Abstract
A series of 1-D lanthanide coordination polymers [Ln(μ3-OH)(pybz)(pa)] n (Ln = Er (1), Tb (2), Gd (3), Hpybz = 4-pyridin-4-yl-benzoic acid, Hpa = 2-picolinic acid) based on [Ln4(μ3-OH)4] cluster units have been hydrothermally synthesized and characterized by single crystal X-ray diffraction, IR, elemental analysis, and thermogravimetric analysis. X-ray crystal structure analyses reveal that 1–3 are isomorphous with tetragonal space group P \( \overline{4} \)21c and comprise tetranuclear Ln–O clusters, in which four Ln3+ centers are joined together by four μ3-bridging hydroxyl groups to form cubane-like [Ln4(μ3-OH)4]8+ cores that are further linked by four μ3-pa− ligands to produce 1-D chains along the c-axis.
Graphical Abstract
A series of 1-D lanthanide coordination polymers [Ln(μ3-OH)(pybz)(pa)] n (Ln = Er (1), Tb (2), Gd (3), Hpybz = 4-pyridin-4-yl-benzoic acid, Hpa = 2-picolinic acid) based on [Ln4(μ3-OH)4] cubanes have been hydrothermally synthesized. For this compound, tetranuclear cubane-like clusters [Ln4(μ3-OH)4]8+ composed of four Ln3+ centers as building blocks are further assembled into 1-D chains along the c-axis.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current increasing interest in designing and synthesizing polynuclear complexes, in particular lanthanide based oxo-hydroxo clusters has been significantly provoked due to their novel structural characteristics and rich electronic, magnetic, optical, and catalytic properties [1–5].Among these lanthanide clusters, Ln4(OH)4 cubane is a common structural motif and can serve as a building block [6–8]. A general class of complexes based on tetranuclear, hydroxo lanthanide cubane-like clusters have recently been prepared, providing further insights into the formation, reactivity, and supramolecular assembly of these compounds [9, 10]. Noticeably, selection of the multifunctional organic ligands is a key point to design and assemble expected complexes. So far, various ligands are used to bulid these cubanes, such as amino acids [11], perchlorates [12], diketonates [13], and carboxylates [14]. The isonicotinic acid (Hin) is a multifunctional bridging ligand as a rigid linear ligand constructing extended frameworks with high structural stability and special topologies. Based on these considerations, we have chosen isonicotinic acid as the bridging ligand, which have successfully obtained a series of lanthanide cluster polymers [Er7(μ3-O)(μ3-OH)6(bdc)3](in)9[Cu3X4] (X = Cl or Br, H2bdc = 1,2-benzenedicarboxylic acid) [15] and [Ln6(μ3-O)2](in)18[Cu8(μ4-I)2(μ2-I)3]·H3O (Ln = Y, Nd, Dy, Gd, Sm, Eu, Tb) [16], nanosized hydroxo lanthanide cluster polymers [Ln14(μ6-O)(μ3-OH)20(in)22Cu6Cl4(H2O)8]·6H2O (Ln = Y, Gd, Dy) [17], and [Er4(in)8(bdc)2(OH)(H2O)5][Cu8I7] [18]. Summing up of these results we have obtained, these compounds are always dense due to the restriction on the length of Hin. As a continuation of our search, we have tried to obtain the more open frameworks under rationally hydrothermal conditions by replacing Hin with the lengthened ligand 4-pyridin-4-yl-benzoic acid. Unexpected, we obtained a series of 1-D lanthanide coordination polymers [Ln(μ3-OH)(pybz)(pa)] n (Ln = Er (1), Tb (2), Gd (3), Hpybz = 4-pyridin-4-yl-benzoic acid, Hpa = 2-picolinic acid). To the best of our knowledge, few 1-D polymers based on Ln4(OH)4 cubanes have been reported.
Experimental
Materials and Methods
All chemicals used during the course of this work were of reagent grade and used as received from commercial sources without further purification. Elemental analyses of C, H, and N were carried out with a Vario EL III elemental analyzer. IR spectra were recorded in the range 400–4000 cm−1 on an ABB Bomem MB102 spectrometer in KBr pellets.
Synthesis of [Er(μ3-OH)(pybz)(pa)] n (1)
A mixture of Er2O3 (0.25 mmol, 0.096 g), Hpybz (0.5 mmol, 0.103 g), Hpa (1 mmol, 0.123 g), H2O (10 mL), and HClO4 (0.5 mmol) was sealed in a 30 mL Teflon-lined bomb at 170 °C for 7 days, then cooled to room temperature. Pink prismatic crystals of 1 were obtained (yield: 44% based on Er2O3). Anal. Calcd for 1, C18H13N2O5Er: C, 42.85; H, 2.60; N, 5.55. Found: C, 42.48; H, 2.89; N, 5.11. IR bands (cm−1) for 1: 3632vs, 3430w, 3066w, 1927w, 1656vs, 1630vs, 1595vs, 1557vs, 1477w, 1406s, 1293m, 1176m, 1011m, 850m, 774vs, 709vs, 685vs, 633m, 548w.
