Abstract
Cardiac output measurement has a long history in haemodynamic management and many devices are now available with varying levels of accuracy. The purpose of the study was to compare the agreement and trending abilities of cardiac output, as measured by transpulmonary thermodilution and calibrated pulse contour analysis, using the VolumeView™ system, continuous thermodilution via a pulmonary artery catheter, and uncalibrated pulse contour analysis, using FloTrac™ with pulmonary artery bolus thermodilution. Twenty patients undergoing off-pump coronary artery bypass surgery using a pulmonary artery catheter and the VolumeView™ and FloTrac™ systems were included in this subgroup analysis of the cardiovascular anaesthesia registry at a single tertiary centre. During surgery, cardiac output was assessed after the induction of anaesthesia, after sternotomy, during the harvesting of grafts, during revascularization of the anterior and posterior/lateral wall, after protamine infusion, and after sternal fixation. In total, 145 sets of measurements were evaluated using Bland–Altman with % error calculation, correlation, concordance, and polar plot analyses. The percentage error (bias, limits of agreement) was 12.6 % (−0.12, −0.64 to 0.41 L/min), 26.7 % (−0.38, −1.50 to 0.74 L/min), 29.3 % (−0.08, −1.32 to 1.15 L/min), and 33.8 % (−0.05, −1.47 to 1.37 L/min) for transpulmonary thermodilution, pulmonary artery continuous thermodilution, calibrated, and uncalibrated pulse contour analysis, respectively, compared with pulmonary artery bolus thermodilution. All pairs of measurements showed significant correlations (p < 0.001), whereas only transpulmonary thermodilution revealed trending ability (concordance rate of 95.1 %, angular bias of 1.33°, and radial limits of agreement of 28.71°) compared with pulmonary artery bolus thermodilution. Transpulmonary thermodilution using the VolumeView™ system provides reliable data on cardiac output measurement and tracking the changes thereof when compared with pulmonary artery bolus thermodilution in patients with preserved cardiac function during off-pump coronary artery bypass surgery.
Trial registration NCT01713192 (ClinicalTrials.gov).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cardiac output (CO) measurement has a long history in haemodynamic management in cardiac surgery patients prone to intraoperative cardiocirculatory disturbances, especially in those with preexisting cardiovascular comorbidities. Intermittent bolus CO by pulmonary artery (PA) thermodilution has been the most commonly accepted clinical standard, but it requires right heart catheterisation, and has many potential risks of complications with its decreasing use in practice over time [1–5]. Today, many other devices with varying levels of accuracy are available for advanced haemodynamic management.
The VolumeView™/EV1000™ system is a recently introduced CO measuring method that consists of a specific thermistor-tipped femoral arterial catheter (VolumeView™ catheter, Edwards Lifesciences LLC, Irvine, CA, USA), connected to a haemodynamic monitor (EV1000™, Edwards Lifesciences LLC). The system is initially calibrated by transpulmonary thermodilution via a bolus injection of cold saline through a central venous line. Then, it provides continuous CO values based on a calibrated pulse contour analysis. It has shown good agreement with the PiCCO2™ system (Pulsion Medical Systems, Munich, Germany), which uses basically the same technique, in critically ill patients [6, 7] and in pigs [8]. However, the level of agreement and trending ability of this new device have not been demonstrated, compared with the PA bolus thermodilution method, especially in patients undergoing cardiac surgery.
The FloTrac™ transducer (Edwards Lifesciences LLC), when connected to an arterial cannula, provides uncalibrated pulse wave-based continuous CO values. It can be used when connected to a Vigileo™ or EV1000™ monitor (both Edwards Lifesciences LLC) to show haemodynamic values, including CO and stroke volume variation. It works with a readily accessible peripheral artery, such as the radial artery. Thus, it can be used without central arterial cannulation, central venous catheterisation, or external calibration. In recent publications, the third-generation FloTrac™ system still showed a lacks of reliable reproducibility, compared with the reference method, in various clinical settings [9–11]. The fourth-generation algorithm improved the reliability of the system, based on updated calibration factors for vascular tone [12]. However, it has shown discrepancies in CO measurements when compared with PA thermodilution after phenylephrine administration in patients undergoing cardiac surgery [13]. In this study, we used the fourth-generation FloTrac™ (verion 4.0)/EV1000™ (version 1.5) system to assess continuous CO values, based on an uncalibrated pulse contour analysis.
The aim of this study was to assess the accuracy, precision, and trending ability of CO measurements using transpulmonary bolus thermodilution (ICOVV) and calibrated pulse contour analysis (CCOVV) using the VolumeView™/EV1000™ system, continuous thermodilution via a PA catheter (CCOPA), uncalibrated pulse contour analysis using the FloTrac™/EV1000™ system (CCOFT), and, as a reference method, PA bolus thermodilution (ICOPA).
