Abstract
To compare the accuracy and trending ability of the cardiac index (CI) measured by FloTrac/Vigileo™ (CIFT) or derived by the Fick equation (CIFick) using E-CAiOVX (enables continuous monitoring of oxygen consumption) with that measured by thermodilution (CITD) in patients with off-pump coronary artery bypass surgery. Twenty-two patients undergoing elective off-pump coronary artery bypass surgery were included. CIFT and CIFick were determined simultaneously at six time-points during off-pump coronary artery bypass surgery. At each time-point, phenylephrine (50 µg) was administered to increase systematic vascular resistance, with CI measured before and after administration (CITD used as reference method). Agreement of each method was evaluated by Bland–Altman analysis, while trending ability was evaluated by four-quadrant plot analysis and polar plot analysis. By Bland–Altman analysis, CIFT and CIFick showed percentage errors of 49.5% and 78.6%, respectively, compared with CITD. Subgroup analysis showed a percentage error between COFT and COTD of 28.9% in patients with a CI ≥ 2.4 L/min/m2, and 78.1% in patients with a CI ≥ 2.4 L/min/m2. The concordance rate of four-quadrant plot analysis was 93.3% for CIFT and 66.7% for CIFick in datasets where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration were included. CIFT and CIFick had wide limits of agreement with CITD, and were below acceptable limits for tracking phenylephrine-induced CI changes. However, subgroup analysis showed improved accuracy and trending ability of CIFT when only points where CITD ≥ 2.4 L/min/m2 were included, while there was no improvement in CIFick accuracy or trending ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With recent technological advances, a number of minimally invasive monitors have been developed for measuring cardiac index (CI). For example, the FloTrac/Vigileo™ measures pulse pressure-derived CI without external calibration, and is widely used perioperatively in operating theaters and intensive care units, at least in part, because of concerns regarding complications related to pulmonary arterial catheterization. However, the accuracy of CI measured by FloTrac/Vigileo™ (CIFT) can be unreliable in some patients, especially those with a high or low systemic vascular resistance (SVR) [1, 2]. The FloTrac/Vigileo™ was upgraded and the algorithm modified several times to overcome this limitation, although it was recently reported to be inaccurate in patients with a low CI, likely because of the high SVR in these patients [3,4,5]. For the fourth-generation FloTrac/Vigileo™, the algorithm was improved by adding a correction factor to follow changes in SVR, which markedly improved the trending ability after phenylephrine administration in patients undergoing cardiac surgery [6]. However, the effect of low CI on the accuracy and trending ability of the fourth-generation FloTrac/Vigileo™ in patients undergoing cardiac surgery remains unclear. Recently, techniques for real time measurement of oxygen consumption (V̇O2) were also developed, allowing calculation of CI using the Fick equation. However, the accuracy and trending ability of CI derived by the Fick equation (CIFick) during cardiovascular surgery is unknown.
A wealth of experimental and clinical studies have validated the accuracy, reliability, and clinical relevance and utility of thermodilution-based CI measurements [7], and bolus thermodilution is the most accepted reference method [8,9,10]. Thus, the aim of the present study was to compare the accuracy and trending ability of CIFT and CIFick with the thermodilution technique (CITD; reference method) in patients undergoing off-pump coronary artery bypass surgery, as well as the effect of low CI on these parameters.
2 Methods
2.1 Patients
This study was prospective observational single center study. The study protocol was approved by the ethics committee of the National Cerebral and Cardiovascular Center, Osaka, Japan, (file number: M26-023-5), and met the guidelines of the Helsinki Declaration. All patients gave informed consent to participate in the study, which included a prospective analysis of 22 patients who underwent off-pump coronary artery bypass surgery from July 2014 to March 2016. All patients underwent off-pump coronary artery bypass surgery under general anesthesia. Exclusion criteria were severe valvular disease, arrhythmias, emergency surgery, or mechanical circulatory support.
