Abstract
Full donor T-cell chimerism (FDTCC) after allogeneic stem cell transplant (allo-SCT) has been associated with improved outcomes in hematologic malignancy. We studied if donor human leukocyte antigen (HLA) mismatch improves achievement of FDTCC because mismatched HLA promotes donor T-cell proliferation where recipient T-cells had been impaired by previous treatment. Patients (N = 138) received allo-SCT with reduced-intensity conditioning (RIC) from 39 HLA mismatched donors (16 unrelated; 23 haploidentical) with post-transplant cyclophosphamide (PTCy) or 99 matched donors (21 siblings; 78 unrelated) with PTCy (N = 18) or non-PTCy (N = 81). Achievement of FDTCC by day 100 was higher with HLA mismatched donors than matched donors (82.1% vs. 27.3%, p < 00,001), which was further improved with 200 cGy total body irradiation (87.9%) or lymphoid (versus myeloid) malignancy (93.8%). Since all mismatched transplants used PTCy, FDTCC was higher with PTCy than non-PTCy (68.4% vs. 25.7%, p < 0.00001), but not in the matched transplant with PTCy (38.9%), negating PTCy as the primary driver. Lymphocyte recovery was delayed with PTCy than without (median on day + 30: 100 vs. 630/µL, p < 0.0001). The benefit of FDTCC was not translated into survival outcomes, especially in myeloid malignancies, possibly due to the insufficient graft-versus-tumor effects from the delayed lymphocyte recovery. Further studies are necessary to improve lymphocyte count recovery in PTCy transplants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of allogeneic stem cell transplantation (allo-SCT) in hematologic malignancy is to maintain remission by graft-versus-tumor (GVT) effect, mainly provided by donor T-cells [1]. Achievement of full donor T-cell chimerism (FDTCC) by post-transplant day + 100 has been reported to be associated with a lower risk of relapse [2], abrogates the adverse impact of pre-transplant measurable residual disease,[3] and prevents relapse if achieved by pre-emptive donor lymphocyte infusion [4]. However, compared to myeloablative conditioning, reduced intensity conditioning (RIC) is associated with lower rates of achieving FDTCC [5] and results in higher rates of relapse [6]. Strategies to accelerate the achievement of FDTCC may optimize GVT and thus improve outcomes in patients transplanted with RIC.
To identify factors that may accelerate FDTCC, we investigated the impact of donor human leukocyte antigen (HLA) mismatch because T-cells are known to proliferate when stimulated by allo-antigens. HLA mismatched between recipient and donor T-cells can result in vigorous T-cell proliferation, recognizing each other as allo-antigens [7]. However, if recipient T-cells have been impaired from pre-transplant treatment or irradiation, donor T cells will exhibit a proliferative advantage and dominate [8]. Thus, we predicted that the achievement of FDTCC is improved when the donor is HLA mismatched and that the intensity of pre-transplant treatment or conditioning of the recipient influences FDTCC.
In this study, we compared the achievement of FDTCC between HLA mismatched and matched donor allo-SCT using RIC. All patients with HLA mismatched donors also received PTCy post-transplant cyclophosphamide (PTCy)[9]-based graft-versus-host disease (GVHD) prophylaxis. On the other hand, the patients with HLA-matched donors received either PTCy or conventional non-PTCy for GVHD prophylaxis because PTCy has been reported to provide safe and effective GVHD prophylaxis in the allo-SCT from haploidentical donors [9], HLA matched donors [10, 11] and mismatched unrelated donors (MMUD) [12]. In this cohort, we demonstrate that the achievement of FDTCC is improved in patients with HLA mismatched donors and evaluate its effect on clinical outcomes in correlation with lymphocyte count recovery.