2 Methods
2.1 Setting
This study was a sub-group analysis of the cardiovascular anaesthesia registry at Seoul National University Hospital. From August 2012 to July 2014, using this registry, we investigated the impact of perioperative haemodynamic and laboratory data on various clinical outcomes. Patients scheduled for cardiovascular surgery (coronary artery bypass, valvular, or aortic surgery) were considered for enrollment in the registry. The Seoul National University Hospital Institutional Review Board approved this study (reference # 1207-111-419) and its ClinicalTrials.gov number is NCT01713192 (registry for perioperative data in patients undergoing cardiac surgery). All patients signed written informed consents and the study was performed according to Good Clinical Practices and in accordance with the Declaration of Helsinki. VolumeView™ sets have been available as the monitoring device of choice for patients undergoing cardiovascular surgery in our hospital, as part of the registry, since May 2014. Thus, this sub-group analysis involved patients included in the registry between May and July 2014.
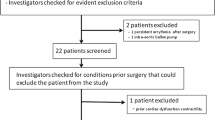
From the registry, this study included 20 patients undergoing off-pump coronary artery bypass (OPCAB) surgery during which the VolumeView™, FloTrac™, and PA catheterisation were used. Double arterial cannulation for both VolumeView™ and FloTrac™ systems was applied only to the patients included in the present analysis among those in the registry. Patients with persistent arrhythmia, severe valvular regurgitation, severe stenosis of the femoral artery on preoperative computed tomographic angiography, significantly impaired ventricular contractility (left ventricular [LV] ejection fraction <30 % by preoperative echocardiography), or any mechanical cardiac support device were excluded. Patients with severely reduced cardiac function were excluded because they are prone to unstable haemodynamics and a high probability of needing support with mechanical devices, such as intra-aortic balloon pump or extra-corporeal membrane oxygenator, during surgery. Patients who underwent lung resection before the present surgery were also excluded.
2.2 Anaesthesia
Patients were monitored with routine haemodynamic monitoring with 5-lead ECG including ST segment analysis, non-invasive blood pressure, peripheral oxygen saturation by pulse oximetry, and cerebral oxygen saturation with a near-infrared spectroscopic sensor on their forehead. Without premedication, a 20-G cannula (Angiocath Plus™, Becton–Dickinson Medical Ltd., Tuas, Singapore) was placed in the right or left radial artery after local skin analgesia with 1 % lidocaine injection, and then connected to a FloTrac™ transducer with EV1000™ monitor. Anaesthesia was induced with midazolam (0.1 mg/kg), vecuronium (0.1 mg/kg) or cisatracurium (0.015 mg/kg), and sufentanil (1 μg/kg). The trachea was intubated and the lungs were ventilated with volume-controlled ventilation with a tidal volume of 6–10 mL/kg and respiration rate of 10–14/min to adjust the arterial partial pressure of carbon dioxide to 35–45 mmHg, positive end-expiratory pressure of 0–8 cm H2O, and the fraction of inspired oxygen started at 0.5 and was adjusted to maintain an arterial partial pressure of oxygen above 90 mmHg.
The right or left femoral artery was catheterized with a thermistor-tipped 4-Fr (16-cm-long) or 5-Fr (20-cm-long) catheter (VolumeView™ catheter), which was then connected to another EV1000™ monitor. The device was initially calibrated by transpulmonary thermodilution with injection of 20 mL of cold (4–7 °C) saline for calibration of the CCOVV, which was not included in the analysis for comparing CO measurements. The averaged value of three consecutive injections within 15 % deviation was accepted for calibration. The time difference between the initial calibration of the system and the first measurement for analysis was similar among the patients.
For all patients, a 9-Fr, 11-cm-long central venous catheter (AVA HF, Edwards Lifesciences LLC, Irvine, CA, USA) was inserted into the right internal jugular vein under ultrasonographic guidance. A 7.5-Fr, 110-cm-long thermodilution PA catheter (Swan-Ganz CCOmbo V, model 774HF75, Edwards Lifesciences LLC) was inserted through the internal jugular lumen and then advanced into the PA during monitoring of the distal and proximal pressure waves. That the tip of the PA catheter was located within the main PA trunk or just the distal portion thereof was confirmed by transoesophageal echocardiography. The PA catheter was connected to a Vigilance™ II monitor (Edwards Lifesciences LLC), and in vivo calibration was performed. All transducers were zeroed to atmospheric pressure. For all monitors, patient-related data (age, sex, height, and weight) were inserted.
