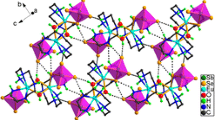
Abstract
The study of polynuclear lanthanide (Ln) complexes has been a field of rapid growth in coordination chemistry. Lanthanide clusters synthesized via a ligand-controlled hydrolytic approach using different flexible α-amino acids have been well summarized. In this chapter, we describe efforts to synthesize lanthanide and lanthanide-transition-metal (Ln-TM) cluster organic frameworks using rigid ligands of isonicotinic acid (HIN), 4-pyridin-4-ylbenzoic acid (HL), nicotinic acid (HNA), and 4-(3-pyridyl)benzoic acid (HL′) under hydrothermal condition. In addition, the synergistic coordination between these rigid ligands with other organic/inorganic ligands has also been discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Heterometallic compounds
- Hydrothermal synthesis
- Lanthanide cluster organic framework
- Rigid ligands
- Synergistic coordination
1 Introduction
Lanthanide (Ln) and lanthanide-transition-metal (Ln-TM) clusters and coordination polymers are of great interest because of their fascinating structures and a variety of applications ranging from luminescent and magnetic materials to their use in homogeneous catalysis [1–7]. At present, hydroxo lanthanide clusters can be synthesized via a ligand-controlled hydrolytic approach with the judiciously chosen supporting ligands to limit the degree of lanthanide hydrolysis and the aggregation of the hydroxo intermediates [8, 9]. To date, a large number of lanthanide clusters from Ln3 to Ln104 have been reported [10–25], in which most of the reported high-nuclearity hydroxo lanthanide clusters are discrete. Obviously, novel lanthanide clusters with interesting structures and exciting properties strongly rely on the innovations in synthetic methodology, developing new synthetic methods continue to be of great importance in this field. Hydrothermal synthesis represents a kind of milder and softer synthetic techniques by employing water as reaction media at relatively low temperature. Hydrothermal synthesis has been widely used in the synthesis of coordination polymers or metal–organic frameworks [26, 27], and extended to grow crystalline lanthanide cluster organic frameworks very recently [28]. Under hydrothermal process, lanthanide oxides can be used as the source of lanthanides in the presence of acid at low pH value, rather than using lanthanide salts in aqueous solution at high pH value.
The coordination chemistry of the copper(I) halides has been of great interest due to their large structural variation and rich electronic/optical properties. Copper(I) halides are inclined to form a variety of inorganic CuX clusters generally based on corner or edge sharing of trigonal planar {CuX3} or tetrahedral {CuX4} subunits, various copper halide cluster motifs from rhomboid Cu2X2 dimers, cubane or stepped cubane Cu4X4 tetramers to Cu36X56 have been well documented [29]. Therefore, it should be rational to introduce CuX clusters into the Ln cluster organic framework to construct fascinating 3D hetero-Ln-TM structures. Linear and rigid ligands with oxygen and nitrogen donors, such as isonicotinic acid (HIN) and 4-pyridin-4-ylbenzoic acid (HL) were selected to make lanthanide and Ln-TM cluster organic frameworks based on the following considerations: (1) They are rigid ligands with oxygen and nitrogen donors on opposite sides, enabling these ligands to act as a linear bridge for the formation of the extended structures. (2) The carboxy group may induce the oxophilic lanthanide ions to undergo hydroxo lanthanide cluster aggregation, while the nitrogen atoms can coordinate to TM ions, and thus extended solids containing hydroxo lanthanide cluster cores and TM ions might be obtained. The hetero-Ln-TM structures consist of both Ln3+ and d10 TM ions (Cu/Ag/Zn), which may expand their applications in photovoltaic and optoelectronic devices, based on their luminescent properties [4]. In addition, the synergistic coordination between HIN/HL and other organic/inorganic ligands also gives rise to a new series of lanthanide cluster organic frameworks. Lanthanide cluster organic frameworks constructed by the analogue nicotinic acid (HNA) and 4-(3-pyridyl)benzoic acid (HL′) have also been discussed (Scheme 1).
2 Lanthanide and Lanthanide-Transition-Metal Cluster Organic Frameworks
2.1 Cluster Organic Frameworks Constructed by Isonicotinic Acid
Hydrothermal reaction of Ln2O3, HIN, and CuCl2 · 2H2O in water in the presence of HClO4 (pH 2) leads to three lanthanide cluster organic frameworks: [Ln14(μ6-O)(μ3-OH)20(IN)22Cu6Cl4 (H2O)8] · 6H2O (Ln = Y, Gd, Dy) [30]. These structures contain the high-nuclearity hydroxo lanthanide cluster [Ln14(μ6-O)(μ3-OH)20(H2O)8]20+, which acts as a building block that combines with copper ions through linear IN− ligands to form a 3D framework. The Gd14 core consists of one octahedral [Gd6(μ6-O)(μ3-OH)8]8+ unit that shares two opposing Gd1 apexes with two novel [Gd5(μ3-OH)6]4+ trigonal bipyramids. The linkages between the Gd14 cores and two different types of copper centers through IN− ligands give rise to an unusual 3D cluster organic framework (Fig. 1).