After induction of anesthesia with fentanyl (1.5–2 µg/kg), midazolam (0.1 mg/kg), and continuous infusion of propofol (4–6 mg/kg/h), 1 mg/kg rocuronium was given to facilitate orotracheal intubation with a cuffed endotracheal tube. All patients were maintained under anesthesia with propofol (4–6 mg/kg/h) and remifentanil (0.3–0.5 µg/kg/min) using mechanical ventilation with a tidal volume of 8–10 ml/kg body weight at a frequency of 8–12 breaths per min, to maintain an end-tidal carbon dioxide at 35–40 mmHg. The fraction of inspiratory oxygen during surgery was maintained at 30–60%. Measurement of V̇O2 was performed using D-lite flow sensors (Datex-Ohmeda Division, Instrumentarium Co., Helsinki, Finland), which attach in-line to an artificial airway and include a side-stream sampling port for gas analysis and spirometry [11]. Continuous gas sampling through a gas sensor connected to a GE CARESCAPE B650 Monitor with an E-CAiOV Airway Module (GE Healthcare, Milwaukee, WI, USA) to provide breath-by-breath analysis of expired air, including measurement of V̇O2 [11, 12].
After anesthesia induction, the radial artery was cannulated, providing hemodynamic monitoring using the FloTrac/Vigileo™ system (version 4.00; Edwards Lifesciences, Irvine, CA, USA). The central venous catheter and the pulmonary arterial catheter (PAC) were inserted by the attending anesthesiologist. Correct positioning of the catheters was confirmed by pressure waves and transesophageal echocardiography. The Vigilance™ monitor (Edwards Lifesciences) was connected to the PAC to measure CI.
2.2 Study protocol
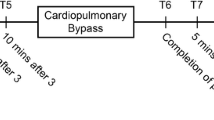
A series of CI data were measured using three different methods at six time-points, as follows: (T1) after anesthesia induction; (T2) after sternotomy; (T3) during anastomosis of the left anterior descending branch of the left coronary artery; (T4) during tilting of the heart; (T5) after protamine injection; and (T6) at the end of surgery. At each point, phenylephrine (50 µg) was administered to increase SVR, and CIs were obtained before and after administration. Twelve CIs were measured per patient.
The reference CI was measured using the conventional PAC thermodilution technique with ice-cold saline (10-mL bolus injection). The average of three consecutive CI measurements with PAC was used as the reference CI. We simultaneously recorded CIFT from the FloTrac/Vigileo™, and standard hemodynamic data [heart rate, mean arterial pressure (MAP), and central venous pressure] and V̇O2 using E-CAiOVX (GE Healthcare). Immediately after the calculation of CITD, blood samples were collected simultaneously from the arterial catheter and the distal port of the PAC. Blood gas analyses were performed on a multi-wavelength optical blood analyzer (ABL800 FLEX; Radiometer Medical A/P/S, Copenhagen, Denmark).
2.3 Determining CIFT using the FloTrac/Vigileo™ system
Stroke volume (SV) was calculated with the FloTrac/Vigileo™ system using arterial pulsatility (standard deviation [SD] of the pulse pressure over a 20-s interval), resistance, and compliance. Cardiac output (CO), CI, and SV were calculated as follows: CO = HR × SV, CI = CO/body surface area, and SV = Κ × pulsatility, respectively, where K is a constant quantifying arterial compliance and vascular resistance. K was derived from a multivariate regression model that included Langewouters’ model of aortic compliance, mean arterial blood pressure, and variance, skewness, and kurtosis of the pressure curve [4, 5, 13]. The rate of adjustment of K was 1 min.
2.4 Determining CIFick using the FloTrac/Vigileo™ system
CIFick was calculated by applying the Fick principles using V̇O2 and the difference between arterial (CaO2) and venous (CvO2) blood oxygen contents. CaO2 and CvO2 were calculated as: hemoglobin [Hb] value × arterial Hb oxygenation × 1.36 and Hb × SvO2 × 1.36, respectively. CO and CI were calculated as: CO = V̇O2/CaO2 − CvO2 and CI = CO/body surface area, respectively.
2.5 Statistical methodology
All data are expressed as mean ± SD or number. Patients’ hemodynamic data were compared using a paired t-test. All statistical analyses were performed with statistical software (EZR statistical software, Saitama Medical Center, Jichi Medical University, Saitama, Japan; available at http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) [14], which is a modified version of the R commander (R Foundation for Statistical Computing, Vienna, Austria). A P value < 0.05 was considered statistically significant.