Anaesthesia was maintained with continuous infusion of propofol (effect site concentration, C e = 1.0–3.0 μg/mL) and remifentanil (C e = 5.0–12.0 ng/mL) using target-controlled infusion with the bispectral index maintained at 40–60. Vecuronium or cisatracurium was infused continuously at 0.04 or 0.05 mg/kg/min for muscle relaxation. Crystalloid or colloid was used as a maintenance fluid and blood products were transfused according to the intraoperative laboratory results.
2.3 Cardiac output measurements
For each patient, CO was measured using three devices (PA catheter, VolumeView™/EV1000™, and FloTrac™/EV1000™) simultaneously at least seven times during the surgery. The measurements were planned to include haemodynamically important time points and were distributed throughout the whole OPCAB procedure, as follows: (1) after induction of anaesthesia, (2) after sternotomy, (3) during the harvesting of vascular grafts, during revascularization of the (4) anterior, and (5) posterior/lateral wall, (6) after protamine infusion, and (7) after sternal fixation.
At each time point, haemodynamic stability was ensured for at least 10 min without bolus injection or change of infusion rate of catecholamine or vasopressor. Thereafter, a set of haemodynamic variables and values of CCOPA, CCOVV, and CCOFT were recorded, and then CO measurements using bolus thermodilution were performed. ICOPA was measured first, and then ICOVV was determined, according to the manufacturer’s recommendation. By performing intermittent transpulmonary thermodilution, the VolumeView™ system was inevitably re-calibrated.
Technique of displaying CCOPA values on monitor starts with intermittent heating of blood flowing through the superior vena cava by an electric filament attached to the PA catheter in a pseudorandom sequence. The resulting change in blood temperature is detected downstream by the thermistor on the tip of the PA catheter, and analyzed using area under the thermodilution curve [2, 3, 14]. During averaging values to reduce noise and improve reliability, it can take up to 12 min to fully register a change in CO on the monitor, restricting the suitability of the system during rapid haemodynamic changes [3, 14].
PA bolus thermodilution was performed with injection of 10 mL of cold (4–7 °C) saline via the proximal lumen of the PA catheter, completed within 10 s per injection, according to the manufacturer’s guidelines. Transpulmonary thermodilution was performed through the VolumeView™/EV1000™ system with injection of 20 mL of cold saline via the distal lumen of the central venous catheter, completed between 2 and 10 s per injection, based on the manufacturer’s guidance (personal communication with the manufacturer, Edwards Lifesciences). Each set of bolus thermodilution determinations consisted of at least three consecutive injections and the averaged values were recorded. In case of a deviation of measured values more than 15 % within a set, two or more injections were added. Injections were spread randomly over the ventilatory cycle [2] and were performed by the same person (YJC) to minimise inter-observer variation. Any intravenous fluids were transiently stopped during bolus thermodilution measurements except for constant infusions of anaesthetics or vasoactive drugs. If haemodynamic stability was not maintained (deviation of arterial pressure or heart rate >10 %) during measurement, the series of data was discarded. If the arterial waveform was transiently damped or analyzing the waveform failed, the series of measurements were abandoned.
2.4 Statistical analysis
Data are presented as means (standard deviation, SD) if normally distributed or medians (interquartile range, IQR) if otherwise, according to the result of Kolmogorov–Smirnov one-sample test for normality.
Bland–Altman analysis was used to assess the agreement of each pair of CO measurements by each method. Bias was defined as the mean difference between the measurements of each paired data. The upper and lower limits of agreement (LOA) were defined as ±1.96 SD of the bias. Correction for multiple measurements per individual was performed according to the distribution of data [15]. Percentage error was calculated as LOA divided by the mean CO of the reference methods × 100 %. To determine the acceptability of a method, we used the 30 % threshold suggested by Critchley et al. [16]. According to the distribution of measurements, Pearson’s r or Spearman’s ρ was obtained to evaluate correlation between values.
For trend analysis, delta CO (ΔCO) was calculated by subtracting the preceding value from the subsequent measurement. Sufficient concordance was assumed when the concordance rate was >92 % [14]. A polar plot was prepared, and angular bias as well as radial LOA were calculated as described by Critchley et al. [17]. A trending ability was assumed to be ‘good’ when the angular bias was within ±5° and radial LOA were <30° [17]. The central zone of insignificant change (ΔCO < 15 % for concordance and <10 % for polar plots) was excluded to avoid statistical noise [14, 17].
The precision of the reference method (ICOPA) was calculated according to the previous description by Cecconi et al. [18]. Briefly, the precision of ICOPA was defined as two times the coefficient of error (CE), where CE is defined as
where CV = coefficient of variation of a single measurement and n = number of measurements. CV was calculated as the SD divided by the mean.