Materials and methods
Patients and study design
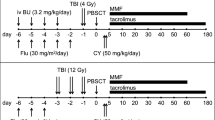
This study included all patients who underwent allogeneic peripheral blood stem cell transplantation (allo-SCT) with reduced-intensity conditioning (RIC) or non-myeloablative regimens for acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), acute lymphoblastic lymphoma (ALL), or lymphoma at our institution between January 2017 and February 2021. Patients who received anti-thymocyte globulin (ATG) or previous allo-SCT were excluded. Donors were assigned as matched (8/8) or mismatched (7/8 or less) based on HLA-A, B, C, and DRB1 loci. All patients with mismatched donors received PTCy GVHD prophylaxis, which consists of cyclophosphamide (Cy) 50 mg/kg × 2 days on post-SCT days + 3 and + 4, followed by tacrolimus (TAC) and mycophenolate mofetil (MMF) starting on day + 5. Patients with matched donors received PTCy or non-PTCy GVHD prophylaxis at the physician’s discretion. Non-PTCy GVHD prophylaxis consisted of TAC 0.03 mg/kg/day (serum concentration of 5 to 10 ng/mL) starting on day − 2 and methotrexate (MTX) 5 mg/kg on days + 1, + 3, + 6, and + 11. Low-dose total body irradiation (200 cGy TBI on day − 1) was included at the physician’s discretion. All patients received filgrastim 5 µg/kg starting on day + 12. Disease risk index [13] was used to predict the outcome.
Outcome evaluation
Neutrophil and platelet recovery was defined as an ANC ≥ 500 cells/µL and platelet count ≥ 20,000 cells/µL [14], respectively. Lymphocyte recovery was defined as the first day of 3 consecutive days with the absolute lymphocyte count (ALC) ≥ 200 cells/µL. Lymphocyte subset study was not tested. Chimerism was tested on peripheral blood total white cells (unfractionated) and T-cell subset (selected by CD3), as previously reported [15]. Briefly, short-tandem repeat or quantitative PCR was used per protocol (approximately 1, 2, 3, 6, and 12 months after transplantation, then annually) or when clinically indicated. Full donor chimerism is defined as donor > 95%[14].
Clinical outcome was evaluated for 2-year overall survival (OS), disease-free survival (DFS), GVHD/relapse-free survival (GRFS) [16], relapse rate (RR), graft failure, transplant-related mortality (TRM), and acute and chronic GVHD. RR, graft failure, TRM, and chronic GVHD were assessed by 2-year cumulative incidence. Acute GVHD was estimated by the cumulative incidence of aGVHD in the first 100 days post-SCT.
Statistics
An unpaired t-test was used to compare the length of time in days of blood cell recovery. Logistic regression and Cox proportional hazard regression with time-dependent covariate was used in multivariate analysis. For the Cox analysis, FDTCC was treated as a time-dependent covariate, and DFS was analyzed as a landmark analysis from day + 30. The statistical software GraphPad Prism and EZR were used for the analysis [17]. OS, DFS, and GRFS were assessed using Kaplan–Meier methods and log-rank tests. RR, graft failure, TRM, and GVHD were assessed using Gray’s test for the cumulative incidence of competing events. TRM, relapse, and engraftment failure were treated as competing risks.
Results
Patient and donor characteristics
A total of 138 patients received allo-SCT from donors who were HLA mismatched (N = 39) or matched (N = 99) (Table 1). In the HLA mismatched group, as compared to the HLA matched group, the patients were younger (p < 0.01), and more patients received 200 cGy TBI the (p < 0.001) or fludarabine (Flu)/Cy (p < 0.001) as part of their RIC, but fewer patients had AML (p < 0.05). Follow-up period, disease risk index, disease status at transplant, and CD34 cell dose were not significantly different. The 39 mismatched donors included 16 MMUD (7/8 matched N = 14; 6/8 matched N = 2) and 23 haploidentical donors (4/8 matched N = 15; 5/8 matched N = 7; and 7/8 matched N = 1). The 99 matched donors included 21 siblings (MSD) and 78 matched unrelated donors (MUD) consisting of 12/12 match (DQ/DP identical, N = 14, 18%), 11/12 match (one DP mismatch, N = 41), and 10/12 match (two DP mismatch, N = 22; one DQ and one DP mismatch N = 1). For conditioning, most received either Flu/Busulfan (Bu) (46.2%) or Flu/Cy (43.6%) in the mismatched group, while most received Flu/Bu (80.8%) in the matched group. Measurable residual disease data was unavailable for this study cohort.
