Abstract
New lanthanide(III) compounds [[Ln(tepa)(Cl)]-[Ln(tepa)(OH)]2(SbSe4)2] n (Ln=Sm, Eu), [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] (Ln=Eu, Gd, Ho) (tepa=tetraethylenepentamine) were prepared by solvothermal methods. Acting as a bidentate μ-1κ:2κ-SbSe4 bridging ligand, the [SbSe4]3− unit interconnects [[Ln(tepa)]2(OH)2]4+ and [Ln(tepa)Cl]2+ (Ln=Sm, Eu) ions to form one-dimensional coordination polymers [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n . The [SbSe4]3− unit acts as monodentate ligand to Ln(III) centers in [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2]. The different coordination modes of the [SbSe4]3− units in [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n and [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] are attributed to the size of Ln3+ ions. The bidentate μ-1κ:2κ-SbSe4 bridging ligand in [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n is observed in the lanthanide complexes of tetraselenidoantimonate ligands for the first time. All compounds exhibit steep band gaps between 2.04 and 2.31 eV at room temperature.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chalcogenides of group 15 metals have drawn increasing interest due to their structural diversity, and potential applications in many areas such as fast-ion conductivity, semiconductivity, photo-catalyst, nonlinear optical material [1–8]. In the past decade, solvothermal synthesis in a coordinative amine media has proven to be a useful approach to the ternary chalcogenidoantimonates containing transition metal (TM) components [9–13], since the cobalt thioantimonate [Co(en)3]CoSb4S8 was prepared by the reaction of CoBr2 and Na3SbS3 in ethylenediamine (en) at 130 °C [14]. The coordinative amine acts as not only the reaction solvent but also the ligand to TMn+ ion in the solvothermal reaction. Composition and structure of the coordinative amine show substantial influence on the combination between TM centers and chalcogenidoantimonate anions. A large number of chalcogenidoantimonates containing free d-block TM complexes have been prepared in bidentate en and tridentate dien (dien=diethylenetriamine) solvents because of the formation of coordination-saturated octahedra [TM(en)3]n+ and [TM(dien)2]n+ complexes ions [15–23]. Only a few ternary chalcogenidoantimonates integrated with TM were prepared in en and dien, and the examples include Cr(en)2SbS3 [24], [Mn2(en)2(Sb2S5)] [25], [Mn2(dien)(Sb2S5)] [26], [Mn4(en)9(SbSe4)4]4− [27], [Mn2(SbSe4)2(en)4(H2O)]2− [28], and (dienH3)[(dienH)MnSb8S15] H2O [29]. On the other hand, the TMn+ ions are easily incorporated with chalcogenidoantimonates using tetradentate tris(2-aminoethyl)amine (tren) and pentadentate tetraethylenepentamine (tepa) as the coligands to the TMn+ centers. The tren or tepa coligands leave one or two coordination sites free for the TMn+ ions to form TM–S or TM–Se bond with the chalcogenidoantimonate anions. As a result, ternary TM-chalcogenidoantimonates were obtained [30–38].
In comparison to the ternary TM-chalcogenidoantimonates, which are constructed from TMn+ ions and chalcogenidoantimonate anions via TM–S or TM–Se bond, the weak interactions between lanthanide (Ln) ions and chalcogenidoantimonate anions make the synthesis of ternary Ln-chalcogenidoantimonates a challenging task. However, our work has demonstrated that the chalcogenidoantimonate anions [SbS4]3− and [SbSe4]3− can coordinate to Ln(III) centers using coordinative amines as co-ligands, and a number of Ln-chalcogenidoantimonates have been solvothermally prepared in en, dien, and trien solvents [39–44]. Unlike the TMn+ metals which exhibit restricted stereochemistry in coordination complexes, the Ln3+ ions are characterized by variable geometries due to their higher coordination numbers [45], which theoretically provides the Ln3+ ions with different structural features from the TMn+ ions in the combination with chalcogenidoantimonate anionic ligands in the presence of the same ethylene polyamines. The Ln3+ ions can form unsaturated complex units with en and dien ligands, as well as with tetradentate trien ligand or en+dien, and en+trien mixed ligands. The [SbS4]3− or [SbSe4]3− anions complete the unsaturated coordination sites of Ln3+ centers via Ln–S or Ln–Se bond formation. Furthermore, the [SbS4]3− or [SbSe4]3− anions can be tuned to coordinate to Ln(III) centers in mono-SbQ4, 1κ2-SbQ4 and μ-1κ,2κ2-SbQ4 (Q=S, Se) coordination modes using ethylene polyamines en, dien and trien as the co-ligands [39–44]. It is important to systematically investigated the synergistic coordination effects of SbQ4 and polyamino ligands on the Ln(III) centers in the preparation of new Ln-containing chalcogenidometalates. By using identical Ln(III) metals and SbQ4 tetrahedra as building blocks, structural diversities could be obtained by virtue of ethylene polyamino ligands with different denticities. The solvothermal syntheses of lanthanide chalcogenidoantimonates in polyamines with higher denticities remain less explored, although a few lanthanide chalcogenidoantimonates were prepared in pentadentate tepa [46–48] and hexadentate pentaethylenehexamine (peha) [49]. Now, the LnCl3 (Ln2O3)/Sb/Se (Ln=Sm, Eu, Gd, Ho) system was investigated in tepa, and new members of the Ln–Sb–Se compound family [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n (Ln=Sm (1a), Eu (1b)), and [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] (Ln=Eu (2a), Gd (2b), Ho (2c)) were prepared using solvothermal methods. The influences of tepa coligands and ionic size of the Ln(III) ions on coordination modes of the tetraselenidoantimonate anion [SbSe4]3−are discussed.