Analyses were performed using the SPSS software (version 21.0.0.0 for Windows, IBM, Armonk, NY, USA), SigmaPlot (version 12.5 for Windows, Systat Software, Inc., San Jose, CA, USA) and MedCalc (Medcalc.org, version 14.8.1, MedCalc Software, Ostend, Belgium). Statistical significance was set at p < 0.05.
3 Results
Among 28 patients assessed for eligibility, 8 were excluded (5 had LV ejection fraction <30 %, 2 had persistent atrial fibrillation, and in 1 patient, the operation was cancelled due to preoperative fever of unknown origin). Thus, 20 patients were enrolled in the study between May and July 2014. Patient characteristics are presented in Table 1. The right internal jugular vein and right femoral artery were catheterised for PA and VolumeView™ catheters in 20 patients. A radial arterial cannula was placed on the right in 3 patients and the left in 17 patients for connection to a FloTrac™ sensor. All patients were infused with nitroglycerine at 0.3–0.5 μg/kg/min according to our institutional protocol. Among the 20 patients, there was no complication related to this study except for one patient who had a small amount of hematoma around the femoral artery, where the VolumeView™ catheter was placed. However, no intervention was required for the hematoma, and the patient was discharged from the hospital on the sixth postoperative day with no further problem.
Among 190 attempts to obtain CO data set, 23 were abandoned due to haemodynamic instability during measurements and 14 due to damped arterial pressure waveforms. Only complete data sets including all five CO measurements were included in the final analysis. Thus, eight sets were discarded due to analysis failure in one of the measurement systems. In total, 145 sets of CO measurements were completed and analysed. At each time point, 13–40 sets were obtained from 11–15 patients (Table 2). Unreliable data affected by haemodynamic instability were excluded and, in some patients, more than one measurement was performed during prolonged procedures: during the harvesting of grafts (T3), up to six sets of measurement per patient were obtained due to the procedure duration (up to 4 h or longer) and relatively stable haemodynamics than at the other time points during the procedure, such as induction of anaesthesia or revascularisation (Table 2). The mean (SD) CO values from each method (ICOPA, ICOVV, CCOVV, CCOPA, and CCOFT) were 4.2 (0.8), 4.1 (0.8), 4.1 (0.9), 3.8 (0.7), and 4.1 (1.0) L/min, respectively. A summary of CO measured by the five methods as well as haemodynamic and volumetric variables according to the perioperative time points are presented in Table 2. All patients received continuous infusion of nitroglycerine of 0.3–0.5 μg/kg/min according to our institution’s normal protocol. Vasopressor (norepinephrine, mean ± SD, 0.02 ± 0.01 μg/kg/min) was infused continuously during 24 % (35/145) of the measurements. The precision of the reference method (ICOPA) was measured to be ±14.4 %.
With the exception of CCOFT, the Bland–Altman analysis revealed agreement (% error <30 %) of ICOVV, CCOVV, and CCOPA, with the highest bias of CCOPA, when compared with ICOPA (Table 3; Fig. 1). Bolus transpulmonary thermodilution using the VolumeView™/EV1000™ system (ICOVV) showed the lowest % error. We found significant correlations between each CO measurement using the different methods and ICOPA (Table 3; Fig. 2). In trend analysis, only ICOVV showed good trending ability with ICOPA in both concordance (concordance rate >95 %) and polar plot (angular bias within ±5° and radial LOA <30°) analysis (Table 3; Figs. 3, 4).