(a) Polyhedral representation of the structure of [Gd14(μ6-O)(μ3-OH)20]20+ core; (b) the overall 3D structure showing the unusual framework. Reproduced from [30] by permission of John Wiley & Sons Ltd
The I− ion has a larger ionic radius than Cl− and Br−, and may favor higher coordination numbers and versatile coordination modes, resulting in a larger copper-iodide cluster. Hydrothermal reactions of Ln2O3, CuI, HIN, and 2-pyrazinecarboxylic acid in water in the presence of HClO4 (pH 2) give the sandwich frameworks: [Ln6(μ3-O)2](IN)18[Cu8(μ4-I)2(μ2-I)3] · H3O (FJ-4, Ln = Y, Nd, Dy, Gd, Sm, Eu, Tb) [31]. Two unusual trinuclear [Ln3(μ3-O)] and tetranuclear [Cu4(μ4-I)] cores are successfully used as secondary building units to make two different nanosized wheels [Ln18(μ3-O)6(CO2)48]6−, Ln18, and [Cu24(μ4-I)6(μ2-I)12]6+, Cu24, with 12-membered rings and a diameter of 26.7 and 26.4 Å, respectively. The wheels are further assembled into 2D Ln18 and Cu24 networks, the linkages between two distinct layered networks of Ln18 and Cu24 wheels by IN− pillars along the c axis giving a series of unprecedented 3D sandwich frameworks (Fig. 2).
(a) Polyhedral view of layered network of Dy18 wheels; (b) polyhedral view of layered network of Cu24 wheels; and (c) view of the layered networks of Dy18 and Cu24 wheels linked by IN− ligands. Reproduced from [31] by permission of John Wiley & Sons Ltd
Dy30I(μ3-OH)24(μ3-O)6(NO3)9(IN)41(OH)3(H2O)38 and Dy104I4(μ3-OH)80 (μ3-O)24(NO3)36(IN)125(OH)19(H2O)167 have been obtained under hydrothermal conditions by incorporation of IN− and NO3 − ligands [32]. [Dy26(μ3-OH)20 (μ3-O)6(NO3)9I]36+ cluster core motif has been observed in their crystal structures, nine NO3 − ligands are incorporated into the cluster core backbone by Dy–O coordination bonds. The size of the Dy26 cluster is 20.47 × 17.20 Å2. The synergistic coordination between the IN− ligands and the trigonal planar geometry NO3 − ligands as surface modifiers inserted into the lanthanide cluster core backbone remarkably improves the dimension of cluster cores.
Dy30I(μ3-OH)24(μ3-O)6(NO3)9(IN)41(OH)3(H2O)38 consists of two Dy26 and two Dy4 clusters, these clusters are further linked by IN− linkers to form the final structure, while Dy104I4(μ3-OH)80(μ3-O)24(NO3)36(IN)125(OH)19(H2O)167 is the first tetramer assembled by the Dy26 clusters and IN linkers (Fig. 3).
(a) View of the structure of Dy26 core; (b) view of the structures of tetramer constructed by lanthanide clusters and IN− linkers. Reprinted with the permission from [32]. Copyright 2007 American Chemical Society
Compared with the reported discrete Ln26 cluster of [Dy26(μ3-OH)20 (μ3-O)6(NO3)9I]36+ [32], Xu et al. replaced NO3 − by CO3 2− to reinforce the huge Ln26 cluster and introduced a third ligand CH3COO− to reduce the steric restriction. Two 3D coordination polymers Zn1.5Dy26(IN)25(CH3COO)8(CO3)11(OH)26(H2O)29 and Zn1.5Gd26(IN)26(CH3COO)7(CO3)11(OH)26(H2O)28 have been hydrothermally synthesized [33]. Structural analysis indicates that the ligands IN−, CH3COO−, and CO3 2− anion make the Ln26 cluster stable. The linkages between nanosized Ln26 cluster and Zn centers through IN ligands result in two novel 3D open framework topologies (Fig. 4).
(a) View of the Ln26 core; (b) the linkages of nanosized Ln26 clusters and zinc centers by IN− ligands. Reprinted with the permission from [33]. Copyright 2010 American Chemical Society
A novel 2D coordination polymer K2[Ho48(IN)46(μ3-OH)84(μ4-OH)4 (μ5-O)2(OAc)4(H2O)14(CO3)Br2] · 2HIN · 20H2O [34] which contains nanosized Ho48 clusters was synthesized and structurally characterized by Xu et al. At the top or bottom of the core structure of Ho48, each cubane-like [Ho4(μ3-OH)4]8+ unit (Fig. 5a) can be described as a tetrahedron, while the middle Ho5 (Fig. 5b) units can be depicted as square pyramids. Six Ho5 units surround the equatorial ring of the Ho48 core via six corner sharing Ho atoms to form the barrel of the drum (Fig. 5c). Each Ho48 cluster is simultaneously bridged to four adjacent Ho48 cores by the IN− ligands to form a large rhombic ring with a length of 26.57 Å (Fig. 6). Similar nanosized Ln48 cluster is also observed in {[Cl2&(NO3)]@[Er48(NA)44(OH)90(N3)(H2O)24]}n · 6nCl · 35nH2O [35], in which Er48 clusters are linked by NA− ligands and N3 − anions to give a square layer, Cl− and NO3 − anions act as templates.