Results and discussion
Syntheses
Solvothermal reactions of Sb, Se with SmCl3 (EuCl3) in tepa at 190 °C for 7 days produced polymeric lanthanide(III) compounds [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n (Ln=Sm (1a), Eu (1b)). The reactions with Ln2O3 as starting material under the same conditions afforded complexes [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] (Ln=Eu (2a), Gd (2b), Ho (2c)). Polyamine tepa not only acts as solvent of the solvothermal reaction, but also takes part in the coordination to Ln(III) centers as coligand. Recently, Zhou reported the complexes [Ln2(tepa)2(μ-OH)2Cl2][[Ln(tepa)]2(μ-OH)2(SbSe4)2] (Ln=Sm, Gd), which were prepared by the reaction at 170 °C for 6 days in tepa using LnCl3 as starting materials [48]. Comparing the Sm and Gd complexes in tepa, reaction conditions and Cl− ion influence the solvothermal syntheses of the Sb/Se system in tepa.
Crystal Structures of 1a and 1b
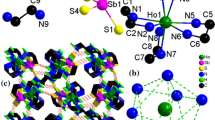
Compounds 1a and 1b crystallize in the monoclinic space group C2/c with four units in the unit cell. They are isostructural and consist of one-dimensional (1-D) coordination polymers constructed by [[Ln(tepa)]2(OH)2]4+, [Ln(tepa)Cl]2+, and [SbSe4]3− fragments. The crystal structure of 1a is illustrated in Figs. 1 and 2. As shown in Fig. 1, Sm(1)3+ ion is coordinated by a pentadentate tepa forming a [Sm(tepa)]3+ unit. Two [Sm(tepa)]3+ units are joined by two μ-OH bridging ligands to form the binuclear [[Sm(tepa)]2(OH)2]4+ complex fragment. The separation of Sm(1)···Sm(1A) in the binuclear unit is 3.8100(7) Å, which is comparable to that observed in [[Sm(en)]3(OH)2]4+ [(Sm···Sm = 3.844(2) Å)] [50]. Sm(2)3+ ion is coordinated by a tepa and a Cl−ligand to form the [Sm(tepa)Cl]2+ fragment. The Sm(2)3+ and Cl− ions are disordered and the occupancies of both ions being refined as 50 % each. The Sb5+ ion binds four Se2− anions with distances in the range of 2.4427(12)–2.4821(10) Å, generating a tetrahedral [SbSe4]3− unit with Se–Sb–Se angles in the range of 105.62(4)°–112.01(5)° (Table 1). The bond lengths and angles are consistent with the corresponding values observed in the selenidoantimonates containing [SbSe4]3− unit [41–44, 48]. Acting as a bidentate μ-1κ:2κ-SbSe4 bridging ligand, the [SbSe4]3− (A) unit interconnects the [[Sm(tepa)]2(OH)2]4+ (B) and [Sm(tepa)Cl]2+ (C) fragments to a neutral coordination polymer [[Sm(tepa)(Cl)][Sm(tepa)(OH)]2(SbSe4)2] n (Fig. 2), in which three fragments are repeated in the order of [–ABACABAC–]. Both Sm(1)3+ and Sm(2)3+ ions are in an eightfold coordination environment, forming a SmN5O2Se and a SmN5Se2Cl polyhedra, respectively (Fig. S1). The bond lengths Sm–N [(2.576(6)–2.638(18) Å)], Sm–Se [(3.0162(9)–3.334(3) Å)], Sm–O [(2.295(5) and 2.328(5) Å)] and Sm–Cl [(3.02(3) Å)] are in the range of those observed in literature [41–44, 48].