Bland–Altman analysis for cardiac output (CO) measured by intermittent transpulmonary thermodilution (ICOVV) and continuous calibrated pulse contour analysis (CCOVV) using the VolumeView™/EV1000™ system, continuous pulmonary artery thermodilution (CCOPA), continuous uncalibrated pulse contour analysis using the FloTrac™/EV1000™ system (CCOFT) compared with intermittent pulmonary artery thermodilution (ICOPA). The solid line represents the bias, the two dashed lines the upper and lower limits of agreement. a Comparison of ICOPA and ICOVV. b Comparison of ICOPA and CCOVV. c Comparison of ICOPA and CCOPA. d Comparison of ICOPA and CCOFT
Correlation analysis for cardiac output (CO) measured by intermittent transpulmonary thermodilution (ICOVV) and continuous calibrated pulse contour analysis (CCOVV) using the VolumeView™/EV1000™ system, continuous pulmonary artery thermodilution (CCOPA), continuous uncalibrated pulse contour analysis using the FloTrac™/EV1000™ system (CCOFT) compared with intermittent pulmonary artery thermodilution (ICOPA). a Comparison of ICOPA and ICOVV. b Comparison of ICOPA and CCOVV. c Comparison of ICOPA and CCOPA. d Comparison of ICOPA and CCOFT
Concordance analysis for changes of cardiac output (CO) measured by intermittent transpulmonary thermodilution (ICOVV) and continuous calibrated pulse contour analysis (CCOVV) using the VolumeView™/EV1000™ system, continuous pulmonary artery thermodilution (CCOPA), continuous uncalibrated pulse contour analysis using the FloTrac™/EV1000™ system (CCOFT) compared with intermittent pulmonary artery thermodilution (ICOPA). Excluded central zone of ΔCO < 15 % is displayed. a Comparison of ICOPA and ICOVV. b Comparison of ICOPA and CCOVV. c Comparison of ICOPA and CCOPA. d Comparison of ICOPA and CCOFT
Polar plot analysis for changes of cardiac output (CO) measured by intermittent transpulmonary thermodilution (ICOVV) and continuous calibrated pulse contour analysis (CCOVV) using the VolumeView™/EV1000™ system, continuous pulmonary artery thermodilution (CCOPA), continuous uncalibrated pulse contour analysis using the FloTrac™/EV1000™ system (CCOFT) compared with intermittent pulmonary artery thermodilution (ICOPA). The green dashed line represents the mean angular bias and blue dashed lines the radial limits of agreement. Excluded central zone of ΔCO < 10 % is displayed. a Comparison of ICOPA and ICOVV. b Comparison of ICOPA and CCOVV. c Comparison of ICOPA and CCOPA. d Comparison of ICOPA and CCOFT
4 Discussion
In the present study, the accuracy, precision and trending ability of four methods of CO measurement were compared to those of ICOPA. During OPCAB surgery, ICOVV, CCOPA, and CCOVV demonstrated acceptable degrees of agreement with ICOPA (% error, 12.6, 26.7, and 29.3 %, respectively). In tracking changes of CO, only ICOVV showed a good trending ability when compared to ICOPA (concordance rate of 96.9 %, angular bias of 1.33°, and radial LOA of 28.71°). This is the first reported study to evaluate the degree of agreement and tracking ability of the VolumeView™ system in comparison with ICOPA in patients undergoing OPCAB surgery.
4.1 Cardiac output measurement during off-pump coronary artery bypass surgery
In cardiac surgery, especially during OPCAB surgery, the beating heart is manipulated and haemodynamic variables change dynamically, especially during revascularisation of the posterior or lateral wall [19]. Moreover, most patients also have underlying coronary artery disease, which can cause profound intraoperative cardiovascular deterioration [19]. Furthermore, there is abnormal vascular compliance in patients with cardiovascular diseases [20]. OPCAB surgery may provide particularly challenging circumstances in which to measure CO and track the changes thereof. In the present analysis, both transpulmonary thermodilution and continuous pulse contour analysis with the VolumeView™ system showed acceptable agreement with standard thermodilution, while only transpulmonary thermodilution using the VolumeView™ system showed good trending ability. The trending ability of a monitoring device can be more helpful in haemodynamic management and optimisation because the patient’s haemodynamic condition and responses may change during the surgical procedure. Our results indicate that transpulmonary thermodilution using the VolumeView™ system provides reliable data on CO in patients undergoing OPCAB surgery.
4.2 Transpulmonary thermodilution and calibrated pulse contour analysis
The PiCCO™ system is based on essentially the same algorithm as the VolumeView™ in measuring transpulmonary thermodilution CO [7]. For calibrated pulse contour analysis, the PiCCO™ system is based on the systolic waveform assessment (Wesseling approach), while the VolumeView™ system uses a combination of systolic and improved diastolic wave portion analysis (Windkessel model) [4, 6]. Several previous studies were performed to validate the accuracy of the PiCCO™ system versus ICOPA. They showed precise and reproducible performance compared with the reference measurement in patients in various clinical situations, such as organ transplantation and cardiac valve surgery, and in patients with reduced cardiac function [21–24]. However, some of them did not provide important information such as % error [22, 23], or calculated CO values from different devices with a shared injection for thermodilution [24]. In a recent study, transpulmonary thermodilution using the PiCCO™ system (ICOPiCCO) showed poor results in measuring (% error of 45 %) and tracking ability (angular bias of 3.9° and radial LOA of 41°) when compared with ICOPA in patients undergoing liver transplantation [25]. Wouters et al. [26] reported that ICOPiCCO using the brachial artery is a reliable method of measuring CO (bias of 0.91 L/min and LOA of ±0.98 L/min in comparison with ICOPA) during OPCAB surgery. While Ostergaard et al. [27] presented a % error of 21.2 % in a comparison of ICOPA and ICOPiCCO in 25 patients undergoing coronary artery bypass graft (CABG) (20 on-pump and 5 off-pump) surgery without evaluating trending ability, Yamashita et al. [28] demonstrated poor agreement (% error, 34–40 %) between continuous pulse wave analysis by the PiCCO™ system (CCOPiCCO) and ICOPA in vasodilatory status during OPCAB surgery. Thus, the PiCCO™ system has not been sufficiently evaluated or demonstrated to be consistent with ICOPA in agreement, or a trend analysis, especially in patients undergoing OPCAB surgery. However, in the present study, transpulmonary thermodilution using the VolumeView™ system showed lower bias and % error than the PiCCO™ device, and good trending ability when compared with ICOPA, during OPCAB surgery. Although the same mathematical algorithm is used to calculate CO [7], transpulmonary thermodilution using the VolumeView™ device may be more suitable than the PiCCO™ system and can be recommended as a reliable CO monitoring method in patients with preserved cardiac function undergoing OPCAB surgery.