Agreement of each method was evaluated by the Bland–Altman method [15]. Bias was defined as the difference between the test method and the reference method, and represents the systematic error between methods. Precision was defined as the SD of the bias, and represents the random error or variability between the techniques. Limits of agreement was defined as the range in which 95% of the differences between the methods were expected to lie, and were calculated as bias ± 2SD. The percentage error was calculated as 2SD of the bias/mean CI of the reference method. The percentage error was considered clinically acceptable, and the tested method (CIFT or CIFick) was regarded as interchangeable with the reference method (CITD), if it was < 30%, as proposed by Critchley et al. [16] To examine the effect of low CI on the association of CIFT with CITD, or of CIFick with CITD, we subdivided the measured CI sets into two groups according to the measured CITD, as follows: (1) CITD < 2.4 L/min/m2 and (2) CITD ≥ 2.4 L/min/m2. The cut off value of 2.4 L/min/m2 was defined according to published literature, where a CI of < 2.2 L/min/m2 represents shock, while a CI > 2.6 L/min/m2 is considered normal [17, 18]. The Bland–Altman method was used for analysis in each group.
We used a four-quadrant plot analysis to examine the trending ability of CIFT and CIFick, which can assess the concordance rate between percentage change in CITD (∆CITD) and the percentage change in CIFT (∆CIFT) or percentage change in CIFick (∆CIFick). The concordance rate was calculated before and after phenylephrine administration at each time-point. As the direction of the change in CI at the center of the plot varied randomly, this can cause unreliability in the trending ability of the test methods. To avoid this limitation, we set an exclusion zone defined as the area of percentage change in CI < 10%, based on a previous study [2]. We then used polar plot analysis to assess the agreement between the two methods, using the angle of the vector with the line of identity (y = x) and the magnitude of change by the length of the vector [19, 20]. The plots used in four-quadrant plot analysis were rotated 45° clockwise. The following statistical variables were calculated from the polar plot analysis: (1) mean angular bias, which represents the average angle between all the polar data points and the polar axis; (2) radial limits of agreement, which represents the radial sector containing 95% of the data points; and (3) angular concordance rate, which represents the percentage of points in the 30° radial zone. A previous study suggested the following acceptance limits for polar plot analysis: (1) angular bias of < ± 5°, (2) radial limit of agreement < ± 30°, and (3) angular concordance rate > 95% [19].
3 Results
Twenty-two patients (three women, 19 men) were enrolled in this study. Patients’ characteristics are shown in Table 1, and hemodynamic data are summarized in Table 2. MAP and systemic vascular resistance (SVRI) significantly increased after phenylephrine administration at each time-point (P < 0.05 for both MAP and SVRI). By contrast, there was no change in CI measured by three methods following phenylephrine administration at each time-point.
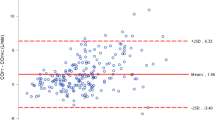
To clarify the accuracy and trending ability of CIFT and CIFick, we first performed a Bland–Altman analysis to compare CIFT and CIFick with CITD. Bland–Altman analysis results for comparisons between CIFT and CITD for all measures (Fig. 1a) showed a percentage error of 49.5%, suggesting that CIFT had a wide limit of agreement with CITD. Bland–Altman analysis results for comparisons between CIFick and CITD for all measures (Fig. 1b) showed a percentage error of 78.6%, suggesting that CIFick also had a wide limit of agreement with CITD.
Next, we divided the measured CI sets into two groups for subanalysis according to the measured CITD, as follows: (1) CITD < 2.4 L/min/m2 (177 sets) and (2) CITD ≥ 2.4 L/min/m2 (87 sets). The percentage error of CIFT improved to 28.9% in the CITD ≥ 2.4 L/min/m2 group, but was 56.2% in the CITD < 2.4 L/min/m2 group (Fig. 2a). This suggests that the FloTrac/Vigileo™ was not accurate in patients with a low CI, particularly those with a CI < 2.4 L/min/m2. By contrast, the percentage error of CIFick was 78.1% in the CITD ≥ 2.4 L/min/m2 group, and 70.5% in the CITD < 2.4 L/min/m2 group, showing acceptable limits in both groups (Fig. 2b). SVRI was significantly higher in patients with a CITD < 2.4 L/min/m2 versus the CITD ≥ 2.4 L/min/m2 group (1798 ± 446 versus 3003 ± 786 dyne s/cm5/m2, respectively; P < 0.001), which may have affected the accuracy of the FloTrac/Vigileo™.