In 1a, the 1-D polymeric chains [[Sm(tepa)(Cl)][Sm(tepa)(OH)]2(SbSe4)2] n run parallel to each other. The chains are interconnected to a layer parallel to the (111) plane of the unit cell via weak N–H···Se hydrogen bonds [N···Se: 3.529(7)–3.648(8) Å; N–H···Se: 138.4°–176.2°] (Fig. 2, Table S6). The N···Se separations and N–H···Se angles are in agreement with reported values observed in Ln tetraselenidoantimonate containing amino coligands [41–44, 48]. The layers are further connected through interlayer N–H···Se interactions to form a 3-D H-bonding network (Fig. S2). Orientations of the neighboring [[Sm(tepa)(Cl)][Sm(tepa)(OH)]2(SbSe4)2] n chains alternate in the same layer.
Crystal structures of 2a–2c
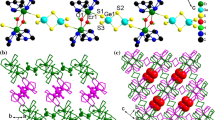
Compounds 2a–2c are isostructural. They are composed of a [[Ln(tepa)(SbSe4)]2(OH)2]2− (Ln=Eu, Gd, Ho) anion and a protonated [H2tepa]2+ cation. The molecular structure of 2a is depicted in Fig. 3. The Eu3+ ion is coordinated by a pentadentate tepa ligand and a monodentate [SbSe4]3− ligand forming a [Eu(tepa)(SbSe4)] unit. Two [Eu(tepa)(SbSe4)] units are linked by two μ-OH bridging groups to generate a binuclear [[Eu(tepa)(SbSe4)]2(OH)2]2− complex anion (Fig. 3). The Eu3+ ion is coordinated by five N, two O, and one Se atoms in a distorted bicapped trigonal prismatic environment (Fig. S3). The bond lengths and angles of the [[Eu(tepa)(SbSe4)]2(OH)2]2− anion are similar to those of 1b (Tables 1, 2). The Ln–Se, Ln–O, and Ln–N bond lengths decrease from Eu to Ho in compounds 2a–2c, due to lanthanide contraction (Table 2). The Se(1), Se(2), and Se(3) atoms have contacts with amino groups NH and NH2 of neighboring [[Eu(tepa)(SbSe4)]2(OH)2]2− units with N···Se separations varying between 3.551(12) and 3.725(11) Å, and N–H···Se angles varying in 142.5°–176.1° (Table S6). In addition, Se(1) atom also interacts with a neighbor hydroxyl group OH− (O···Se = 3.494(8) Å, O–H···Se = 168.2°). Each [[Eu(tepa)(SbSe4)]2(OH)2]2− unit contact four neighbors with N–H···Se and O–H···Se hydrogen bonds. As a result, the [[Eu(tepa)(SbSe4)]2(OH)2]2− units are connected to a layer perpendicular to the a axis (Fig. 4a). The protonated [H2tepa]2+ cations are located between the layers, and interact with the layer via N–H···Se hydrogen bonds (Fig. 4b).
