Abstract
We evaluated the safety and efficacy of donor lymphocyte infusion (DLI) with granulocyte colony-stimulating factor priming and short-term immunosuppressive agents for prophylaxis of relapse in patients with advanced leukemia after human leukocyte antigen (HLA)-mismatched T cell-replete hematopoietic stem cell transplantation (HCT). Twenty-nine patients received prophylactic DLI at a median 75 (33–120) days after HCT. Acute graft-vs-host disease (GVHD) grades 3–4 occurred in six patients, and all cases were controlled. Eleven patients were alive and relapse-free with a probability of leukemia-free survival (LFS) of 37.3 ± 9.6% at 3 years. Chronic GVHD was associated with a lower relapse rate and higher probability of LFS. Prophylactic-modified DLI is feasible in patients with advanced leukemia to prevent relapse after HLA-mismatched HCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adult patients with advanced hematologic malignancies after allogeneic hematopoietic stem cell transplantation (HCT) have a poor prognosis because of a high rate of relapse and transplant-related mortality [1, 2]. Donor lymphocyte infusion (DLI) after allogeneic HCT exhibits definite antileukemia effects in this group of patients [1–3]. DLI can, however, be followed by a high rate of severe graft-vs-host disease (GVHD) [3] and thus is not generally used for prophylaxis of relapse.
We previously reported that infusion of granulocyte colony-stimulating factor (G-CSF)-primed peripheral blood progenitor cells (GPBPCs) instead of unprimed lymphocytes exhibited a comparable or stronger graft-vs-leukemia (GVL) effect and comparable or reduced incidence of GVHD, rarely accompanied by pancytopenia [4]. When DLI with GPBPCs was combined with the use of short-term immunosuppressant therapy, such as short-term cyclosporine A (CsA) or methotrexate (MTX) for GVHD prophylaxis, the incidence of fatal GVHD complicated with DLI decreased further [5]. This modification made prophylactic DLI infusion in human leukocyte antigen (HLA)-matched HCT possible, decreasing relapse incidence in patients with high-risk leukemia after HLA-identical sibling HCT [6]. In our recent reports, this modified DLI has also been successfully used for the treatment of relapse after HLA-mismatched/haploidentical T cell-replete HCT [7]. Based on these findings, we assessed modified DLI for prophylaxis of relapse of leukemia in patients with advanced leukemia after HLA-mismatched/haploidentical T cell-replete HCT to evaluate its safety and efficacy.
Materials and Methods
Eligibility Criteria
Patients were included if they fulfilled at least one of the following criteria defining advanced hematological malignancies: (1) acute leukemia in the first complete remission (CR1) with unfavorable cytogenetic abnormality, a positive Philadelphia (Ph) chromosome, (2) acute leukemia beyond CR2 status or in the nonremission state, or (3) chronic myelogenous leukemia (CML) in blast phase (BP) [8, 9]. Further inclusion criteria were HLA-mismatched/haploidentical HCT without in vitro T cell depletion. To be eligible, HCT recipients had to have been typed at the allele level as all of these, HLA-A, HLA-B, HLA-C, and HLA-DRB1. Patients with any of the following were excluded: creatinine clearance less than 80 ml/min, bilirubin or transaminases greater than three times the upper limit of the normal range, left ventricular ejection fraction less than 50%, and pregnancy before transplantation, uncontrolled GVHD, uncontrolled infection, any evidence of hematological relapse, or refusal of the patient before DLI.
Patient Characteristics
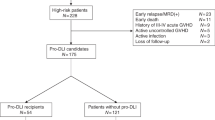
We enrolled patients with hematological malignancies suitable for allo-HSCT, who had no HLA-identical related or unrelated donors. Family donors were ranked on the bases of best HLA match, age (youngest preferred), relationship (mother preferred), gender (same preferred), and health status (better preferred). From November of 2002 to October of 2005, 29 patients with advanced leukemia were enrolled and allocated on an intention-to-treat basis to HLA-mismatched/haploidentical T cell-replete HCT followed by prophylactic modified DLI. The Peking University Review Board approved GPBPC cryopreservation, remobilization, and infusion. All 29 healthy donors and the 29 recipients signed the consent form for stem cell collection and transplantation. Patient characteristics are summarized in Table I. All patients were mismatched at the allele level for HLA-A, HLA-B, HLA-C, and HLA-DRB1. Twelve patients were mismatched in four loci, 14 patients were mismatched in three loci, and three in two loci. Eleven patients had been diagnosed with acute myeloid leukemia (AML; ten in a nonremission state, one in CR1 with Philadelphia chromosome [Ph+]), 13 had a diagnosis of acute lymphoblastic leukemia (ALL; eight in nonremission state, two in CR3, three in CR1 with Ph+), and five patients were diagnosed with CML in BP. The median age of the 29 HCT recipients was 29 years (range, 12–49 years).