We then examined the trending ability of CIFT and CIFick using four-quadrant plot analysis. The concordance rate was 85.9% for CIFT (Fig. 3a) and 63.6% for CIFick (Fig. 3b). These concordance rates were below 92%, which is considered the acceptable cut-off [20]. Next, we examined the trending ability of CIFT and CIFick using polar plots analysis, which showed a mean angular bias of − 0.6° and 10.1°, respectively, radial limits of agreement of 39.7° and 64.1°, respectively, and concordance rates of 73.1% and 57.4%, respectively (Fig. 4a, b). Both of the angular concordance rates were below the acceptable limit (> 95%).
When we included the 38 points where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration, the concordance rate of the four-quadrant plot analysis was 93.3% for CIFT (Fig. 5a) and 66.7% for CIFick (Fig. 5b). The concordance rate for CIFT was within the acceptable limit (> 92%), which is considered to represent a good trending ability. As for the polar plot analysis, the angular concordance rate of CIFT was 90.9%, which was near the acceptable limit (> 95%) (Fig. 6a). The angular concordance rate of CIFick was 38.9%, below the acceptable limit (Fig. 6b).
Four-quadrant plot analysis of trending ability using 38 datasets where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration. a ∆CIFT versus ∆CITD. b ∆CIFick versus ∆CITD. The plots at the center represent an exclusion zone (shaded black area) set as the percentage change in cardiac output < 10%
Polar plots analysis of trending abilities using 38 datasets where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration. a ∆CIFT versus ∆CITD. b ∆CIFick versus ∆CITD. The angle from the axis (0°) shows agreement between the two methods. The shaded black area represents an exclusion zone of 10%
4 Discussion
This study investigated the accuracy and trending ability of CI derived using the fourth-generation FloTrac/Vigileo™ (CIFT) and the Fick method (CIFick) in patients undergoing off-pump coronary bypass surgery by comparing with CITD. Results showed that both CIFT and CIFick had a wide limit of agreement compared with CITD, and that the trending ability of CIFT and CIFick were below the acceptable limits for tracking phenylephrine-induced CI changes. However, subgroup analysis revealed that the accuracy of CIFT and the trending ability of CIFT both improved when only points where CITD ≥ 2.4 L/min/m2 were included. By contrast, there were no improvements in accuracy or trending ability in CIFick with subgroup analysis.
The accuracy of the fourth-generation FloTrac/Vigileo™ has been investigated in various clinical situations, including cardiac surgery, cardiac resynchronization therapy implantation surgery, and other elective surgeries requiring continuous arterial pressure monitoring [4,5,6, 21, 22]. The ability of FloTrac/Vigileo™ to follow changes in SVR was improved using the new algorithm. However, the calculation used for CI measurement is that same as previous versions. Thus, there is still a wide limit of agreement between CI measured by FloTrac/Vigileo™ and the reference method in previous studies [4,5,6, 21, 22], likely because the accuracy of FloTrac/Vigileo™ is strongly influenced by vascular tone. Our finding of an overall percentage error between CIFT and CITD of 49.5% (suggesting that CIFT had a wide limit of agreement with CITD) was comparable with those studies.
The trending ability of the fourth-generation FloTrac/Vigileo™ system after increased vasomotor tone induced by phenylephrine was reported to be markedly improved compared with previous versions [6, 21]. Our study revealed a concordance rate of 85.9%, which was less than the accepted cut-off of 92% [20]. However, if we included only points where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration, the concordance rate of the four-quadrant plot analysis improved to 93.3%. Further, the percentage error of CIFT improved to 28.9% in the CITD ≥ 2.4 L/min/m2 group. These improvements may relate to the high SVRI in the CITD < 2.4 L/min/m2 group. SVRI was significantly higher in patients with a CITD < 2.4 L/min/m2 versus the CITD ≥ 2.4 L/min/m2 group (1798 ± 446 versus 3003 ± 786 dyne s/cm5/m2, respectively; P < 0.001). As a high SVRI can affect the accuracy of the FloTrac/Vigileo™, this may cause the inaccuracy of CIFT in patients with a CITD < 2.4 L/min/m2. Overall, these findings suggest that FloTrac/Vigileo™ may be more accurate and precise in patients with a higher CI.