Donor chimerism
In peripheral blood total white cells, full donor chimerism was achieved by day + 100 in the majority of patients with HLA mismatched or matched donors (89.7% vs. 80.8%, p = 0.2). The achievement of total cell full donor chimerism was not significantly different by conditioning regimens, disease type, GVHD prophylaxis, or patient age (data not shown).
By day + 100, the percentage of patients who achieved T-cell chimerism was much higher in those with HLA mismatched donors than with matched donors (FDTCC: 82.1% vs. 27.3%, p < 00,001, Table 2, Fig. 1A–C). Achievement of FDTCC with HLA mismatched donors was higher regardless of the patients’ conditioning regimens (88.2% vs. 70.0%among FluCy patients, p < 0.05; 77.3% vs. 25.9% among non-PTCy patients, p < 0.00001), ages (91.8% vs. 21.2% among patients < 60 years old, p < 0.0001; 82.4% vs. 30.4% among patients ≥ 60 years-old, p < 0.001), disease types (73.9% vs. 22.7% among myeloid patients, p < 0.00001; 93.8% vs. 41.7% among lymphoid malignancy patients, p < 0.01), GVHD prophylaxis (82.1% vs. 38.9% among PTCy patients, p < 0.01), and TBI (87.9% vs. 40.0% among TBI patients, p < 0.02; 50.0% vs 25.9% among non-TBI patients, p = 0.2) (Table 2). The percentage of patients who achieved FDTCC by day + 100 was higher in those who received TBI versus those who did not in both the mismatched and matched groups (87.9% vs. 50.0%, p = 0.2 in the mismatched group; 40.0% vs. 25.9% in the matched group, p = 0.3).
Cumulative incidence for total T-cell chimerism achievement was shown for HLA mismatched, or HLA matched allo-SCT (both myeloid and lymphoid malignancies combined, (A), for myeloid disease only (acute myeloid leukemia or myelodysplastic syndrome, (B), and for lymphoid disease only (acute lymphocytic leukemia or lymphoma, (C). In addition, HLA mismatched allo-SCT was stratified into the patients who received TBI or those who did not
Since all patients with mismatched donors had received PTCy, higher achievement of FDTCC was seen in the PTCy patients than in the non-PTCy patients (68.4% vs. 25.7%, p < 0.00001). However, when the PTCy patients were stratified, achievement of FDTCC was higher only with the mismatched but not with matched donors (82.1% vs. 38.9%, p < 0.01). Thus, the improvement of FDTCC is mediated by the donor HLA mismatch but not the use of PTCy. Dose-dependent effect of HLA mismatch was not evident, as FDTCC was not significantly different between ≤ 6/8 and 7/8 matched donors (87.5% vs. 73.3%, p = 0.6).
By multivariate analysis, factors predicting FDTCC by day + 100 were lymphoid disease over myeloid disease and haploidentical/MMUD donors over MUD donors (p < 0.05, Table 3). No significant influence was detected by disease risk index, use of PTCy, and lymphocyte count ≥ 200/µL by day + 30.
Peripheral blood cell count recovery
Cell count recovery was delayed in the HLA mismatched group as compared to the matched group (Table 4). However, all patients with HLA mismatch donors also received PTCy, which has been known to delay cell count recovery. In fact, the PTCy patients, as compared to non-PTCy patients, had a delayed median recovery of neutrophils (18 vs. 14 days), platelets (23 vs. 13 days), and lymphocytes ≥ 200/µL (40 vs. 15 days) (all p < 0.0001). Median lymphocyte count on day + 30 was significantly lower in the PTCy patients than in non-PTCy patients (100 vs. 630/μL, p < 0.0001). On the other hand, in the 57 PTCy patients, the median time to count recovery was not different between the HLA mismatched and matched groups. Thus, the delayed recovery is due to the GVHD prophylaxis but not the HLA mismatch.