Transplantation Procedures
All patients underwent HLA-mismatched/haploidentical T cell-replete transplantation. Transplantation was performed using our recently reported protocol [10]. The Peking University Review boards approved the study protocols. Conditioning regimens consisted of cytosine arabinoside (4 g m−2 day−1, intravenous [i.v.]) on days −10 and −9, busulfan (12 mg/kg, orally in 12 doses) on days −8, −7, and −6, cyclophosphamide (1.8 g m−2 day−1, i.v.) on days −5 and −4, semustine (250 mg/kg, i.v.) on day −3, and anti-human thymocyte immunoglobulin (2.5 mg kg−1 day−1, i.v. from SangStat, rabbit) on days −5 through −2.
The source of stem cells was the mixture of G-CSF-mobilized bone marrow and G-CSF-mobilized PBSCs.
All transplant recipients received CsA, mycophenolate mofetil (MMF), and short-term MTX for GVHD prophylaxis [10]. Follow-up assessment of engraftment and chimerism was determined by cytogenetic analyses and the variable number of tandem repeats (VNTRs) of bone marrow aspirations in the first, second, and third months after transplantation and every 2–6 months thereafter. Progenitor cells and cell subtypes in the grafts were determined by two- or three-color staining in flow cytometry using monoclonal antibodies specific for CD34, CD3, CD4, CD8, and CD56 cells, as described by Liu et al. [11].
Stem Cell Collection
Donors were mobilized with G-CSF (Filgrastim, Kirin, Japan) at 5 μg/kg daily injected subcutaneously for 6 consecutive days. Stem cells from bone marrow were collected on the fourth day of mobilization and the target mononuclear cell (MNC) count was 3–4 × 108 cells/kg recipient weight. In case of ABO major blood group incompatibility, red cells in the marrow were removed by hydroxyethyl sediment manipulation. On the fifth and sixth days, GPBPCs were collected with a COBE Blood Cell Separator (Spectra LRS, COBE BCT, Lakewood, CO) at a rate of 80 ml/min from a total blood volume of 10 l. The target numbers of cells of greater than 4 × 108 MNCs/kg or 2 × 106 CD34+ cells/kg were planned for transplantation. Bone marrow was infused in patients within 6 h after collection, and the PBSC product was infused freshly. The extra harvested cells were cryopreserved with dimethyl sulfoxide in a nitrogen tank. An automated counter instrument was used to count MNCs. The numbers of CD34+, CD3+, CD4+, and CD8+ cells contained in the collected product were counted by flow cytometry. The reasons for cryopreservation of GPBPCs at harvest for potential DLI were convenience for donors and cost effectiveness.
Protocol of Modified DLI
The protocol included two elements. (1) G-CSF-primed PBPCs instead of unprimed donor lymphocyte harvests were used; DLI with GPBPCs was planned from day 30 after transplantation in patients without the following exclusion criteria. Before the prophylactic DLI, serious infection had to be cleared and no serious organ failure present. Infusion with serial dose increments of CD3+ cells was adopted to minimize GVHD and maximize GVL effect. At least 14 days after the first prophylactic DLI, the second infusion with an escalated dose of CD3+ cells was given if the patient did not have active GVHD, infection, or relapse. The medians of MNCs and CD3+ cells infused for prophylactic DLI were 1.0 × 108 (range 0.5–3.0 × 108/kg) and 0.49 × 108/kg (range 0.2–1.4 × 108/kg) for the first infusion, respectively, as well as 1.3 × 108 (range 1–5.82 × 108/kg) and 0.7 × 108/kg (range 0.5–2.91 × 108/kg), respectively, for the second infusion. Chimerism status was examined before and after prophylactic DLI, which was assessed by VNTR in polymerase chain reaction analysis or by fluorescence in situ hybridization (FISH). (2) Short-term immunosuppressive agents were used for the prevention of GVHD after DLI. At the beginning of the study, five patients who received DLI did not have any immunosuppressant to prevent GVHD. Twenty patients received CsA (blood concentration of 150–250 ng/ml for 2–4 weeks), two received CsA combined with MTX (10 mg once per week for 2–4 weeks), and one patient received tacrolimus for the prevention of prophylactic DLI-associated GVHD.