a A view of the layer constructed by [[Eu(tepa)(SbSe4)]2(OH)2]2− moieties via O–H···Se and N–H···Se interactions (shown in dashed lines) in 2a. b Crystal packing of 2a viewed along the b axis. The SbSe4 unit is shown in purple tetrahedron. Hydrogen atoms of CH2 groups are omitted for clarity (color figure online)
In our previous studies on solvothermal syntheses of Ln(III) selenidoantimonates, we have found that the tetraselenidoantimonate [SbSe4]3− anion can be tuned to coordinate to Ln(III) centers with varying coordination modes using ethylene polyamino coligands like en, dien, trien or their mixtures [41–44]. Being multidentate chelating ligands with N-donor atoms, ethylene polyamines are prone to chelating the Ln(III) ions, but usually leave one or more coordination sites free due to steric hindrance of the polyamines. The numbers of left coordination sites are related to the structure of ethylene polyamine and the size of Ln(III) ion. The [SbSe4]3−anion coordinates to the remaining coordination sites and completes the coordination environment around the Ln(III) ions. As a result, Ln(III)–SbSe4 complexes with different coordination modes of the [SbSe4]3− ligand are obtained. Detailed investigation of the Ln/Sb/Se system in en, for instance, gave two types of Ln(III) compounds [Ln(en)4(SbSe4)] (Ln=La, Pr, Nd) and [Ln(en)4]SbSe4·0.5en (Ln=Sm, Eu, Gd) [41–43].The former contains a 9-coordinated Ln(III) ion with a N8 + Se donor set and a monodentate [SbSe4]3− ligand, while the later contains a 8-coordinated Ln(III) ion with N8 donor set and a free [SbSe4]3−anion. A similar investigation in dien solvent also produced two types of Ln(III) compounds [Ln(dien)2(μ-1κ2:2κ-SbSe4)]n (Ln=La, Pr, Nd) and [Ln(dien)2(1κ2-SbSe4)] (Ln = Sm, Eu, Gd), which contain a 9-coordinated Ln(III) ion with a N6 + Se3 donor set and a 8-coordinated Ln(III) ion with a N6 + Se2 donor set, respectively [41, 42]. Different coordination modes of the [SbSe4]3− anion across the lanthanide series are also observed in en + dien and en + trien mixtures [41, 44]. It is worthy to note that La3+–Nd3+ ions always possess coordination number of nine, while the ions beyond Nd3+ possess coordination number of eight [41–44]. Now, the solvothermal syntheses in tepa solvent gave two types of Ln(III)–SbSe4 compounds 1a, 1b, and 2a–2c, in which μ-1κ:2κ-SbSe4 and mono-SbSe4 ligands were obtained. The bidentate bridging μ-1κ:2κ-SbSe4 ligand in 1a, 1b features a new coordination mode observed in Ln/Sb/Se systems. It is notable that 2a is the first Ln(III) selenidoantimonate containing a 9-coordinated Sm(III) ion with a N6 + Se3 donor set. In summary, the coordination mode of [SbSe4]3− is a result of synergetic effect of the structure of ethylene polyamine and the size of Ln(III) ion.
Solid state absorption spectra
Solid state optical diffuse reflection spectra of 1a–2c were measured on powder samples at room temperature. The absorption data were calculated from the reflectance using the Kubelka–Munk function [51]. The obtained spectra of the complexes show well-defined abrupt absorption edges from which the band gaps can be estimated at 2.07, 2.18, 2.24, 2.22, and 2.31 eV for 1a, 1b, 2a–2c, respectively (Figs. 5, 6), showing that the title compounds exhibit potential semi-conducting properties. The band gaps (E g) are similar to those of [Ln(dien)2(1κ2-SbSe4)] (Ln=Sm, Eu, Gd) (E g: 2.19–2.28 eV) [41, 42], but are much higher than those of the layered copper selenidoantimonate compounds Cs2Cu2Sb2Se5 (E g: 1.2–1.3 eV) [52], Cu2SbSe3·0.5en (E g 1.58 eV), and Cu2SbSe3·en (E g 1.61 eV) [53].
Conclusion
In summary, the ternary system Ln/Sb/Se (Ln=Sm, Eu, Gd, Ho) was investigated in tepa solvent under solvothermal conditions. Two types of Ln-tetraselenidoantimonate complexes with general formula [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n and [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] have been prepared. The [SbSe4]3− anion adopts μ-1κ:2κ-SbSe4 and mono-SbSe4 coordination modes in the two types of Ln-tetraselenidoantimonate compounds, respectively. The coordination modes are different from those of the Ln-tetraselenidoantimonates, which were prepared in bidentate en and tridentate dien solvents. This observation shows synergetic effects of ethylene polyamines on the combination between Ln3+ and [SbSe4]3− ions.
Experimental
All starting chemicals were of analytical grade and used as purchased. Elemental analyses were conducted using an MOD 1106 elemental analyzer. The micropobe analysis by energy dispersive X-ray spectroscopy (EDXS) was performed on a Hitachi S-4700 spectrometer. Fourier transform infrared (FT-IR) spectra were recorded with a Nicolet Magna-IR 550 spectrometer in dry KBr discs over the 4000–400 cm−1 range. Room-temperature optical diffuse reflectance spectra of the powder samples were obtained with a Shimadzu UV-3150 spectrometer. Absorption (α/S) data were calculated from the reflectance using the Kubelka–Munk function α/S = (1 − R 2)/2R [51], where R is the reflectance at a given energy, α is the absorption, and S is the scattering coefficient.