In the PTCy group, we explored additional factors that may have affected cell count recovery. The delay in count recovery was more pronounced with age ≥ 60 than < 60 years especially in neutrophil counts (neutrophil: 19.5 vs. 16 days, p < 0.05; platelet: 25 vs. 21 days, p = 0.1; lymphocyte: 40.5 vs. 36 days, p = 0.3). The other factors, such as conditioning, TBI, and disease type, did not affect the recovery (data not shown).
Clinical outcome
Patients who received an HLA mismatched allo-SCT showed no difference in 2-year OS (71.7% vs. 52.2%, p = 0.07). Two-year disease-free survival was better in the HLA mismatched group than in the HLA-matched group in DFS (63.9% vs. 43.0%, p < 0.05) and GRFS (45.7% vs. 21.9%, p < 0.05) (Table 2). The difference between mismatched and matched donors was much more pronounced in patients with lymphoid malignancy. In contrast, the difference in patients with a myeloid malignancy was not statistically significant (p = 0.3 for OS, p = 0.4 for DFS, p = 0.06 for GRFS). Graft failure, TRM, and acute/chronic GVHD were not statistically significantly different between mismatched and matched donor allo-SCT recipients.
Discussion
In this study, we demonstrated that the achievement of FDTCC by day + 100 was improved with HLA mismatched donors. Our results also suggested that using 200 cGy TBI conditioning and having a lymphoid malignancy further improve the achievement of FDTCC. One possible explanation for this finding is that recipient T-cells were more extensively damaged (or suppressed) in patients who received more lymphotoxic conditioning, such as TBI-containing conditioning regimens, resulting in a competitive advantage for the proliferation of donor T-cells. Donor mismatch at HLA-A, B, C, and DRB1 in haploidentical and MMUD effectively improved the achievement of FDTCC. In contrast, DP mismatch in the 64 MUD patients (82.1%) was not sufficient to enhance the achievement of FDTCC compared to the 21 patients with matched sibling donors (25.1% vs. 19.0%, p = 0.6). Improved regulatory T-cells (T-reg) expansion has been reported with the use of PTCy [18], which could improve the FTDCC. However, the reported ratio of regulatory T-cell: conventional CD4 T-cell after PTCy haploidentical allo-SCT is only from 0.05 to 0.2 (approximately less than 10% of total T-cells). Thus, this may be insufficient to explain the magnitude of FDTCC difference between HLA mismatched vs. matched groups (82.1% vs. 27.3% by day + 100).
It has been reported that higher T-cell chimerism achievement is associated with using PTCy in a cohort of 21 sickle cell disease or thalassemia haploidentical transplant recipients [19] and a cohort consisting of 10 matched and 20 haploidentical transplant recipients [20]. However, our data indicated that the PTCy was rather a confounding factor with mismatched donors, arising from the fact that the patients with mismatched donors were in the PTCy group. The FDTCC, DFS, and GRFS were better with lymphoid diseases than myeloid disease, and in lymphoid disease, with mismatched donors than with matched donor transplants (Table 2). The better survival of the lymphoid disease compared with myeloid malignancies was consistent with the previous report in the non-myeloablative haploidentical SCT with PTCy [9]. However, the better outcome with mismatched donor transplant in lymphoid disease was not consistent with the previous larger retrospective study [21].
The outcome from mismatched donor transplant with PTCy has been equivalent but never superior to matched donor transplant regardless of lymphoid [21] or myeloid disease [22, 23]. The limited size of our cohort and heterologous disease types may have contributed to this discrepancy. On the other hand, the outcome of mismatched donor transplant in myeloid disease was equivalent to matched donor transplant in our study, which is consistent with previous larger studies [24, 25]. Thus, the documented benefit of FDTCC in non-PTCy settings [2, 3, 6] was not demonstrated in the HLA mismatch transplant with PTCy. This could be explained by the delayed lymphocyte recovery in HLA mismatched patients with PTCy, which is a known risk for infection, relapse [26], and poor outcomes in the allo-SCT with conventional non-PTCy GVHD prophylaxis [27,28,29,30].