Therapy of GVHD after DLI
Acute GVHD of grade II or higher was treated with methylprednisolone (0.5–1 mg/kg per day). Patients who manifested skin reactions caused by GVHD were treated with MTX combined with methylprednisolone. Prednisone and CsA were the first-line therapy for patients with chronic GVHD. When there was an inadequate or absent response to primary therapy, MMF, tacrolimus, azathioprine, thalidomide, or anti-CD25 monoclonal antibody (Daclizumab; Roche, Basel, Switzerland) were administered.
Evaluations and Definitions
HCT recipients were nursed in isolation rooms with laminar airflow systems. Prophylactic trimethoprim/sulfamethoxazole, acyclovir, and fluconazole were taken to prevent infection with Pneumocystis carinii, herpes simplex, and fungi, respectively. Red blood products were infused into patients who had hemoglobin levels below 70 g/l and/or platelet counts less than 20 × 109/l. All blood products were irradiated before use.
The severity of acute and chronic GVHD was diagnosed with the standard criteria [12, 13]. However, GVHD was diagnosed as acute or chronic according to clinical features of the affected organs rather than based on time after DLI. Myelosuppression was defined as hypocellular marrow with leukocyte counts below 1.0 × 109/l, platelet counts below 20 × 109/l, or reticulocyte counts below 0.2%.
Neutrophil recovery was defined as the number of days to achieve an absolute neutrophil count greater than 0.5 × 109/l for 3 consecutive days. Platelet engraftment was defined as the time to achieve platelets greater than 20 × 109/l without requiring a blood transfusion for 7 continuous days. At day +30 after DLI, disease response and chimerism were assessed in peripheral blood and bone marrow. Because thrombocyte regeneration could be postponed by factors other than leukemia and cytotoxic therapy (i.e., GVHD, viral infection, drugs), complete remission (CR) was defined as less than 5% blasts without evidence of dysplasia in bone marrow and more than 1,500 neutrophils per microliter in peripheral blood. Donor chimerism in unfractionated bone marrow was compared before and after prophylactic DLI, using FISH in gender-mismatched and VNTR analysis in gender-matched transplantations. Relapse was defined as hematological recurrence of disease. Death from leukemia was defined as death with refractory disease after transplantation or as death from any cause after relapse after transplantation. Transplantation-related mortality was defined as death during continuous post-transplant remission.
Supportive Care and Follow-up
During hospitalization in a laminar airflow unit, clinical status, adverse events, and hematological and clinical biochemical parameters were monitored daily. After discharge from the hospital, patients were seen in the outpatient clinic one or two times a week up to day 100 and at gradually longer intervals thereafter. All patients with hematologic malignancies were examined at the first, second, third, sixth, and twelfth month after transplantation of bone marrow for morphology, chimerism, and immunophenotype. Toxicities were graded according to the World Health Organization criteria.
Statistical Analysis
Results were analyzed on October 20, 2007. Overall survival (OS) at 3 years from transplantation was the primary endpoint of the study. Secondary endpoints included leukemia-free survival (LFS, defined as survival in continuous CR after transplantation), nonrelapse mortality, and incidence of relapse, as well as incidence and severity of acute and chronic GVHD. The day of stem cell transfusion was counted as day 0, and all intervals were calculated based on this date.
Numeric variables were analyzed as categories considering their value below or above the median of the entire cohort, as indicated in “Results.” Acute and chronic GVHD were analyzed as time-dependent variables. OS and LFS were estimated using the Kaplan–Meier method. The SPSS 13.0 software package was used for data analysis.