Synthesis of [Tb(μ3-OH)(pybz)(pa)] n (2)
The colorless crystals of 2 were prepared by a similar method as used in the synthesis of the crystals of 1 except that Er2O3 was replaced by Tb4O7 (0.125 mmol, 0.093 g). The resulting colorless prismatic crystals of 2 were obtained (yield: 32% based on Tb4O7). Anal. Calcd for 2, C18H13N2O5Tb: C, 43.57; H, 2.64; N, 5.64. Found: C, 43.22; H, 2.93; N, 5.27. IR bands (cm−1) for 2: 3625vs, 3414w, 3065w, 1927w, 1651vs, 1630vs, 1595vs, 1557vs, 1477w, 1409s, 1297m, 1186m, 1015m, 854s, 774vs, 708vs, 678vs, 637m, 552w.
Synthesis of [Gd(μ3-OH)(pybz)(pa)] n (3)
The colorless crystals of 3 were prepared by a similar method as used in the synthesis of the crystals of 1 except that Er2O3 was replaced by Gd2O3 (0.25 mmol, 0.091 g). The resulting colorless prismatic crystals of 3 were obtained (yield: 30% based on Gd2O3). Anal. Calcd for 3, C18H13N2O5Gd: C, 43.71; H, 2.65; N, 5.66. Found: C, 43.43; H, 2.93; N, 5.36. IR bands (cm−1) for 3: 3630vs, 3418w, 3066w, 1925w, 1655vs, 1632vs, 1595vs, 1557vs, 1479w, 1409s, 1297m, 1187m, 1023m, 855s, 774vs, 710vs, 679vs, 639m, 556w.
X-Ray Crystallography
Crystal structure determinations by X-ray diffraction were performed on a Siemens SMART-CCD diffractometer with graphite-monochromated MoKα (λ = 0.71073 Å) radiation in the ω scanning mode at room temperature. The program SADABS was used for the absorption correction [19]. The structures were solved by the direct method and refined on F 2 by full-matrix least-squares methods using the SHELX97 program package [20, 21]. In 1–3, all non-hydrogen atoms were refined anisotropically. Hydrogen atoms were introduced in calculated positions and refined isotropically. In 1, a total of 23660 reflections (2.04 ≤ θ ≤ 27.48o) were collected with 3824 unique ones (R int = 0.0360), of which 3799 reflections with I > 2σ (I) were used for structural elucidation. At convergence, R 1(wR 2) was 0.0369(0.0990) and the goodness-of-fit was 1.085.
The final Fourier map had a minimum and maximum of −1.794 and 0.805 eÅ−3. Experimental detail for the structural determinations of 1 is presented in Table 1. Selected bond distances and bond angles data for 1 are listed in Table 2. Crystallographic data for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre, CCDC-734922, 743114, 743260 for compound 1–3. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (int. code) +44(1223)336-033; e-mail for inquiry: deposit@ccdc.cam.ac.uk).
Result and Discussion
X-ray crystal structure analyses reveal that 1–3 are isomorphous and crystallize in tetragonal space group P \( \overline{4} \)21c (crystal data shown in Table 1). Therefore, only the structure of 1 is described in details. The asymmetric unit of 1 contains one unique Er3+ ion, one μ3-OH− one pybz− ligand, and one pa− ligand. Er1 is eight-coordinated, and the coordination geometry is close to that of a bicapped trigonal prism: two carboxylate oxygen atoms (OCOO, O1, O2) from two pybz− ligands, two carboxylate oxygen atoms (OCOO, O3, O4), and one nitrogen atoms(N2) from two pa− ligands and three μ3-O(O5, O5a, O5b) from three μ3-bridging hydroxyl groups (Fig. 1a).
The Er–O distances range from 2.314(6) to 2.419(6) Å and the Er–N bond length is 2.528(7) Å (Table 2). The O–Er–O angles range from 69.8(2) to 147.0(2)°, while the O–Er–N angles vary from 66.2(2) to 143.84(2)°. The exclusive pybz− ligand behaves as μ2-pybz− mode linking two Er3+ ions in two monodentate modes, while the μ3-pa− ligand links two Er3+ ions in a bidentate and a monodentate mode.
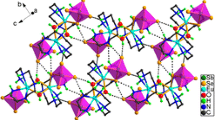
Compound 1 is based on a tetranuclear cluster composed of four crystallographically unique Er3+ centers which are joined together by four μ3-bridging hydroxyl groups (O5, O5a, O5b, and O5c) leading to the formation of a cubane-like [Er4(μ3-OH)4]8+ core (i.e., four Er3+ centers are bonded together by another four hydroxyl groups placed in opposite vertices of the cuboidal arrangement) surrounded by four chelating pybz− ligands and eight bridging pa− ligands (Fig. 1b). In the cubane-like tetranuclear cluster the four Er3+ ions are located at the four apexes of a fairly regular tetrahedron, Er···Er distances ranging between 3.6941(6) and 3.7630(5) Å (average of 3.73 Å). We note that these structural features are comparable with those found in compounds comprising related distorted [Er4(μ3-OH)4]8+ cluster cores [6–8]. In addition, these clusters as building blocks are further assembled into a 1-D chain along the c-axis (Fig. 2), while four μ3-pa− ligands act as bridges between adjacent two [Er4(μ3-OH)4]8+ clusters in this framework. Most interestingly, neighboring paralleled chains are linked together via weak π–π stacking leading to a 3-D supramolecular network structure and the centeroid–centeroid distance between them is 3.824 Å (Fig. 3). Small channels occupied by ligands emerge along the c-axis (Fig. 4).