The PTCy patients in this study developed severe lymphopenia and delayed lymphocyte recovery compared to non-PTCy patients. All PTCy patients also received TAC and MMF for GVHD prophylaxis. Significant lymphopenia has been previously reported in patients who received PTCy + TAC + MMF by 1-month post-SCT as compared to non-PTCy patients, who gradually recovered lymphocytes after 2 months post-SCT [18]. In comparison, the patients who received haploidentical allo-SCT with PTCy, sirolimus, and MMF were reported to have a median lymphocyte count of 469/µL on day + 30 [31], higher than our patients (100/µL on day + 30) as well as in the previous report. [18] Thus, we suspect that the combination of PTCy and TAC is the cause of delayed lymphocyte recovery. Another potential advantage of sirolimus is its ability to expand Treg, which plays an important role in GVHD prevention [32]. This is contrary to the calcineurin inhibitors that suppress Treg activity [33]. The application of sirolimus may lead to a faster lymphocyte recovery, possibly taking better advantage of HLA-mismatch-associated improved FDTCC, resulting in better survival outcomes in PTCy transplants.
In conclusion, the achievement of FDTCC can be improved by donor HLA mismatch in the allo-SCT with RIC. However, the benefit of FDTCC was not evident in clinical outcomes in allo-SCT recipients with myeloid malignancies. This may be due to insufficient GVT from the delayed lymphocyte recovery. Therefore, strategies to improve lymphocyte count recovery may be the key to taking advantage of FDTCC.
Data Availability
Raw data were generated at Penn State Cancer Institute. Derived data supporting the findings of this study are available from the corresponding author KM on request.
References
Blazar BR, Hill GR, Murphy WJ (2020) Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD. Nat Rev Clin Oncol 17(8):475–492
Peterlin P, Delaunay J, Guillaume T et al (2015) Complete donor T cell chimerism predicts lower relapse incidence after standard double umbilical cord blood reduced-intensity conditioning regimen allogeneic transplantation in adults. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 21(1):180–184
Craddock C, Jackson A, Loke J et al (2021) Augmented reduced-intensity regimen does not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol 39(7):768–778
Caldemeyer LE, Akard LP, Edwards JR et al (2017) Donor lymphocyte infusions used to treat mixed-chimeric and high-risk patient populations in the relapsed and nonrelapsed settings after allogeneic transplantation for hematologic malignancies are associated with high five-year survival if persistent full donor chimerism is obtained or maintained. Biol Blood Marrow Transplant 23(11):1989–1997
Reshef R, Hexner EO, Loren AW et al (2014) Early donor chimerism levels predict relapse and survival after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol Blood Marrow Transplant 20(11):1758–1766
Scott BL, Pasquini MC, Fei M et al (2021) Myeloablative versus reduced-intensity conditioning for hematopoietic cell transplantation in acute myelogenous leukemia and myelodysplastic syndromes-long-term follow-up of the BMT CTN 0901 clinical trial. Transplant Cell Ther 27(6):483 e481-483 e486
Bain B (1968) Mixed Leucocyte Cultures and histocompatibility testing. J R Coll Physicians Lond 3(1):25–32
Farrell C, Honeyman M, Hoadley C (1988) An analysis of the effect of HLA-DP in the mixed lymphocyte reaction. J Immunogenet 15(5–6):243–250
Luznik L, O’Donnell PV, Symons HJ et al (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplantat 14(6):641–650
Battipaglia G, Labopin M, Hamladji RM et al (2021) Post-transplantation cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation from HLA-identical sibling donors: a retrospective analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer 127(2):209–218
Mielcarek M, Furlong T, O’Donnell PV et al (2016) Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 127(11):1502–1508
Battipaglia G, Labopin M, Kroger N et al (2019) Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood 134(11):892–899