Results
Patient and Donor Characteristics
All patients achieved CR and complete allogeneic engraftment as confirmed by VNTR or FISH after HLA-mismatched HCT. Fifteen donors were parents, two were children, and the other 12 donors were HLA-mismatched siblings of the patients.
The median time to achieve myeloid engraftment was 18 (9–25) days, and time to achieve platelet engraftment was 26 (9–42) days.
All patients received prophylactic DLI after allogeneic HCT. As shown in Table I, the first prophylactic modified DLI was administered between 33 and 120 days (median 75 days) after transplantation in 26 patients. It was administered in 18 patients before day 90 after HCT. One patient received DLI on day 200 and another on day 330 because of the finding of immunotype of leukemia cells by flow cytometry with normal chromosome and morphology of bone marrow during follow-up. Another patient received DLI on day 429 after HCT, 75 days after CR after relapse after transplantation and reinduction with chemotherapy. Prophylactic DLI was performed 35 times among these patients after HCT. Twenty-three patients received only one DLI because of the occurrence of GVHD, infection, or relapse; only six patients received two rounds of DLI treatments because there was no sign of GVHD, even after discontinuation of immunosuppressant for 2–4 weeks.
GVHD and Pancytopenia
Before DLI, 8 of the 29 patients developed acute GVHD grade 1 to 2 after HLA-mismatched HCT at a median of 23.5 days (range: 16–40 days). All acute GVHD was controlled by methylprednisolone (0.5–1 mg kg−1 day−1, i.v.) or combined with MTX. The target organs were skin and liver. Twenty-one patients did not have GVHD before receiving prophylactic DLI.
After the prophylactic DLI, seven patients had no GVHD. Two patients developed acute GVHD grade 1, seven developed acute GVHD grade 2, four developed acute grade 3, and two developed grade 4. All cases of GVHD were controlled thereafter. Acute GVHD occurred in the above 15 patients at a median of 30 (7–90) days after DLI. Twelve out of the 16 patients developed acute GVHD after discontinuation of CsA or MTX (for GVHD prophylaxis) after the DLI, at a median of 44.5 (15–77) days after discontinuation of immunosuppressive agents. Five patients at the beginning of the study did not receive the short-term immunosuppressive agents as GVHD prophylaxis after DLI. Two developed acute GVHD grade 2, and one developed grade 4. Two of the three patients with acute GVHD were alive free of leukemia with a follow-up of 1,115 and 1,852 days after the first DLI; the other patient was free of leukemia at 44 days after DLI until she died of infection. The other two patients who did not have GVHD relapsed at 155 and 325 days after DLI, respectively. After the prophylactic DLI, the cumulative incidence of acute GVHD grade 1 to 2 was 41.5 ± 10.3%, and that of grade 3 to 4 was 28.4 ± 9.2%.
Chronic GVHD occurred in seven patients with a cumulative incidence of 48.1 ± 10.1%, and four of these patients had the extensive type of chronic GVHD. The median time of occurrence of chronic GVHD was 60 (27–80) days after DLI. The cumulative incidence of chronic GVHD and extensive chronic GVHD with competing risks of relapse and death was 27.59 ± 8.47 and 10.34 ± 5.76%, respectively. Limited chronic GVHD occurred in three patients at 40, 60, and 60 days, respectively, after DLI. Six of the seven cases developed chronic GVHD after the tapering or discontinuation of CsA or MTX. The patients who did not receive GVHD prophylaxis and developed acute GVHD grade 4 after DLI had extensive chronic GVHD 60 days after DLI. Factors were analyzed that may influence the occurrence of GVHD in univariate analysis, including the diagnosis and disease status before transplantation, age, gender, and relationship of the patients and donors, mismatched loci, and numbers of MNCs and CD3+ cells infused in DLI, and no risk factors were found.
The nadir of white blood cell count occurred 10 (2–27) days after DLI, and that of platelet count was 9 (2–27) days after DLI. Myelosuppression occurred in five patients. The white blood cell count decreased to less than 2.0 × 109/l in five patients and to less than 1.0 × 109/l in two patients. The platelet count decreased to less than 50 × 109/l in seven patients and to less than 20 × 109/l in five patients.