(a) The {Ho4} cluster unit. (b) The {Ho5} cluster unit. (c) The drum-like core structure of {Ho48} cluster. Reproduced from [34] by permission of The Royal Society of Chemistry
The layered structure connected by IN ligands. Reproduced from [34] by permission of The Royal Society of Chemistry
Xue et al. obtained two 3D heterometallic coordination polymers, Ln4 (μ3-OH)2Cu6I5(IN)8(OAc)3 (Ln = Nd, Pr; HOAc = acetic acid) under hydrothermal conditions [36]. The Ln6 and Ln2 cores are connected alternately to form a nanosized Ln16 wheel with an eight-membered ring with the size of 12.59 and 9.13 Å, OAc− ligand shows two different coordination modes. The transition-metal cluster moiety is the 1D chain formed by Cu6I5 clusters. It is interesting that the linkage between the 2D lanthanide wheel cluster layers and the 1D copper halide cluster chains by IN− ligands gives rise to a 3D coordination framework (Fig. 7).
(a) View of the 2D cluster network constructed by lanthanide wheel clusters with an eight-membered ring; (b) view of the 3D coordination framework based on the linkage of 2D neodymium cluster layers and 1D copper-iodine cluster chains by IN− linkers. Reprinted with the permission from [36]. Copyright 2007 American Chemical Society
If a second ligand 1,2-benzenedicarboxylic acid (H2bdc) were to be introduced, the synergistic coordination between IN and bdc ligands leads to two new lanthanide cluster organic frameworks, [Er7(μ3-O)(μ3-OH)6(bdc)3](IN)9[Cu3X4] (X = Cl/Br, FJ-2a/b) [37]. The Er4 and the Er2 cores are alternately linked from a nanosized [Er36(μ3-OH)30(μ3-O)6(bdc)6]54+ (Er36), this wheel-shaped building block of Er36 with an 18-membered ring is currently the largest lanthanide wheel (Fig. 8a). Remarkably, six bdc2− ligands are trapped in the inner of the 18-membered ring (Fig. 8a). Each Er36 cluster is linked to surrounding clusters and forming a highly ordered layered cluster network with hexagonal, honeycomb arrays (Fig. 8b). The linkages between 2D hybrid cluster polymers and copper clusters by IN− ligands give rise to an unprecedented 3D sandwich framework (Fig. 8c).
(a) View of the Er36 wheel; (b) view of giant wheel clusters linked to form layered cluster network; and (c) view of sandwich framework based on linkages of 2D cluster layers and Cu cluster pillars by IN− ligands. Reproduced from [37] by permission of John Wiley & Sons Ltd
The synergistic coordination between IN− and 2,5-pyridinedicarboxylic acid gives a new lanthanide cluster organic framework, Er4(OH)4Cu5I4(IN)6(NA)(2,5-pdc) · 0.3H2O (HNA = nicotinic acid, 2,5-pdc = 2,5-pyridinedicarboxylic acid) [38]. This compound consists of two distinct building blocks of inorganic 1D [Ln4(OH)4]n 8n+ cluster polymers and [Cu10I8]2+ clusters. The inorganic 1D [Ln4(OH)4]n 8n+ chains are further connected to each other by 2,5-pdc2− into 2D layers in the ab plane. The linkage between layered Ln networks and [Cu10I8]2+ clusters by IN− and NA− pillars along the c axis forms an unprecedented 3D framework (Fig. 9). It is interesting that decarboxylation occurred in the ortho position and 2,5-pdc2− was partially transformed into NA− under hydrothermal conditions.
(a) View of the inorganic [Ln4(OH)4]n 8n+ chain; (b) 2D Ln–organic layer; and (c) the 3D framework. Reprinted with the permission from [38]. Copyright 2008 American Chemical Society
2.2 Cluster Organic Frameworks Constructed by 4-(4-Pyridyl)benzoic Acid
To make new Ln cluster organic frameworks for potential applications, an expanded ligand with a benzene spacer between the two coordinating moieties of HIN, 4-pyridin-4-ylbenzoic acid (HL), is employed, with the expectation that this lengthened ligand is capable of avoiding steric crowding around metal clusters. Heptanuclear trigonal-prismatic Ln clusters derived from HL, [Ln7(μ3-OH)8L9(H2O)6] · 4ClO4 · 3HL · nH2O (Ln = Y, La, Gd, Yb, n = 6; Ln = Dy, Er, n = 4), were made by the hydrothermal treatment of Ln2O3 and HL at 190°C for 7 days in the presence of HClO4 (pH 2) [39]. The heptanuclear cluster core, [Y7(μ3-OH)8]13+ (Y7) core, can be described as two Y4(OH)4 cubanes sharing a Y atom, in contrast to previously reported trigonal antiprismatic Ln7 cores [40]. In the structure, each Y7 core connects six nearest neighbors with a distance of 16.955 Å by the ligands to produce a 2D Ln cluster organic layer possessing a thickness about 10.92 Å along the b axis (Fig. 10).