In addition, we examined the unadjusted relationship between CITD and the ratio of the discrepancy of CI (i.e., either (CIFT−CITD)/CITD or (CIFick − CITD)/CITD) using a cubic spline function to identify any inflection point (Supplementary Fig. 1, Supplementary Fig. 2). Both the cubic splines related to the CITD and CI discrepancy were negatively sloped, showing that CI had a significant effect in the discrepancy of CI (P < 0.05). There was an inflection point at approximately 2.3–2.6 L/min/m2 (Supplementary Fig. 1), after which the discrepancy of CI ((CIFT − CITD)/CITD) almost plateaued around zero. By contrast, this inflection point was not seen in Supplementary Fig. 2. In addition to CITD, we investigated the impact of heart rate, MAP, and SVRI on the discrepancy of CI by applying the cubic spline function for these data. Apart from the impact of SVRI on (CIFT − CITD)/CITD (Supplementary Fig. 3), there was no association between the parameters and CI discrepancy (data not shown). These findings suggest that only SVRI affected the accuracy of the FloTrac/Vigileo™, resulting in the inaccuracy in patients with high SVRI.
We also investigated the accuracy and trending ability of CI measured by the Fick equation using V̇O2 derived from E-CAiOVX in patients undergoing off-pump coronary artery bypass. However, both the accuracy and trending ability were less than the acceptable limits, suggesting that these two methods are not interchangeable. This inaccuracy may relate to metabolic consumption of oxygen by the lung itself [23, 24], or diffusive loss of oxygen through the visceral pleura during the surgery [25, 26]. We maintained the fraction of inspiratory oxygen during surgery at 30–60%, which will increase the partial pressure of alveolar oxygen, and may lead to increased oxygen loss through the visceral pleura. These factors contribute to V̇O2 measured at the level of the mouth, but are not part of the net uptake of oxygen by the blood as it traverses the lung [24]. Thus, these confounders would not be affected by CI, which may explain the lack of improvement in accuracy and trending ability of CIFick in patients with a CITD ≥ 2.4 L/min/m2.
We acknowledge several limitations in our study. First, our sample size was relatively small, and there were only 38 data sets with a CITD ≥ 2.4 L/min/m2 for the subanalysis of trending ability. Second, catecholamine use was left at the discretion of the attending anesthesiologist. Norepinephrine (0.02–0.08 µg/kg/min) was used in all patients at T4, and low-dose dopamine (2.2–4.2 µg/kg/min) was used in all patients at T5 and T6, with or without norepinephrine (0.01–0.02 µg/kg/min). This may have affected the SVRI of patients, and contributed to the inaccuracy of the FloTrac/Vigileo™. To overcome this limitation, we excluded the T4, T5, and T6 datasets to conduct a Bland–Altman sensitivity analysis (four-quadrant plot), which showed a percentage error of 36.8% for CIFT and 69.6% for CIFick, and a concordance rate of 86.4% for CIFT and 73.3% for CIFick. Polar plots analysis for CIFT and CIFick showed a mean angular bias of 3.0° and 14.7°, respectively, radial limits of agreement of 39.8° and 43.9°, respectively, and a concordance rate of 73.1% and 76.3%, respectively. Further, if we included 23 datasets with CITD ≥ 2.4 L/min/m2 for subanalysis, Bland–Altman analysis showed percentage errors of 25.0% for CIFT and 83.6% for CIFick. If we included the nine points where CITD ≥ 2.4 L/min/m2 before and after phenylephrine administration, the concordance rate of the four-quadrant plot analysis was 100% for CIFT and 60.0% for CIFick. As for the polar plot analysis, the angular concordance rate was 100% for CIFT and 66.7% for CIFick. These subgroup-sensitivity analyses are comparable with those of the total data sets, suggesting that our data are robust. Finally, when sampling blood for measurements, we took care to avoid bubbles, and performed measurements immediately to avoid metabolic consumption of oxygen. However, it remains possible that these factors may have affected the accuracy of CaO2 or CvO2.