4.3 Continuous thermodilution using a pulmonary artery catheter
In previous studies, Della Rocca et al. [22] compared CCOPA and ICOPA with resultant bias of 0.02 (2SD LOA, 1.48) L/min during liver transplantation and with 0.15 (1.39) L/min during lung transplantation surgery [23]. However, Vilchez-Monge et al. [25] reported no agreement (% error of 64 %) and limited trending ability (angular bias of 2.6° and radial LOA of 40°) of CCOPA compared to ICOPA in patients undergoing liver transplantation. Moreover, Halvorsen et al. [29] described poor agreement (% error, 32–50 %) between CCOPiCCO and CCOPA during OPCAB surgery, which requires positioning of the heart and the use of a stabilising device. In the current study, CCOPA demonstrated poor trending ability versus ICOPA during the OPCAB procedure. Although haemodynamic stability and constant use of infusates or vasoactive agents were confirmed prior to reading CCOPA values, the previously described time delay of CCOPA [3, 14], combined with dynamic haemodynamic changes inherent to surgical procedures [19, 26], may have affected the trending ability of this measurement system in patients undergoing OPCAB surgery.
4.4 Uncalibrated pulse contour analysis
The main advantage of an uncalibrated pulse wave analysis system is its simplicity in use (no requirement for a specific catheter) and minimal invasiveness (use of a readily accessible peripheral artery) [9]. The FloTrac™ sensor, via a conventional arterial catheter, derives stroke volume from the pulse pressure of the arterial pulse wave, after correcting for the compliance and the resistance of the vasculature [30]. However, in the absence of external calibration, the system also has innate shortcomings in evaluating cardiac performance values accurately. Although Mehta et al. [31] reported % error of 29 % between FloTrac™ system (version 1.07) and ICOPA in 12 OPCAB patients, the first and second generation FloTrac™ devices had shown insufficient reliability (range of % error, 31–54 % and 21.6–69 %, respectively) in CO measurement [11]. Despite the improvement in evaluation of vasomotor tone [especially in low systemic vascular resistance (SVR) state], the third generation FloTrac™ system also has shown inconsistent results in validation studies [9, 11]. While Vasdev et al. [32] demonstrated an acceptable agreement of CCOFT (version 3.02), compared with ICOPA (% error, 20 %) in patients undergoing on-pump CABG, Desebbe et al. [10] reported poor agreement between CCOFT (version 3.01) and ICOPA (% error, 66.5 %) in cardiac surgery patients. In the present study, we also observed the limits of an uncalibrated system, even with the most updated algorithm (FloTrac™ system, version 4.0), in measuring absolute CO values and in tracking their changes. Vasopressor use in 24 % of all measurements might have some influence on arterial elastance, and thus affected pulse wave analysis, especially CCOFT, which was not calibrated regularly, in the current results. However, our results are consistent with a previous study by Suehiro et al. [13], in which the authors reported limited performance of FloTrac™ system (version 4.0) in agreement (% error, 55.4 %) and tracking ability (concordance rate, 87.0 %) compared to ICOPA during cardiac surgery.
4.5 Study limitations
Our study had several limitations. We excluded patients with arrhythmia for accurate analysis of the arterial pulse waveform. However, many patients of older age or who require cardiac procedures frequently have various arrhythmias, including atrial fibrillation, in clinical practice. We also excluded patients with impaired ventricular contraction or with an intra-aortic balloon pump or other ventricular supporting device. Thus, the results of the present study may not be representative of those with arrhythmias, reduced cardiac function, or with supporting devices. It should be considered that we excluded such patients before applying our results to such patients or seeking to interpret or generalise these findings.