View of the 2D Ln cluster organic layer constructed by Y7 cluster and L−. Reprinted with the permission from [39]. Copyright 2013 American Chemical Society
Two pillared-layer cluster organic frameworks, [Ln5(μ3-OH)4(μ-H2O)Cu8I8L11] · H2O (Ln = Dy, Eu), have been made by employing lanthanide oxide and copper(I) halide as the source of lanthanide and transitional metal under hydrothermal condition [41]. There are two distinct nanoscale crown-like clusters in the structure, one is hydroxo lanthanide [Dy10(μ3-OH)8]22+ (Dy10) cluster and the other is copper(I) halide [Cu16I16] (Cu16) cluster. The Dy10 cluster can be intuitively regarded as a slightly slipped sandwich configuration. Each half of the sandwich contains a roughly planar set of five Dy3+ ions in a trapezoid arrangement, which can be viewed as three edge-sharing triangles with each bearing a capped μ3-OH group. The Dy10 core has an external diameter of 1.2 nm and an inner olive-shaped 4-ring with a diameter of 0.7 nm. The Dy10 cores are bridged by water molecules to be a ribbon-like chain along the [0 1 0] direction. The adjacent inorganic chains with reverse orientation are extended via L− ligands to generate Ln cluster organic layer on the bc plane. The Cu16 cores and the Ln cluster organic layers are pillared by the L− ligands to generate a fascinating 3D pillared-layer cluster organic framework. From the topological point of view, these compounds represent an intriguing example of a binodal (8,14)-connected net considering the Dy10 and Cu16 connectors as the nodes, revealing that they are typical high dimensional frameworks with high connected net based on high-nuclearity nodes (Fig. 11).
View of the framework constructed by Dy10 and Cu16 clusters. Reprinted with the permission from [41]. Copyright 2014 American Chemical Society
The synergistic coordination between two different organic ligands, as well as inorganic and organic ligands, leads to two types of cluster organic frameworks: [La6(μ3-OH)2(ox)3L12Cu11(μ3-X)6(μ2-X)3] · 8H2O (X = Br/Cl, FJ-21 a/b; ox = oxalate); [Ln4(OAc)3(H2O)4L9][Cu(μ3-I)]@[Cu10(μ3-I) (μ4-I)6(μ5-I)3] · 7H2O (Ln = Pr/Nd/Sm/Eu, FJ-22 a/b/c/d; OAc = acetate) [42]. FJ-21 a/b were made by hydrothermal treatment of lanthanum oxalate, CuX2 (X = Br/Cl), and HL at 200°C for 5 days in the presence of HClO4 (pH 2). The secondary building unit (SBU) of Ln wheel in FJ-21a is edge-sharing trinuclear unit [La3(μ3-OH)]8+ (La3). Every La3 core is linked by three ox ligands and nine L ligands. Six La3 cores with reverse orientation are alternately linked by six ox ligands to form an [La18(μ3-OH)6(ox)6]36+ (La18) wheel having a diagonal dimension of about 2.3 nm and a thickness of 0.4 nm, respectively. The SBU of CuI wheel in FJ-21a is Cu-centered edge-sharing truncated cubane [Cu4(μ3-Br)6]2−. Six Cu4 cores are connected by halide bridges into a nanosized neutral [Cu24(μ3-Br)18(μ2-Br)6] (Cu24) wheel with 12-ring and a diameter of 2.0 nm. Two different kinds of the wheel cluster layers of La18 and Cu24 are pillared by L− ligands to give rise to a 3D sandwich framework.
FJ-22 was made by hydrothermal reaction of Ln2O3, CuI, sodium acetate, and HL at low pH value under the same reaction conditions as FJ-21. The SBUs of Ln wheel in FJ-22d are edge-sharing tetranuclear [Eu4(OAc)3]9+ (Eu4) cores in compressed tetrahedral geometry. Six Eu4 cores are alternately linked edge-to-edge by twelve L ligands to generate an [Eu24(OAc)18(COO)12]42+ (Eu24) wheel with a diameter of 3.0 nm and a thickness of 0.4 nm. Six Cu4 cores are linked alternately to form a nanosized [Cu24(μ4-I)12(μ5-I)6]6+ (Cu24) wheel with 6-ring and a diameter of 2.8 nm. Therefore, the 3D sandwich framework of FJ-22d can be understood as the strictly alternating of Eu24 and Cu24 wheel cluster layers pillared by L ligands. Obviously, the synergistic coordination between organic ligands, L and oxalate/acetate, leads to the formation of La18 and Ln24 wheels, while the synergistic coordination between organic L and inorganic Br/I ligands results in Cu wheels for FJ-21 and FJ-22, respectively (Fig. 12).