In conclusion, we found that both CIFT and CIFick had a wide limit of agreement with CITD, and that the trending ability of CIFT and CIFick were below the acceptable limits for tracking phenylephrine-induced CI changes. However, subgroup analysis revealed an improvement in the accuracy and trending ability of CIFT when only points where CITD ≥ 2.4 L/min/m2 were included, while there were no improvements for CIFick.
References
Biancofiore G, Critchley LA, Lee A, Yang XX, Bindi LM, Esposito M, Bisa M, Meacci L, Mozzo R, Filipponi F. Evaluation of a new software version of the FloTrac/Vigileo (version 3.02) and a comparison with previous data in cirrhotic patients undergoing liver transplant surgery. Anesth Analg. 2011;113(3):515–22. https://doi.org/10.1213/ANE.0b013e31822401b2.
Suehiro K, Tanaka K, Funao T, Matsuura T, Mori T, Nishikawa K. Systemic vascular resistance has an impact on the reliability of the Vigileo-FloTrac system in measuring cardiac output and tracking cardiac output changes. Br J Anaesth. 2013;111(2):170–7. https://doi.org/10.1093/bja/aet022.
Maeda T, Yoshitani K, Inatomi Y, Ohnishi Y. Inaccuracy of the FloTrac/Vigileo system in patients with low cardiac index. J Cardiothorac Vasc Anesth. 2014;28(6):1521–6. https://doi.org/10.1053/j.jvca.2014.04.013.
Hattori K, Maeda T, Masubuchi T, Yoshikawa A, Ebuchi K, Morishima K, Kamei M, Yoshitani K, Ohnishi Y. Accuracy and Trending ability of the fourth-generation FloTrac/Vigileo system in patients with low cardiac index. J Cardiothorac Vasc Anesth. 2017;31(1):99–104. https://doi.org/10.1053/j.jvca.2016.06.016.
Maeda T, Hattori K, Sumiyoshi M, Kanazawa H, Ohnishi Y. Accuracy and trending ability of the fourth-generation FloTrac/Vigileo system in patients undergoing abdominal aortic aneurysm surgery. J Anesth. 2018;32(3):387–93. https://doi.org/10.1007/s00540-018-2491-y.
Suehiro K, Tanaka K, Mikawa M, Uchihara Y, Matsuyama T, Matsuura T, Funao T, Yamada T, Mori T, Nishikawa K. Improved performance of the fourth-generation flotrac/vigileo system for tracking cardiac output changes. J Cardiothorac Vasc Anesth. 2015;29(3):656–62. https://doi.org/10.1053/j.jvca.2014.07.022.
Pugsley J, Lerner AB. (2010) Cardiac output monitoring: is there a gold standard and how do the newer technologies compare? Semin Cardiothorac Vasc Anesth 14 (4):274–282. https://doi.org/10.1177/1089253210386386.
Thiele RH, Bartels K, Gan TJ. Cardiac output monitoring: a contemporary assessment and review. Crit Care Med. 2015;43(1):177–85. https://doi.org/10.1097/CCM.0000000000000608.
Squara P, Cecconi M, Rhodes A, Singer M, Chiche JD. Tracking changes in cardiac output: methodological considerations for the validation of monitoring devices. Intensive Care Med. 2009;35(10):1801–8. https://doi.org/10.1007/s00134-009-1570-9.
Joosten A, Desebbe O, Suehiro K, Murphy LS, Essiet M, Alexander B, Fischer MO, Barvais L, Van Obbergh L, Maucort-Boulch D, Cannesson M. Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysis dagger. Br J Anaesth. 2017;118(3):298–310. https://doi.org/10.1093/bja/aew461.
Seckeler MD, Hirsch R, Beekman RH 3rd, Goldstein BH. A new predictive equation for oxygen consumption in children and adults with congenital and acquired heart disease. Heart. 2015;101(7):517–24. https://doi.org/10.1136/heartjnl-2014-306378.
Masuda T, Kuramoto M, Tanimoto H, Yamamoto K, Ikeshima S, Kitano Y, Kuroda D, Shimada S, Baba H. Intraoperative baseline oxygen consumption as a prognostic factor in emergency open abdominal surgery. J Crit Care. 2016;32:42–7. https://doi.org/10.1016/j.jcrc.2015.11.014.