All patients in our study were infused with intravenous nitroglycerine (0.3–0.5 μg/kg/min) throughout the surgery according to our institutional protocol. Yamashita et al. [28] observed limited agreement between CCOPiCCO and ICOPA (% error of 34–40 %) in patients with vasodilation induced by prostaglandin E1 infusion during OPCAB surgery. Suehiro et al. [33] also discussed that CCOFT (version 3.02) showed poor agreement (% error, 46.3 %), as well as poor tracking ability, compared with ICOPA in the subgroup of patients with low SVR during cardiac surgery. In the current study, the vasodilatory effect of nitroglycerine infusion might have influenced the estimation of CO using a pulse wave-analysing algorithm (CCOVV and CCOFT) due to the possible alteration in arterial waveform with decreased SVR [28]. It should be considered when interpreting the present results that one could recognise better performance of pulse wave analysis method when the use of a vasodilating agent is excluded in those patients.
Furthermore, during repeated measures of intermittent transpulmonary thermodilution, the VolumeView™ system was repeatedly re-calibrated during the study period. This effect could have enhanced the accuracy of measurement with the device; thus, the results in this study may not represent a conventional method of continuous CO measurement in clinical practice. Nevertheless, pulse contour analysis, even with repeated re-calibration, failed to show good agreement with the standard method in trending ability. This should be taken into account when it is used in the clinical setting without regular intermittent re-calibration during measuring and monitoring CO.
5 Conclusions
During OPCAB surgery, CO measured by transpulmonary thermodilution and calibrated pulse contour analysis using the VolumeView™/EV1000™ system, and continuous PA thermodilution through a PA catheter demonstrated acceptable agreement with CO measured by PA bolus thermodilution. However, only transpulmonary thermodilution through the VolumeView™/EV1000™ system showed good trending ability in comparison with PA bolus thermodilution. Transpulmonary thermodilution using the VolumeView™ system provides reliable data on CO measurements and tracking the changes thereof when compared with PA bolus thermodilution in patients with preserved cardiac function during OPCAB surgery.
References
Finfer S, Delaney A. Pulmonary artery catheters. BMJ. 2006;333:930–1.
de Waal EE, Wappler F, Buhre WF. Cardiac output monitoring. Curr Opin Anaesthesiol. 2009;22:71–7.
Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK. Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth Analg. 2010;110:799–811.
Hofer CK, Rex S, Ganter MT. Update on minimally invasive hemodynamic monitoring in thoracic anesthesia. Curr Opin Anaesthesiol. 2014;27:28–35.
Koo KK, Sun JC, Zhou Q, Guyatt G, Cook DJ, Walter SD, Meade MO. Pulmonary artery catheters: evolving rates and reasons for use. Crit Care Med. 2011;39:1613–8.
Bendjelid K, Marx G, Kiefer N, Simon TP, Geisen M, Hoeft A, Siegenthaler N, Hofer CK. Performance of a new pulse contour method for continuous cardiac output monitoring: validation in critically ill patients. Br J Anaesth. 2013;111:573–9.
Kiefer N, Hofer CK, Marx G, Geisen M, Giraud R, Siegenthaler N, Hoeft A, Bendjelid K, Rex S. Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit Care. 2012;16:R98.
Bendjelid K, Giraud R, Siegenthaler N, Michard F. Validation of a new transpulmonary thermodilution system to assess global end-diastolic volume and extravascular lung water. Crit Care. 2010;14:R209.
Tsai YF, Liu FC, Yu HP. FloTrac/Vigileo system monitoring in acute-care surgery: current and future trends. Expert Rev Med Devices. 2013;10:717–28.
Desebbe O, Henaine R, Keller G, Koffel C, Garcia H, Rosamel P, Obadia JF, Bastien O, Lehot JJ, Haftek M, Critchley LA. Ability of the third-generation FloTrac/Vigileo software to track changes in cardiac output in cardiac surgery patients: a polar plot approach. J Cardiothorac Vasc Anesth. 2013;27:1122–7.
Suehiro K, Tanaka K, Matsuura T, Funao T, Yamada T, Mori T, Nishikawa K. The Vigileo-FloTrac System: arterial waveform analysis for measuring cardiac output and predicting fluid responsiveness: a clinical review. J Cardiothorac Vasc Anesth. 2014;28:1361–74.
Ji F, Li J, Fleming N, Rose D, Liu H. Reliability of a new 4th generation FloTrac algorithm to track cardiac output changes in patients receiving phenylephrine. J Clin Monit Comput. 2015;29:467–73.
Suehiro K, Tanaka K, Mikawa M, Uchihara Y, Matsuyama T, Matsuura T, Funao T, Yamada T, Mori T, Nishikawa K. Improved performance of the fourth-generation FloTrac/Vigileo system for tracking cardiac output changes. J Cardiothorac Vasc Anesth. 2015;29:656–62.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111:1180–92.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011;25:536–46.