The frameworks of FJ-21 and FJ-22 consist of two different kinds of nanosized Ln and Cu wheel cluster units. Reproduced from [42] by permission of John Wiley & Sons Ltd
Two sandwiched cluster organic frameworks, Eu6(OH)2Cu9I6L12(ox)3 · H2O · ClO4 (FJ-23, ox = oxalate) and Eu6Cu7I7L12(OAc)6(H2O)2 · 2H2O (FJ-24, OAc = acetate), have been successfully made [43]. In FJ-23, the [Eu18(μ3-OH)6(ox)6]36+ wheel contains six edge-to-edge equilateral triangles [Eu3(μ3-OH)]8+ SBUs. While in FJ-24, the [Eu18(OAc)18]36+ wheel is made up of six vertex-sharing compressed tetrahedral [Eu4(OAc)3]9+ SBUs. In FJ-23 and FJ-24, the graphene-like wheel cluster layers are linked through shape-matching trigonal prism metalloligands into 5-connected BN nets (Figs. 13 and 14). The second harmonic generation (SHG) measurements show that the SHG coefficients of FJ-23 and FJ-24 are about 0.15 and 0.2 times as large as that of KH2PO4 (KDP).
(a) Polyhedral view of the graphene-like Eu18 wheel cluster layer in FJ-23; (b) ball/stick view of the Cu3 SBU; (c, d) the coordinate environment of the Cu3L6 and 3(CuL2) metalloligands in FJ-23; (e) the overall pillared-layer framework of FJ-23. (f–i) Zoomed images at the left show the side and top view of the ClO4 − ions located in the narrow hexagonal channels. Reproduced from [43] by permission of John Wiley & Sons Ltd
(a) Polyhedral view of the Eu@Eu18 wheel cluster layer in FJ-24; (b) ball/stick view of the star-shape I@Cu6 SBU; (c, d) the coordinate environment of the I@Cu6L6 and 3(CuL2) metalloligands in FJ-24; (e) the overall pillared-layer framework of FJ-24; and (f) top view of the FJ-24. Reproduced from [43] by permission of John Wiley & Sons Ltd
Two supertetrahedral cluster organic frameworks (SCOFs), 2(Ln4Cu10I8L18)∙8H3O∙9H2O (Ln = Sm, Gd) were made by hydrothermal reaction of Ln2O3, CuI, and HL ligands at 180°C for 3 days [44]. A prominent structural feature of these two compounds is the presence of tetrahedral [Sm4(COO)6] (Sm4) and supertetrahedral T3-[Cu10I8] (Cu10) clusters. Each Sm4 tetrahedron is linked to six adjacent Cu10 supertetrahedra via 18 carboxyl groups, and each Cu10 supertetrahedron is bridged to six nearest Sm4 tetrahedra by 18 pyridine nitrogen atoms, the overall framework exhibits a twofold interpenetrated pcu net (Fig. 15). The proton conductivity at 30°C is 7.1 × 10−6 S/cm at 30% RH. When the temperature increases to 80°C, the conductivity dramatically rises to 1.4 × 10−3 S/cm (Fig. 16).
(a) View of the inducement of Ln(III) tetrahedral and Cu(I) supertetrahedral clusters; (b) 3D framework along the a-axis. Reproduced from [44] by permission of John Wiley & Sons Ltd
Arrhenius plots of the proton conductivity under 95% RH. Reproduced from [44] by permission of John Wiley & Sons Ltd
2.3 Cluster Organic Frameworks Constructed by Nicotinic Acid
Koner et al. obtained a new Gd26 cluster based 3D framework, {[Gd26(μ6-CO3)9(NA)32(μ3-OH)26](NO3)2 · 3(H2O)}n via hydrothermal synthesis [45]. Five distorted cubane cores are attached to each other through six Gd3+ ions to give the Gd26 clusters. The dimension of Gd26 cluster shell including the organic ligands is around 2.32(4) nm. The Gd26 clusters are then connected to each other by NA− ligands forming a 3D framework. The compound catalyzes the heterogeneous epoxidation of olefinic substrates including α,β-unsaturated ketones (Fig. 17). Similar nanosized Ln26 clusters have been observed in lanthanide-transition-metal–organic frameworks, [Dy26Cu3(NA)24(CH3COO)8(CO3)11(OH)26(H2O)14]Cl · 3H2O and [Tb26NaAg3(NA)27(CH3COO)6(CO3)11(OH)26Cl(H2O)15] · 7.5H2O [46]. In these compounds, Ln26 clusters and Cu+/[Ag3Cl]2+ centers are connected by NA− bridges to give rise to 3D perovskite-like and 2D structures, respectively (Fig. 18).
(a) The structure of Gd26 cluster; (b) 3D framework along the a-axis; and (c) reaction profile for the epoxidation of olefins with tBuOOH catalyzed in acetonitrile media. Reproduced from [45] by permission of John Wiley & Sons Ltd
(a) The 3D coordination structure constructed by {Dy26} clusters and Cu centers and its perovskite-like topological structure; (b) the 2D coordination layer constructed by {Tb26} clusters and [Ag3Cl]2+ bridges and its topological structure. Reproduced from [46] by permission of John Wiley & Sons Ltd
Hong et al. reported two 2D coordination polymers based on huge 36-metal pure lanthanide clusters, {[Ln36(NA)36(OH)49(O)6(NO3)6(N3)3(H2O)20]Cl2 · 28H2O}n (Ln = Gd, Dy) [47]. Six tetrahedral Ln4 clusters adopt an up and down arrangement and form a cyclohexane chair-like Ln24 cluster. The Ln36 cluster can be viewed as the aggregation of two types of cluster units of one wheel-like Ln24 unit and two identical tripod-like Ln6 units (Fig. 19). The nanosized Ln36 clusters are then connected to each other by NA− ligands to form a square layer. These compounds show a large MCE of 39.66 J kg−1 K−1 and slow relaxation of the magnetization, respectively.