Relapse
After a median follow-up of 915 days (range, 252–1805 days) after HCT, 13 patients experienced leukemia relapse at a median of 147 days (range, 48–458 days) after transplantation. The median interval from the first DLI to onset of relapse was 139 days (range, 11–383 days). There was hematological relapse in 12 cases and extramedullary relapse in one. The patient with extramedullary relapse received chemotherapy (fludarabine and cyclophosphamide) followed by DLI and was alive with a follow-up of 90 days after diagnosis of relapse, with chronic GVHD. Nine of the 13 cases of relapse occurred within the first year after transplantation. The cumulative incidence of 1-year relapse was 51.3%. Three patients who received DLI twice relapsed at 14, 112, and 311 days, respectively, after the last DLI. The 11 patients with hematological relapse all died of relapse within 2 months after relapse.
The relationship between occurrence of GVHD and relapse was observed as follows. Four of the 13 patients who relapsed did not have GVHD after DLI. They relapsed at 10, 22, 155, and 325 days after DLI, respectively. Immunosuppressive agents were discontinued in the latter two patients on days 30 and 135 after DLI. The patient with extramedullary relapse was diagnosed during the process of active chronic GVHD. Among the other eight patients with hematological relapse, one patient receiving DLI twice relapsed at 14 days after the second DLI during active chronic GVHD. Two patients relapsed 11 and 87 days after DLI, respectively, each during acute GVHD grade 4 and grade 2, respectively, without discontinuation of CSA. Five patients who developed acute GVHD grade 2 to 3 relapsed 40, 139, 176, 362, and 383 days after DLI, respectively, and their immunosuppressive agents were discontinued at 35, 91, 86, 162, and 80 days after DLI, respectively.
Follow-up and Outcome
Through October 20, 2007, 12 out of the 29 patients were alive, and 11 were alive without disease recurrence, for a median duration of LFS of 932 days (range, 250–1567 days). Four out of the 11 patients with refractory AML, 5 out of 13 with ALL, and two out of five with CML survived free of relapse. Seventeen patients died. The causes of death were recurrent disease (n = 11 patients), infection (n = 5 patients), and cardiac infarction (n = 1 patient). The probability of LFS was 37.3 ± 9.6% at 3 years (Fig. 1). The patients who developed chronic GVHD after the DLI had higher LFS values (80.1 ± 10.1 vs 36.5 ± 14.8%, p = 0.010; Fig. 1). The probability of relapse in patients with chronic GVHD was lower than that in patients without chronic GVHD (28.6 ± 14.4 vs 62.3 ± 13.8%, p = 0.052; Fig. 2).
Discussion
For patients with advanced leukemia, the opportunity to achieve CR with conventional chemotherapy is limited, and long-term survival is almost unachievable. Allogeneic HCT is recommended for these patients because of poor results with conventional chemotherapy [14–16]. However, its success is limited by a high incidence of disease recurrence for these patients with advanced leukemia [17–19]. DLI exerts a GVL effect after allogeneic HCT. This outcome suggested that the strategy against disease recurrence might be converted from a therapeutic DLI to prophylactic DLI for those with advanced hematological malignancies. In most trials of HLA-matched HCT, the majority of DLI recipients developed GVHD, so there was special concern about fatal GVHD after DLI because of HLA disparity in HLA-mismatched transplantation. Up to now, there has been no report on prophylactic DLI in patients receiving HLA-mismatched HCT.
The current study was initiated to assess the feasibility and efficacy of modified prophylactic DLI in HLA-mismatched HCT without in vitro T cell depletion. The cumulative incidence of acute GVHD grades 1 to 2 and grades 3 to 4 after the modified prophylactic DLI is higher than that which we reported for HLA-mismatched HCT with CSA, MMF, and short-term MTX as GVHD prophylaxis [10]. However, we note that most prophylactic DLI was administered before day 90 after transplantation, when a high incidence of acute GVHD was commonly seen. Twelve out of the 16 patients who developed acute GVHD had stopped the prophylactic immunosuppressive agents within 4 weeks after DLI. We know that even in HLA-matched HCT, the immunosuppressive agents are usually tapered or discontinued within a half-year after allogeneic transplantation. Thus, the incidence of acute GVHD after the modified DLI in HLA-mismatched HCT was acceptable. The use of G-CSF-primed PBPCs instead of unprimed lymphocytes may be one explanation for the acceptable incidence of GVHD. The effect of in vivo G-CSF application on T cell function has been extensively explored. Our serial study has confirmed that in vivo G-CSF application indirectly induces a decrease in T cell proliferation and the type II helper T cell polarization in the cytokine profile [20–23]. Furthermore, T cell hyporesponsiveness induced by in vivo G-CSF may be related to a selective decrease of DC1 and the downregulation of CD28/B7 costimulatory signals. This mechanism may contribute to the antigen-specific hyporesponsiveness of T cells in G-CSF-mobilized harvest [20–23].