Langewouters GJ, Wesseling KH, Goedhard WJ. The pressure dependent dynamic elasticity of 35 thoracic and 16 abdominal human aortas in vitro described by a five component model. J Biomech. 1985;18(8):613–20.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. https://doi.org/10.1038/bmt.2012.244.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15(2):85–91.
Gillies M, Bellomo R, Doolan L, Buxton B. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Critical Care. 2005;9(3):266–79. https://doi.org/10.1186/cc3024.
Abualsaud AO, Lowe BS, Guo K, Marelli AJ, Kaouache M, Guo L, Jutras L, Martucci G, Therrien J. Cardiac output as a predictor in congenital heart disease: are we stating the obvious? Int J Cardiol. 2016;210:143–8. https://doi.org/10.1016/j.ijcard.2016.02.071.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011;25(3):536–46. https://doi.org/10.1053/j.jvca.2011.01.003.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111(5):1180–92. https://doi.org/10.1213/ANE.0b013e3181f08a5b.
Ji F, Li J, Fleming N, Rose D, Liu H. Reliability of a new 4th generation FloTrac algorithm to track cardiac output changes in patients receiving phenylephrine. J Clin Monit Comput. 2015;29(4):467–73. https://doi.org/10.1007/s10877-014-9624-0.
Lin SY, Chou AH, Tsai YF, Chang SW, Yang MW, Ting PC, Chen CY. Evaluation of the use of the fourth version FloTrac system in cardiac output measurement before and after cardiopulmonary bypass. J Clin Monit Comput. 2017. https://doi.org/10.1007/s10877-017-0071-6.
Smithies MN, Royston B, Makita K, Konieczko K, Nunn JF. Comparison of oxygen consumption measurements: indirect calorimetry versus the reversed Fick method. Crit Care Med. 1991;19(11):1401–6.
Peyton PJ, Robinson GJ. Measured pulmonary oxygen consumption: difference between systemic oxygen uptake measured by the reverse Fick method and indirect calorimetry in cardiac surgery. Anaesthesia. 2005;60(2):146–50. https://doi.org/10.1111/j.1365-2044.2004.04044.x.
Shepard JW Jr, Minh VD, Dolan GF. Gas exchange in nonperfused dog lungs. J Appl Physiol. 1981;51(5):1261–7. https://doi.org/10.1152/jappl.1981.51.5.1261.
Light RB. Intrapulmonary oxygen consumption in experimental pneumococcal pneumonia. J Appl Physiol. 1988;(1985) 64(6):2490–5. https://doi.org/10.1152/jappl.1988.64.6.2490.
Acknowledgements
We thank GE Healthcare for rental of the E-CAiOVX (GE Healthcare, WI, US), and Jane Charbonneau, DVM, from Edanz Group (http://www.edanzediting.com/ac), for editing a draft of this manuscript. We also thank the resident doctors who cared for the patients in this study.
Funding
This study was supported in part by JSPS KAKENHI (Grant Number JP17K11100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10877_2018_217_MOESM1_ESM.pdf
Supplementary Figure S1. Cubic spline function curves of the relationship between CITD and CI discrepancy (i.e., (CIFT − CITD)/CITD). Shaded areas represent 95% confidence intervals. (PDF 192 KB)
10877_2018_217_MOESM2_ESM.pdf
Supplementary Figure S2. Cubic spline function curves of the relationship between CITD and CI discrepancy (i.e., (CIFick − CITD)/CITD). Shaded areas represent 95% confidence intervals. (PDF 191 KB)
10877_2018_217_MOESM3_ESM.pdf
Supplementary Figure S3. Cubic spline function curves of the relationship between SVRI and CI discrepancy (i.e., (CIFT − CITD)/CITD). Shaded areas represent 95% confidence intervals. (PDF 207 KB)
Rights and permissions
About this article
Cite this article
Maeda, T., Hamaguchi, E., Kubo, N. et al. The accuracy and trending ability of cardiac index measured by the fourth-generation FloTrac/Vigileo system™ and the Fick method in cardiac surgery patients. J Clin Monit Comput 33, 767–776 (2019). https://doi.org/10.1007/s10877-018-0217-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0217-1