(a) The 36-metal Gd(III) cluster; (b) illustration of the structure of Ln36 cluster. Reproduced from [47] by permission of The Royal Society of Chemistry
2.4 Cluster Organic Frameworks Constructed by 4-(3-Pyridyl)benzoic Acid
Two series of wheel cluster organic frameworks (WCOFs), La6Cu3ClL′12(ox)3(OH)2 · 8H2O (FJ-25; ox = oxalate) and La6Cu4X3L′12(ox)3(OH)2 · H3O (FJ-26/27; X = Br/I), are successfully made using 4-(3-pyridyl)benzoic acid (HL′) as ligands [48]. In these compounds, μ3-OH bridge three La3+ ions to form edge-sharing trinuclear [La3(μ3-OH)]8+ (La3) secondary building units (SBUs). The La3 SBUs are linked by ox2− ligands into a 63 graphene-like La18 wheel TBUs, TBUs are further linked by different kinds of pillars to give the whole frameworks (Fig. 20).
View of the La18 TBU and auxiliary pillars in FJ-25 and FJ-26/27. Reproduced from [48] by permission of The Royal Society of Chemistry
3 Summary
This chapter has provided a brief overview of the preparation and structures of lanthanide and Ln-TM cluster organic frameworks using rigid ligands under hydrothermal condition. These compounds show intriguing architectures with several structural types: (1) lanthanide clusters and coordination polymers linked via both Ln–O and Ln–N bonds and (2) Ln-TM heterometallic compounds constructed by lanthanide and different transition metal clusters/ions, in which these rigid ligands act as a linear bridge to form the heterometallic Cluster organic frameworks. The chapter broadens the research from discrete clusters to extended frameworks, which are different to the reported high-nuclearity Ln-TM clusters constructed by flexible ligands of Schiff-base and amino acids, in which the formation of mixed Ln-TM nanosized discrete clusters is usually observed with an investigation on the nature of the magnetic exchange interactions between 3d and 4f ions [3, 49]. The second ligand also plays an important role in the synthetic procedures, the inorganic anions can be used as templates or employed as surface modifiers inserted into the lanthanide cluster core backbone and improve the dimension of cluster cores. To date, the application of these compounds is mainly focused on magnetism and less involved in other aspects [50]. Further investigations in this area are necessary to use these large lanthanide and transition metal clusters to obtain porous cluster organic frameworks, and extend their uses in catalysis and adsorption processes.
Abbreviations
- 2,5-pdc:
-
2,5-Pyridinedicarboxylic acid
- H2bdc:
-
1,2-Benzenedicarboxylic acid
- HIN:
-
Isonicotinic acid
- HL:
-
4-Pyridin-4-ylbenzoic acid
- HL′:
-
4-(3-Pyridyl)benzoic acid
- HNA:
-
Nicotinic acid
- HOAc:
-
Acetic acid
- Ln:
-
Lanthanide
- ox:
-
Oxalate
- TM:
-
Transition metal
References
Chen L, Jiang FL, Zhou K, Wu MY, Hong MC (2015) Metal–organic frameworks based on lanthanide clusters. Struct Bond 163:145–184
Zhang Z, Zheng Z (2015) Nanostructured and/or nanoscale lanthanide metal-organic frameworks. Struct Bond 163:297–368
Kong XJ, Long LS, Zheng ZP, Huang RB, Zheng LS (2010) Keeping the ball rolling: fullerene-like molecular clusters. Acc Chem Res 43:201–209
Zhang SW, Cheng P (2015) Recent advances in the construction of lanthanide–copper heterometallic metal–organic frameworks. CrystEngComm 17:4250–4271
Roesky PW, Canseco-Melchor G, Zulys A (2004) A pentanuclear yttrium hydroxo cluster as an oxidation catalyst. Catalytic oxidation of aldehydes in the presence of air. Chem Commun 738–739
Bünzli J-CG, Piguet C (2002) Lanthanide-containing molecular and supramolecular polymetallic functional assemblies. Chem Rev 102:1897–1928
Shi W, Liu K, Cheng P (2015) Transition–lanthanide heterometal–organic frameworks: synthesis, structures, and properties. Struct Bond 163:231–264
Wang RY, Zheng ZP, Jin TZ, Staples RJ (1999) Coordination chemistry of lanthanides at “high” pH: synthesis and structure of the pentadecanuclear complex of europium(III) with tyrosine. Angew Chem Int Ed 38:1813–1815
Ma BQ, Zhang DS, Gao S, Jin TZ, Yan CH, Xu GX (2000) From cubane to supercubane: the design, synthesis, and structure of a three-dimensional open framework based on a Ln4O4 cluster. Angew Chem Int Ed 39:3644–3646