The other possible explanation is the use of short-term immunosuppressive agents. With the use of these agents as prophylaxis for GVHD after therapeutic DLI in HLA-mismatched HSCT, the incidence of GVHD grades 3–4 between patients with or without GVHD prophylaxis was reported to be 8.3 vs 62.50% after DLI [7]. However, it is still not clear what the duration of immunosuppression should be or what types of immunosuppressive agents should be used for the prophylactic, modified DLI to reduce the incidence of fatal GVHD without influencing the GVL effect. Further studies are needed to address this.
All acute GVHD occurred after the modified prophylactic DLI had been controlled, no death because of GVHD occurred, and the incidence of chronic GVHD was also acceptable, which further indicated the safety of this protocol. No profound, lasting pancytopenia was observed after the prophylactic DLI, which is in accordance with the observations of Sohn et al. [24]. The reason may be the relatively high percentage of CD34+ cells contained in the infused product. Thus, the modified prophylactic DLI after HLA-mismatched/haploidentical T cell-replete HCT in patients with advanced leukemia is safe and feasible in terms of GVHD and pancytopenia, but a larger series will be needed to prove the safety and feasibility of this modified DLI protocol.
Thirteen patients relapsed after prophylactic DLI, and their disease status may have contributed to the high rate of relapse. In this report, 18 of the patients had acute leukemia and were refractory to multiagent chemotherapy and in the nonremission state before transplantation. Our previous data showed that a lower load of malignant cells in bone marrow was associated with a lower rate of relapse after prophylactic DLI in HLA-matched HCT [6]. Another factor that may relate to relapse was disease category, such as Ph+ acute leukemia, which is considered insensitive to DLI [25, 26]. In the present study, a value of 37.3 ± 9.6% for the 2-year probability of LFS was comparable and even more promising than results in previous studies [8]. For patients with advanced leukemia, it is difficult to evaluate the efficacy of prophylactic DLI with a single-arm study; however, it is also difficult to conduct controlled studies involving this group of patients. Thus, a greater number of cases are needed to evaluate more clearly the efficacy of the modified prophylactic DLI, but the safety of the protocol is acceptable.
The data in the present study were not analyzed with multivariate regression because of the limited number of cases. Univariate analysis identified chronic GVHD as the factor associated with a lower rate of relapse and higher LFS, which suggested the presence of a GVL effect associated with chronic GVHD after the modified DLI. In addition, the fact that prophylaxis of GVHD after DLI was not a risk factor for relapse suggested that the prophylaxis of GVHD with short-term immunosuppressive agents might not abrogate or influence the GVL effect.
In summary, this study is the first to our knowledge to show that the modified DLI approach can be safely used for prophylaxis of leukemia relapse in patients with advanced leukemia even after HLA-mismatched T cell-replete HCT. More cases and more stem cell transplant units are needed to participate in this clinical setting to confirm the results.
References
Robak T, Wrzesien-Kus A. The search for optimal treatment in relapsed and refractory acute myeloid leukemia. Leuk Lymphoma 2002;43:281–91.
Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone Marrow Transplant 2000;26:1157–63.
Slavin S, Naparstek E, Nagler A. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood 1996;87:2195–204.
Huang XJ, Guo NL, Ren HY, Xu LP, Chen H, Liu KY, et al. An improved anti-leukemic effect achieved with donor progenitor cell infusion for relapse patients after allogeneic bone marrow transplantation. Chinese Med J 2003;116:736–41.