Tris(tetraethylenepentamine)bis(tetraselenidoantimonate)dihydroxochlorotrisamarium(III) (1a, C24H71ClN15O2Sb2Se8Sm3)
SmCl3 (128 mg, 0.5 mmol), 61 mg Sb (0.5 mmol), and 158 mg Se (2 mmol) were dispersed in 3 cm3 of tepa by stirring, and the dispersion was loaded into a Teflon-lined stainless steel autoclave of 10 cm3 volume. The reaction was run at 190 °C for 7 days. Upon cooling to ambient temperature, orange prism crystals of 1a were filtered off, washed with ethanol, and stored under a vacuum (42 % yield based on Sb). Elemental analyses results of the crystals are consistent with the stoichiometry of C24H71ClN15O2Sb2Se8Sm3. EDXS analysis gave the heavy atom component of Sm3.21Sb1.94Se8.14Cl. IR (KBr): \(\bar{v}\) = 3696 (w), 3550 (m), 3426 (w), 3304 (w), 3130 (w), 2855 (w), 2202 (w), 1635 (w), 1589 (m), 1528 (m), 1435 (w), 1287 (s), 1131 (m), 1051 (m), 952 (s), 716 (m), 618 (m), 585 (m), 473 (w), 425 (w) cm−1.
Tris(tetraethylenepentamine)bis(tetraselenidoantimonate)dihydroxochlorotrieuropium (III) (1b, C24H71ClEu3N15O2Sb2Se8)
Orange block crystals of 1b were obtained with a procedure similar to the synthesis of 1a, except that EuCl3 was used instead of SmCl3 (45 % yield based on Sb). Elemental analysis results of the crystals are consistent with the stoichiometry of C24H71ClEu3N15O2Sb2Se8. EDXS analysis gave the heavy atom component of Eu3.13Sb2.07Se8.21Cl. IR (KBr): \(\bar{v}\) = 3696 (w), 3550 (m), 3304 (w), 3130 (w), 2947 (w), 2855 (w), 2202 (w), 1635 (w), 1589 (m), 1528 (m), 1435 (w), 1287 (s), 1131 (m), 1020 (m), 970 (s), 952 (s), 838 (m), 716 (m), 618 (m), 585 (m), 473 (w), 425 (w) cm−1.
3,6,9-Triazaundecamethylenediammonium μ-dihydroxobis[(tetraethylenepentamine)(tetraselenidoantimonate)europate(III)] (2a, C24H73Eu2N15O2Sb2Se8)
Orange block crystals of 2a were obtained with a procedure similar to the synthesis of 1a, except that Eu2O3 was used instead of SmCl3 (44 % yield based on Sb). Elemental analysis results of the crystals are consistent with the stoichiometry of C24H73Eu2N15O2Sb2Se8. EDXS analysis gave the heavy atom component of EuSb2.11Se4.12. IR (KBr): \(\bar{v}\) = 3605 (w), 3429 (m), 2929 (w), 2840 (w), 2360 (w), 1810 (w), 1721 (w), 1638 (m), 1571 (m), 1480 (m), 1423 (w), 1380 (w), 1304 (s), 1114 (m), 1051 (m), 913 (w), 854 (w), 810 (s), 713 (w), 592 (s), 493 (w), 406 (w) cm−1.
3,6,9-Triazaundecamethylenediammonium μ-dihydroxobis[(tetraethylenepentamine)(tetraselenidoantimonate)gadolinate(III)] (2b, C24H73Gd2N15O2Sb2Se8)
Yellow prism crystals of 2b were obtained with a procedure similar to the synthesis of 1a, except that Gd2O3 was used instead of SmCl3 (48 % yield based on Sb). Elemental analysis results of the crystals are consistent with the stoichiometry of C24H73Gd2N15O2Sb2Se8. EDXS analysis gave the heavy atom component of GdSb1.98Se4.05. IR (KBr): \(\bar{v}\) = 3697 (w), 3425 (s), 2945 (w), 2843 (w), 2083 (m), 1711 (w), 1639 (s), 1571 (w), 1495 (s), 1424 (m), 1380 (w), 1313 (s), 1190 (w), 1116 (m), 1050 (w), 958 (w), 889 (w), 807 (w), 761 (s), 692 (m), 592 (s), 492 (m), 419 (m) cm−1.