Wang WH, Tian HR, Zhou ZC, Feng YL, Cheng JW (2012) Two unusual chiral lanthanide-sulfate frameworks with helical tubes and channels constructed from interweaving two double-helical chains. Cryst Growth Des 12:2567–2571
Guo FS, Chen YC, Mao LL, Lin WQ, Leng JD, Tarasenko R, Orendáč M, Prokleška J, Sechovský V, Tong ML (2013) Anion-templated assembly and magnetocaloric properties of a nanoscale {Gd38} cage versus a {Gd48} barrel. Chem Eur J 19:14876–14885
Hu FL, Jiang FL, Zheng J, Wu MY, Pang JD, Hong MC (2015) Magnetic properties of 3D heptanuclear lanthanide frameworks supported by mixed ligands. Inorg Chem 54:6081–6083
Guo PH, Liu J, Wu ZH, Yan H, Chen YC, Jia JH, Tong ML (2015) Single-molecule-magnet behavior in a [2 × 2] grid DyIII 4 cluster and a dysprosium-doped YIII 4 cluster. Inorg Chem 54:8087–8092
Thielemann DT, Wagner AT, Rösch E, Kölmel DK, Heck JG, Rudat B, Neumaier M, Feldmann C, Schepers U, Bräse S, Roesky PW (2013) Luminescent cell-penetrating pentadecanuclear lanthanide clusters. J Am Chem Soc 135:7454–7457
Zhang L, Zhao L, Zhang P, Wang C, Yuan SW, Tang JK (2015) Nanoscale {LnIII 24ZnII 6} triangular metalloring with magnetic refrigerant, slow magnetic relaxation, and fluorescent properties. Inorg Chem 54:11535–11541
Chang LX, Xiong G, Wang L, Cheng P, Zhao B (2013) A 24-Gd nanocapsule with a large magnetocaloric effect. Chem Commun 49:1055–1057
Canaj AB, Tzimopoulos DI, Philippidis A, Kostaki GE, Millios CJ (2012) A strongly blue-emitting heptametallic [DyIII 7] centered-octahedral single-molecule magnet. Inorg Chem 51:7451–7453
Joarder B, Mukherjee S, Xue SF, Tang JK, Ghosh SK (2014) Structures and magnetic properties of two analogous Dy6 wheels with electron-donation and -withdrawal effects. Inorg Chem 53:7554–7560
Alexandropoulos DI, Fournet A, Cunha-Silva L, Mowson AM, Bekiari V, Christou G, Stamatatos TC (2014) Fluorescent naphthalene diols as bridging ligands in LnIII cluster chemistry: synthetic, structural, magnetic, and photophysical characterization of LnIII 8 “Christmas Stars”. Inorg Chem 53: 5420–5422
Ren YX, Zheng XJ, Li LC, Yuan DQ, An M, Jin LP (2014) Three-dimensional frameworks based on dodecanuclear Dy − hydroxo wheel cluster with slow relaxation of magnetization. Inorg Chem 53:12234–12236
Addamo M, Bombieri G, Foresti E, Grillone MG, Volpe M (2004) Assembling process of charged nonanuclear cationic lanthanide(III) clusters assisted by dichromium decacarbonyl hydride. Inorg Chem 43:1603–1605
Sang RL, Xu L (2013) Unprecedented infinite lanthanide hydroxide ribbons [Ln3(μ3-OH)3]n 6n+ in a 3-D metal-organic framework. Chem Commun 49:8344–8346
Dong J, Cui P, Shi PF, Cheng P, Zhao B (2015) Ultrastrong alkali-resisting lanthanide-zeolites assembled by [Ln60] nanocages. J Am Chem Soc 137:15988–15991
Kong XJ, Wu Y, Long LS, Zheng LS, Zheng Z (2009) A chiral 60-metal sodalite cage featuring 24 vertex-sharing [Er4(μ3-OH)4] cubanes. J Am Chem Soc 131:6918–6919
Peng JB, Kong XJ, Zhang QC, Orendáč M, Prokleška J, Ren YP, Long LS, Zheng ZP, Zheng LS (2014) Beauty, symmetry, and magnetocaloric effect-four-shell keplerates with 104 lanthanide atoms. J Am Chem Soc 136:17938–17941
Cui Y, Yue Y, Qian G, Chen B (2012) Luminescent functional metal-organic frameworks. Chem Rev 112:1126–1162
Sun YQ, Zhang J, Chen YM, Yang GY (2005) Porous lanthanide-organic open frameworks with helical tubes constructed from interweaving triple-helical and double-helical chains. Angew Chem Int Ed 44:5814–5817
Zheng XJ, Jin LP, Gao S (2004) Synthesis and characterization of two novel lanthanide coordination polymers with an open framework based on an unprecedented [Ln7(μ3-OH)8]13+ cluster. Inorg Chem 43:1600–1602
Lu JY (2003) Crystal engineering of Cu-containing metal–organic coordination polymers under hydrothermal conditions. Coord Chem Rev 246:327–347
Zhang MB, Zhang J, Zheng ST, Yang GY (2005) A 3D coordination framework based on linkages of nanosized hydroxo lanthanide clusters and copper centers by isonicotinate ligands. Angew Chem Int Ed 44:1385–1388
Cheng JW, Zhang J, Zheng ST, Yang GY (2008) Linking two distinct layered networks of nanosized {Ln18} and {Cu24} wheels through isonicotinate ligands. Chem Eur J 14:88–97