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM. Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies—with specific reference to the measurement of cardiac output. Crit Care. 2009;13:201.
Chassot PG, van der Linden P, Zaugg M, Mueller XM, Spahn DR. Off-pump coronary artery bypass surgery: physiology and anaesthetic management. Br J Anaesth. 2004;92:400–13.
Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012;1(4):11.
Staier K, Wilhelm M, Wiesenack C, Thoma M, Keyl C. Pulmonary artery vs. transpulmonary thermodilution for the assessment of cardiac output in mitral regurgitation: a prospective observational study. Eur J Anaesthesiol. 2012;29:431–7.
Della Rocca G, Costa MG, Pompei L, Coccia C, Pietropaoli P. Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth. 2002;88:350–6.
Della Rocca G, Costa MG, Coccia C, Pompei L, Di Marco P, Vilardi V, Pietropaoli P. Cardiac output monitoring: aortic transpulmonary thermodilution and pulse contour analysis agree with standard thermodilution methods in patients undergoing lung transplantation. Can J Anaesth. 2003;50:707–11.
Friesecke S, Heinrich A, Abel P, Felix SB. Comparison of pulmonary artery and aortic transpulmonary thermodilution for monitoring of cardiac output in patients with severe heart failure: validation of a novel method. Crit Care Med. 2009;37:119–23.
Vilchez-Monge AL, Tranche Alvarez-Cagigas I, Perez-Pena J, Olmedilla L, Jimeno C, Sanz J, Bellon Cano JM, Garutti I. Cardiac output monitoring with pulmonary Vs transpulmonary thermodilution during liver transplantation. Interchangeable methods? Minerva Anestesiol. 2014;80:1178–87.
Wouters PF, Quaghebeur B, Sergeant P, Van Hemelrijck J, Vandermeersch E. Cardiac output monitoring using a brachial arterial catheter during off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2005;19:160–4.
Ostergaard M, Nielsen J, Rasmussen JP, Berthelsen PG. Cardiac output–pulse contour analysis vs. pulmonary artery thermodilution. Acta Anaesthesiol Scand. 2006;50:1044–9.
Yamashita K, Nishiyama T, Yokoyama T, Abe H, Manabe M. The effects of vasodilation on cardiac output measured by PiCCO. J Cardiothorac Vasc Anesth. 2008;22:688–92.
Halvorsen PS, Sokolov A, Cvancarova M, Hol PK, Lundblad R, Tonnessen TI. Continuous cardiac output during off-pump coronary artery bypass surgery: pulse-contour analyses vs pulmonary artery thermodilution. Br J Anaesth. 2007;99:484–92.
Chamos C, Vele L, Hamilton M, Cecconi M. Less invasive methods of advanced hemodynamic monitoring: principles, devices, and their role in the perioperative hemodynamic optimization. Perioper Med. 2013;2:19.
Mehta Y, Chand RK, Sawhney R, Bhise M, Singh A, Trehan N. Cardiac output monitoring: comparison of a new arterial pressure waveform analysis to the bolus thermodilution technique in patients undergoing off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2008;22:394–9.
Vasdev S, Chauhan S, Choudhury M, Hote MP, Malik M, Kiran U. Arterial pressure waveform derived cardiac output FloTrac/Vigileo system (third generation software): comparison of two monitoring sites with the thermodilution cardiac output. J Clin Monit Comput. 2012;26:115–20.
Suehiro K, Tanaka K, Funao T, Matsuura T, Mori T, Nishikawa K. Systemic vascular resistance has an impact on the reliability of the Vigileo-FloTrac system in measuring cardiac output and tracking cardiac output changes. Br J Anaesth. 2013;111:170–7.
Acknowledgments
The authors thank the Medical Research Collaborating Centre for their advice concerning the statistical analyses. Twenty VolumeView™ sets used in this study were provided free of charge by the manufacturer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The contents of this study have not been published elsewhere nor are being submitted elsewhere. The manuscript has been read and approved by all co-authors, and does not have any potential conflicts of interest including commercial relationships such as consultation and equity interests.
Ethical approval
All procedures performed in the study involving human participates were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cho, Y.J., Koo, CH., Kim, T.K. et al. Comparison of cardiac output measures by transpulmonary thermodilution, pulse contour analysis, and pulmonary artery thermodilution during off-pump coronary artery bypass surgery: a subgroup analysis of the cardiovascular anaesthesia registry at a single tertiary centre. J Clin Monit Comput 30, 771–782 (2016). https://doi.org/10.1007/s10877-015-9784-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9784-6