3,6,9-Triazaundecamethylenediammonium μ-dihydroxobis[(tetraethylenepentamine)(tetraselenidoantimonate)holmate(III)] (2c, C24H73Ho2N15O2Sb2Se8)
Yellow chip crystals of 2c were obtained with a procedure similar to the synthesis of 1a, except that Ho2O3 was used instead of SmCl3 (49 % yield based on Sb). Elemental analysis results of the crystals are consistent with the stoichiometry of C24H73Ho2N15O2Sb2Se8. EDXS analysis gave the heavy atom component of HoSb2.15Se4.09. IR (KBr): \(\bar{v}\) = 3429 (w), 3210 (m), 2946 (w), 2871 (w), 1571 (m), 1442 (m), 1361 (w), 1310 (w), 1260 (m), 1114 (m), 1080 (s), 1009 (m), 966 (m), 882 (m), 831 (w), 740 (w), 657 (s), 574 (m), 535 (s), 471 (m), 419 (w) cm−1.
X-ray structure determination
Data were collected on a Rigaku Saturn (for 1a, 1b, 2b, 2c) or a Rigaku Mercury (for 2a) CCD diffractometer, using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) with a ω-scanning mode to a maximum 2θ value of 50.70°. An empirical absorption correction was applied for all compounds using the multi-scan method. All crystal structures were solved using SHELXS-97 [54], and refinement was performed against F 2 using SHELXL-97 [55]. All non-hydrogen atoms were refined anisotropically. Ln(2) and Cl(1) atoms in 1a and 1b are disordered, and the occupancies of both disordered atoms were refined as 50 and 50 %. The occupancies of the disordered atoms N(6) and N(7) of protonated H2tepa cations in 1a and 1b were refined as 60 and 40 %, while the corresponding disordered atoms in 2c were refined as 50 and 50 %. The hydrogen atoms were added geometrically and refined using the riding model. Crystallographic, experimental, and analytical data for the title compounds are listed in Table 3.
Crystallographic data for the structural analyses have been deposited with the Cambridge Crystallographic Data Center, CCDC Nos. 1057960, 1057961, 1057962, 1057963, and 1057964. These data can be obtained free of charge via https://summary.ccdc.cam.ac.uk/structure-summary-form.
References
Wachhold M, Kanatzidis MG (1999) Inorg Chem 38:3863
Wachhold M, Kanatzidis MG (2000) Inorg Chem 39:2337
Bera TK, Jang JI, Song J-H, Malliakas CD, Freeman AJ, Ketterson JB, Kanatzidis MG (2010) J Am Chem Soc 132:3484
Xiong WW, Athresh EU, Ng YT, Ding JF, Wu T, Zhang QC (2013) J Am Chem Soc 135:1256
Seidlhofer B, Spetzler V, Näther C, Bensch W (2012) J Solid State Chem 187:269
Schaefer M, Näther C, Lehnert N, Bensch W (2004) Inorg Chem 43:2914
Kiebach R, Pienack N, Ordolff ME, Studt F, Bensch W (2006) Chem Mater 18:1196
Liu GN, Guo GC, Chen F, Wang SH, Sun J, Huang JS (2012) Inorg Chem 51:472
Sheldrick WS, Wachhold M (1998) Coord Chem Rev 176:211
Li J, Chen Z, Wang RJ, Proserpio DM (1999) Coord Chem Rev 190–192:707
Sheldrick WS (2000) J Chem Soc Dalton Trans 3041
Seidlhofer B, Pienack N, Bensch W (2010) Z Naturforsch 65b:937
Zhou J, Dai J, Bian GQ, Li CY (2009) Coord Chem Rev 253:1221
Stephan HO, Kanatzidis MG (1996) J Am Chem Soc 118:12226
Vaqueiro P, Chippindale AM, Powell AV (2004) Inorg Chem 43:7963
Lees RJE, Powell AV, Chippindale AM (2005) Polyhedron 24:1941
Bensch W, Näther C, Stähler R (2001) Chem Commun 5:477
Stähler R, Bensch W (2002) Z Anorg Allg Chem 628:1657