Huang XJ, Liu DH, Xu LP, Chen H, Liu KY, Han W, et al. Prophylactic infusion of donor granulocyte colony stimulating factor mobilized peripheral blood progenitor cells after allogeneic hematological stem cell transplantation in patients with high-risk leukemia. Leukemia 2006;20:365–8.
De Lima M, Bonamino M, Vasconcelos Z, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transplant 2001;27:73–8.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica 2007;92:414–7.
Mengarelli A, Iori A, Guglielmi C, et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica 2002;87:52–8.
Appelbaum FR. Who should be transplanted for AML. Leukemia 2001;15:680–2.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006;38:291–7.
Liu Y, Chen S, Yu H. Standardization and quality control in flow cytometric enumeration of CD34(+) cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2000;8:302–6.
Flower ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disase. Hematol Oncol Clin North Am 1999;13:1091–112.
Keith MS. Graft-vs.-host disease. In: Thomas ED, Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. 3rd ed. Malden, MA: Blackwell; 2004. p. 635–64.
Singhal S, Powles R, Henslee-Downey PJ, et al. Allogeneic transplantation from HLA-matched sibling or partially HLA-mismatched related donors for primary refractory acute leukemia. Bone Marrow Transplant 2002;29:291–5.
Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000;96:4075–83.
Estey EH. Treatment of relapsed and refractory acute myeloid leukemia. Leukemia 2000;14:476–9.
Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol 1992;10:1723–9.
Christoph S, Michael S, Rainer S, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006;108:1092–9.
Alessandrino EP, Bernasconi P, Caldera D. Chemotherapy and donor peripheral blood progenitor cells for acute leukemia in early relapse after allogeneic bone marrow transplantation. Bone Marrow Transplant 1999;23:607–12.
Chen SH, Li X, Huang XJ. Effect of recombinant human granulocyte colony-stimulating factor on T-lymphocyte function and the mechanism of this effect. Int J Hematol 2004;79:178–84.
Huang XJ, Chang YJ, Zhao XY. In vivo induction of T-cell hyporesponsiveness and alteration of immunological cells of bone marrow grafts using granulocyte colony-stimulating factor. Haematologica 2004;89:1517–24.
Huang XJ, Chang YJ, Zhao XY. A direct comparison of immunological characteristics of granulocyte colony-stimulating factor (G-CSF)-primed bone marrow grafts and G-CSF-mobilized peripheral blood grafts. Haematologica 2005;90:715–6.
Huang XJ, Chang YJ, Zhao XY. Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transplant Immunol 2007;17:193–7.
Sohn SK, Jung JT, Kim DH, Lee NY, Seo KW, Chae YS, et al. Prophylactic growth factor-mobilized donor lymphocyte infusion using cells reserved at the time of transplantation after allogeneic peripheral blood stem cell transplantation in patients with high-risk hematologic malignancies. Cancer 2002;94:18–24.
Yanada M, Naoe T, Iida H. Myeloablative allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: significant roles of total body irradiation and chronic graft-versus-host disease. Bone Marrow Transplant 2005;36:867–72.
Ko BS, Tang JL, Tsai W. Philadelphia chromosome-positive acute lymphoblastic leukemia in Taiwan. Ann Hematol 2001;80:510–5.
Acknowledgments
This work is supported by the National Outstanding Young Scientist’s Foundation of China (grant no. 30725038), Hi-tech Research and Development Program of China (no. 2006AA02Z4A0), and Program for Innovative Research Team in the University (No. IRT0702).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao-Jun Huang: involved in conception and design, revising the article critically, and final approval of the version to be published; Dai-Hong Liu: performed research, analysis, and interpretation of data and drafting of the article and gave final approval of the version to be published; the other authors: performed research and gave final approval of the version to be published; the authors reported no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Huang, XJ., Liu, DH., Liu, KY. et al. Modified Donor Lymphocyte Infusion after HLA-Mismatched/Haploidentical T Cell-replete Hematopoietic Stem Cell Transplantation for Prophylaxis of Relapse of Leukemia in Patients with Advanced Leukemia. J Clin Immunol 28, 276–283 (2008). https://doi.org/10.1007/s10875-007-9166-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-007-9166-z