Gu XJ, Xue DF (2007) Surface modification of high-nuclearity lanthanide clusters: two tetramers constructed by cage-shaped {Dy26} clusters and isonicotinate linkers. Inorg Chem 46:3212–3216
Huang L, Han LJ, Feng WJ, Zheng L, Zhang ZB, Xu Y, Chen Q, Zhu DR, Niu SY (2010) Two 3D coordination frameworks based on nanosized huge Ln26 (Ln = Dy and Gd) spherical clusters. Cryst Growth Des 10:2548–2552
Chen L, Guo JY, Xu X, Ju WW, Zhang D, Zhu DR, Xu Y (2013) A novel 2-D coordination polymer constructed from high-nuclearity waist drum-like pure Ho48 clusters. Chem Commun 49:9728–9730
Wu M, Jiang F, Yuan D, Pang J, Qian J, AL-Thabaiti SA, Hong M (2014) Polymeric double anion template Er48 nanotubes. Chem Commun 50:1113–1115
Gu XJ, Xue DF (2007) 3D coordination framework [Ln4(μ3-OH)2Cu6I5(IN)8(OAc)3] (IN = Isonicotinate): employing 2D layers of lanthanide wheel clusters and 1D chains of copper halide clusters. Inorg Chem 46:5349–5353
Cheng JW, Zhang J, Zheng ST, Zhang MB, Yang GY (2006) Lanthanide-transition-metal sandwich framework comprising {Cu3} cluster pillars and layered networks of {Er36} wheels. Angew Chem Int Ed 45:73–77
Cheng JW, Zheng ST, Yang GY (2008) Incorporating distinct metal clusters to construct diversity of 3D pillared-layer lanthanide-transition-metal frameworks. Inorg Chem 47:4930–4935
Fang WH, Cheng L, Huang L, Yang GY (2013) A series of lanthanide-based cluster organic frameworks made of heptanuclear trigonal-prismatic cluster units. Inorg Chem 52:6–8
Canaj AB, Tsikalas GK, Philippidis A, Spyros A, Milios CJ (2014) Heptanuclear lanthanide [Ln7] clusters: from blue-emitting solution-stable complexes to hybrid clusters. Dalton Trans 43:12486–12494
Fang WH, Yang GY (2014) Pillared-layer cluster organic frameworks constructed from nanoscale Ln10 and Cu16 clusters. Inorg Chem 53(11):5631–5636
Fang WH, Cheng JW, Yang GY (2014) Two series of sandwich frameworks based on two different kinds of nanosized lanthanide(III) and copper(I) wheel cluster units. Chem Eur J 20:2704–2711
Fang WH, Zhang L, Zhang J, Yang GY (2015) Construction of cluster organic frameworks with bnn hexagonal BN topologies. Chem Eur J 21:15511–15515
Fang WH, Zhang L, Zhang J, Yang GY (2016) Water stable homochiral cluster organic frameworks built by two kinds of large tetrahedral cluster units. Chem Eur J 22:2611–2615
Sen R, Hazra DK, Mukherjee M, Koner S (2011) Gd26 cluster consisting of distorted cubane cores: synthesis, structure and heterogeneous catalytic epoxidation of olefins. Eur J Inorg Chem 2826–2831
Zhang Y, Huang L, Miao H, Wan HX, Mei H, Liu Y, Xu Y (2015) Hydrothermal synthesis, structure, and optical properties of two nanosized Ln26@CO3 (Ln = Dy and Tb) cluster-based lanthanide–transition-metal–organic frameworks (Ln MOFs). Chem Eur J 21:3234–3241
Wu M, Jiang F, Kong X, Yuan D, Long L, Al-Thabaiti SA, Hong M (2013) Two polymeric 36-metal pure lanthanide nanosized clusters. Chem Sci 4:3104–3109
Fang WH, Zhang L, Zhang J, Yang GY (2016) Halogen dependent symmetry change in two series of wheel cluster organic frameworks built from La18 tertiary building units. Chem Commun 52:1455–1457
Zhou Y, Hong M, Wu X (2006) Lanthanide–transition metal coordination polymers based on multiple N and O-donor ligands. Chem Commun 135–143
Huang Y, Jiang F, Hong M (2009) Magnetic lanthanide–transition-metal organic–inorganic hybrid materials: from discrete clusters to extended frameworks. Coord Chem Rev 253:2814–2834
Acknowledgements
This work was supported by the NSFC (no. 91122028, 21571016, and 21471130), the NSFC for Distinguished Young Scholars (no. 20725101), and the NSF of Zhejiang Province (no. LY13B010002).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cheng, JW., Yang, GY. (2016). Hydrothermal Synthesis of Lanthanide and Lanthanide-Transition-Metal Cluster Organic Frameworks via Synergistic Coordination Strategy. In: Zheng, Z. (eds) Recent Development in Clusters of Rare Earths and Actinides: Chemistry and Materials. Structure and Bonding, vol 173. Springer, Berlin, Heidelberg. https://doi.org/10.1007/430_2016_13
Download citation
DOI: https://doi.org/10.1007/430_2016_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53301-7
Online ISBN: 978-3-662-53303-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)