Stähler R, Mosel BD, Eckert H, Bensch W (2002) Angew Chem Int Ed 41:4487
Yue CY, Lei XW, Ma YX, Sheng N, Yang YD, Liu GD, Zhai XR (2014) Cryst Growth Des 14:101
Stähler R, Näther C, Bensch W (2003) J Solid State Chem 174:264
Jin QY, Zhu AM, Pan YL, Jia DX, Zhang Y, Gu JS (2009) Z Anorg Allg Chem 635:139
Jia DX, Zhang Y, Zhao QX, Deng J (2006) Inorg Chem 45:9812
Schur M, Rijnberk H, Näther C, Bensch W (1999) Polyhedron 18:101
Schur M, Bensch W (2002) Z Naturforsch 57b:1
Engelke L, Stähler R, Schur M, Näther C, Bensch W, Pöttgen R, Möller MH (2004) Z Naturforsch 59b:869
Bensch W, Näther C, Schur M (1997) Chem Commun 18:1773
Almsick TV, Sheldrick WS (2006) Z Anorg Allg Chem 632:1413
Yue CY, Lei XW, Zang HP, Zhai XR, Feng LJ, Zhao ZF, Zhao JQ, Liu XY (2014) CrystEngComm 16:3424
Möller K, Näther C, Bannwarth A, Bensch W (2007) Z Anorg Allg Chem 633:2635
Stähler R, Bensch W (2001) Eur J Inorg Chem 3073
Schaefer M, Kurowski D, Pfitzner A, Näther C, Rejai Z, Möller K, Ziegler N, Bensch W (2006) Inorg Chem 45:3726
Schaefer M, Stähler R, Kiebach WR, Näther C, Bensch W (2004) Z Anorg Allg Chem 630:1816
Schaefer M, Näther C, Bensch W (2004) Monatsh Chem 135:461
Stähler R, Bensch W (2001) J Chem Soc Dalton Trans 2518
Schaefer M, Engelke L, Bensch W (2003) Z Anorg Allg Chem 629:1912
Lichte J, Lühmann H, Näther C, Bensch W (2009) Z Anorg Allg Chem 635:2021
Nie L, Xiong WW, Li PZ, Han JY, Zhang GD, Yin SM, Zhao YL, Xu R, Zhang QC (2014) J Solid State Chem 220:118
Pan YL, Chen JF, Wang J, Zhang Y, Jia DX (2010) Inorg Chem Commun 13:1569
Tang WW, Chen RH, Zhao J, Jiang WQ, Zhang Y, Jia DX (2012) CrystEngComm 14:5021
Jia DX, Jin QY, Chen JF, Pan YL, Zhang Y (2009) Inorg Chem 48:8286
Chen RH, Tang WW, Jiang WQ, Zhang Y, Jia DX (2013) J Coord Chem 66:650
Jia DX, Zhu AM, Jin QY, Zhang Y, Jiang WQ (2008) J Solid State Chem 181:2370
Zhao J, Liang JJ, Pan YL, Zhang Y, Jia DX (2011) J Solid State Chem 184:1451
Cassol A, Bernardo PDi, Portanova R, Tolazzi M, Tomat G, Zanonato P (1992) J Chem Soc Dalton Trans 469
Zhou J, An LT, Hu FL, Liu X, Zhao RQ, Lin JW (2012) CrystEngComm 14:5544
Zhou J, Hu FL, An LT, Liu X, Meng CY (2012) Dalton Trans 41:11760
Xiao HP, Zhou J, Zhao RQ, Zhang WB, Huang Y (2015) Dalton Trans 44:6032
Liu Y, Tang CY, Han JY, Shen YL, Lu JL, Jia DX (2015) Inorg Chem Commun 60:103
Jin QY, Chen JF, Pan YL, Zhang Y, Jia DX (2010) J Coord Chem 63:1492
Wendlandt WW, Hecht HG (1966) Reflectance spectroscopy. Interscience Publishers, New York
Chen Z, Wang RJ, Dilks KJ, Li J (1999) J Solid State Chem 147:132
Chen Z, Dilks RE, Wang RJ, Lu JY, Li J (1998) Chem Mater 10:3184
Sheldrick GM (1997) SHELXS-97 program for solution of crystal structures. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97 program for refinement of crystal structures. University of Göttingen, Germany
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21171123), and the project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, P., Liu, S., Han, J. et al. Solvothermal syntheses, crystal structures, and properties of new lanthanide compounds based on tetraselenidoantimonate and tetraethylenepentamine mixed ligands. Monatsh Chem 148, 209–216 (2017). https://doi.org/10.1007/s00706-016-1777-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1777-8