As shown in Fig. 5, the strong and acuity absorption band around 3632 cm−1 and the broad absorption band in the range of 3000–3500 cm−1 in 1 are assigned as the characteristic peaks of OH vibration. The strong vibrations appearing around 1630, 1595, and 1557 cm−1 , corresponds to the asymmetric and symmetric stretching vibrations of the carboxylate group, respectively. The absence of strong bands ranging from 1690 to 1730 cm−1 indicates that the ligands are deproponated.
The thermogravimetric analysis was carried out in flowing dry air atmosphere with a heating rate of 10 °C min−1 in the temperature range of 30–1000 °C. As shown in Fig. 6, compound 1 can be stable up to 360 °C. Above this temperature, two steps of weight loss were observed until 580 °C. In the temperature range 360–580 °C, the weight loss corresponds to the successive release of organic ligands. At 580 °C, the coordination networks of complex 1 decompose completely. Assuming that the residue corresponds to Er2O3, the observed weight (38.2%) is in good agreement with the calculated value (37.9%).
Conclusions
In summary, a series of 1-D lanthanide coordination polymers based on [Ln4(μ3-OH)4] cubanes have been synthesized under hydrothermal conditions. For this compound, tetranuclear cubane-like clusters [Ln4(μ3-OH)4]8+ composed of four Ln3+ centers as building blocks are further assembled into 1-D chains along the c-axis. This work provides a rational route for the construction of fascinating lanthanide coordination polymers based on hydroxo lanthanide clusters, and the further work is continuing in this area.
References
R. Sessoli, D. Gatteschi, A. Caneschi, and M. A. Novak (1993). Nature 365, 141.
D. Gatteschi, A. Caneschi, R. Sessoli, and A. Cornia (1996). Chem. Soc. Rev. 25, 101.
A. P. Alivisatos (1996). Science 271, 933.
D. Gatteschi, L. Pardi, A. L. Barra, A. Müller, and J. Döring (1991). Nature 354, 463.
A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud, and G. Christou (2004). Angew. Chem. Int. Ed. 43, 2117.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, C. H. Yan, and G. X. Xu (2000). Angew. Chem. Int. Ed. 39, 2169.
R. Y. Wang, H. Liu, M. D. Carducci, T. Z. Jin, C. Zheng, and Z. P. Zheng (2001). Inorg. Chem. 40, 2743.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, and C. H. Yan (2000). New J. Chem. 24, 251.
V. Baskar and P. W. Roesky (2006). Dalton Trans. 676.
F. N. Shi, L. Cunha-Silva, T. Trindade, F. A. Paz, and J. Rocha (2009). Cryst. Growth Des. 9, 2098.
R. Y. Wang, H. D. Selby, H. Liu, M. D. Carducci, T. Z. Jin, Z. P. Zheng, J. W. Anthis, and R. J. Staples (2002). Inorg. Chem. 41, 278.
J. C. Plakatouras, L. Baxter, M. B. Hursthouse, K. M. Abdul Malik, J. McAleese, and S. R. Drake (1994). Chem. Soc. Chem. Commun. 2455.
X. M. Chen, Y. L. Wu, Y. X. Tong, Z. D. Sun, and N. Hendrickson (1997). Polyhedron 16, 4265.
T. Dube, S. Gambarotta, and G. Yap (1998). Organometallics 17, 3967.
J. W. Cheng, J. Zhang, and G. Y. Yang (2006). Angew. Chem. Int. Ed. 45, 73.
J. W. Cheng, S. T. Zheng, and G. Y. Yang (2008). Chem. Eur. J. 14, 88.
M. B. Zhang, J. Zhang, and G. Y. Yang (2005). Angew. Chem. Int. Ed. 44, 1385.
J. W. Cheng, J. Zhang, and G. Y. Yang (2007). Inorg. Chem. 46, 10261.
G. M. Sheldrick SHELXS97, Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXL97, Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
Acknowledgments
The authors are thankful for the financial supports from the National Natural Science Fund for Distinguished Young Scholars of China (no. 20725101), the NNSF of China (no. 50872133), the 973 Program (no. 2006CB932904), the NSF of Fujian Province (nos. E0510030 and 2008F3120), and the Knowledge Innovation Program from CAS (no. KJCX2.YW.H01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, ZL., Fang, WH. & Yang, GY. A Series of 1-D Lanthanide Coordination Polymers Based on [Ln4(μ3-OH)4] (Ln = Er, Tb, Gd) Cluster Units. J Clust Sci 20, 725–733 (2009). https://doi.org/10.1007/s10876-009-0276-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-009-0276-z