac output monitoring in septic shock: evaluation of the third-generation Flotrac-Vigileo. J Clin Monit Comput. 2013;27(3):273–9.
Slagt C, et al. Cardiac output measured by uncalibrated arterial pressure waveform analysis by recently released software version 3.02 versus thermodilution in septic shock. J Clin Monit Comput. 2013;27(2):171–7.
Biancofiore G, et al. Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br J Anaesth. 2009;102(1):47–54.
Monnet X, et al. Third-generation FloTrac/Vigileo does not reliably track changes in cardiac output induced by norepinephrine in critically ill patients. Br J Anaesth. 2012;108(4):615–22.
Maeda T, et al. Inaccuracy of the FloTrac/Vigileo system in patients with low cardiac index. J Cardiothorac Vasc Anesth. 2014;28(6):1521–6.
Suehiro K, et al. Improved performance of the fourth-generation FloTrac/Vigileo system for tracking cardiac output changes. J Cardiothorac Vasc Anesth. 2015;29(3):656–62.
Thiele RH, Durieux ME. Arterial waveform analysis for the anesthesiologist: past, present, and future concepts. Anesth Analg. 2011;113(4):766–76.
Pauca AL, Kon ND, O’Rourke MF. The second peak of the radial artery pressure wave represents aortic systolic pressure in hypertensive and elderly patients. Br J Anaesth. 2004;92(5):651–7.
Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955;3(6):623–32.
Pauca AL, et al. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102(4):1193–8.
Karamanoglu M, et al. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14(2):160–7.
Ji F, et al. Reliability of a new 4th generation FloTrac algorithm to track cardiac output changes in patients receiving phenylephrine. J Clin Monit Comput. 2015;29(4):467–73.
Shih BF, et al. Cardiac output assessed by the fourth-generation arterial waveform analysis system is unreliable in liver transplant recipients. Transplant Proc. 2016;48(4):1170–5.
Cho YJ, et al. Comparison of cardiac output measures by transpulmonary thermodilution, pulse contour analysis, and pulmonary artery thermodilution during off-pump coronary artery bypass surgery: a subgroup analysis of the cardiovascular anaesthesia registry at a single tertiary centre. J Clin Monit Comput. 2016;30(6):771–82.
Stern DH, et al. Can we trust the direct radial artery pressure immediately following cardiopulmonary bypass? Anesthesiology. 1985;62(5):557–61.
Baba T, et al. Radial artery diameter decreases with increased femoral to radial arterial pressure gradient during cardiopulmonary bypass. Anesth Analg. 1997;85(2):252–8.
Fuda G, et al. Risk factors involved in central-to-radial arterial pressure gradient during cardiac surgery. Anesth Analg. 2016;122(3):624–32.
Hiraoka H, et al. Changes in drug plasma concentrations of an extensively bound and highly extracted drug, propofol, in response to altered plasma binding. Clin Pharmacol Ther. 2004;75(4):324–30.
Takizawa E, et al. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth. 2006;96(2):179–85.
McMurray TJ, et al. Propofol sedation after open heart surgery. A clinical and pharmacokinetic study. Anaesthesia. 1990;45(4):322–6.
Maitre PO, et al. Pharmacokinetics of midazolam in patients recovering from cardiac surgery. Eur J Clin Pharmacol. 1989;37(2):161–6.
Barry AE, Chaney MA, London MJ. Anesthetic management during cardiopulmonary bypass: a systematic review. Anesth Analg. 2015;120(4):749–69.
Cameron D. Initiation of white cell activation during cardiopulmonary bypass: cytokines and receptors. J Cardiovasc Pharmacol. 1996;27(Suppl 1):S1–5.
Morse DS, Adams D, Magnani B. Platelet and neutrophil activation during cardiac surgical procedures: impact of cardiopulmonary bypass. Ann Thorac Surg. 1998;65(3):691–5.
Bendjelid K, et al. Continuous cardiac output monitoring after cardiopulmonary bypass: a comparison with bolus thermodilution measurement. Intensive Care Med. 2006;32(6):919–22.
Zollner C, et al. Continuous cardiac output measurements do not agree with conventional bolus thermodilution cardiac output determination. Can J Anaesth. 2001;48(11):1143–7.
Della Rocca G, et al. Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth. 2002;88(3):350–6.
Della Rocca G, et al. Cardiac output monitoring: aortic transpulmonary thermodilution and pulse contour analysis agree with standard thermodilution methods in patients undergoing lung transplantation. Can J Anaesth. 2003;50(7):707–11.
Acknowledgements
The authors wish to acknowledge the Biostatistical Center for Clinical Research (Grant CLRPG3D0043), Research Services Center for Health Information (Grant CIRPD1D0031) from Chang Gung Memorial Hospital, and Mr. Shih-Hao Liu for statistical consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Chang Gung Medical Foundation Institutional Review Board in Taiwan (registration number: 104-7177B) and with the 1964 Helsinki declaration.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lin, SY., Chou, AH., Tsai, YF. et al. Evaluation of the use of the fourth version FloTrac system in cardiac output measurement before and after cardiopulmonary bypass. J Clin Monit Comput 32, 807–815 (2018). https://doi.org/10.1007/s10877-017-0071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-017-0071-6