Armand P, Kim HT, Logan BR et al (2014) Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 123(23):3664–3671
Kharfan-Dabaja MA, Kumar A, Ayala E et al (2021) Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the american society for transplantation and cellular therapy. Transplant Cell Ther 27(8):642–649
Tyler J, Kumer L, Fisher C et al (2019) Personalized chimerism test: selection of short tandem repeat or quantitative PCR depending on patient’s chimerism status. J Mol Diagn: JMD 21(3):483–490
Holtan SG, DeFor TE, Lazaryan A et al (2015) Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125(8):1333–1338
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48(3):452–458
Rambaldi B, Kim HT, Reynolds C et al (2021) Impaired T- and NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv 5(2):352–364
Fitzhugh CD, Hsieh MM, Taylor T et al (2017) Cyclophosphamide improves engraftment in patients with SCD and severe organ damage who undergo haploidentical PBSCT. Blood Adv 1(11):652–661
Retiere C, Willem C, Guillaume T et al (2018) Impact on early outcomes and immune reconstitution of high-dose post-transplant cyclophosphamide vs anti-thymocyte globulin after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation. Oncotarget 9(14):11451–11464
Al Malki MM, Yang D, Labopin M et al (2020) Comparing transplant outcomes in ALL patients after haploidentical with PTCy or matched unrelated donor transplantation. Blood Adv 4(9):2073–2083
Kunacheewa C, Ungprasert P, Phikulsod P et al (2020) Comparative efficacy and clinical outcomes of haploidentical stem cell transplantation to other stem sources for treatment in acute myeloid leukemia and myelodysplastic syndrome patients: a systematic review and meta-analysis. Cell Transplant 29:963689720904965
Rashidi A, Hamadani M, Zhang M-J et al (2019) Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv 3(12):1826–1836
Ambinder AJ, Jain T, Tsai HL et al (2022) HLA-matching with PTCy: a reanalysis of a CIBMTR dataset with propensity score matching and donor age. Blood Adv 6(14):4335–4346
Gooptu M, Romee R, St Martin A et al (2021) HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood 138(3):273–282
Velardi E, Tsai JJ, van den Brink MRM (2021) T cell regeneration after immunological injury. Nat Rev Immunol 21(5):277–291
Powles R, Singhal S, Treleaven J et al (1998) Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood 91(9):3481–3486
Fu Q, Xu LP, Zhang XH et al (2016) Early lymphocyte recovery predicts superior outcomes after unmanipulated haploidentical blood and marrow transplant for acute myeloid leukemia. Clin Transplant 30(8):954–958
Le Blanc K, Barrett AJ, Schaffer M et al (2009) Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplantat 15(9):1108–1115
Chang YJ, Zhao XY, Huo MR et al (2011) Clinical impact of absolute lymphocyte count on day 30 after unmanipulated haploidentical blood and marrow transplantation for pediatric patients with hematological malignancies. Am J Hematol 86(2):227–230
Bejanyan N, Pidala JA, Wang X et al (2021) A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv 5(5):1154–1163
Peccatori J, Forcina A, Clerici D et al (2015) Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia 29(2):396–405
Coenen JJ, Koenen HJ, van Rijssen E et al (2007) Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant 39(9):537–545
Acknowledgements
The authors would like to thank the patients who participated in this study and the technicians who participated in the experimental procedures.
Funding
This work was supported by the Arlene Witmer Memorial Fund for Bone Marrow Cancer Research, the John and Denise Gilliland Fund for the Cancer Genetics Program, G. R. Sponaugle Employee Cancer Research Fund, Earl “Bumps” Clouser Memorial Lymphoma Research Endowment, and Richard E. and Stephanie A. Ziegler Charitable Foundation Endowment in Hematology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were by the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cioccio, J., Rakszawski, K., Zheng, H. et al. Donor HLA mismatch promotes full donor T-cell chimerism in the allogeneic stem cell transplant with reduced-intensity conditioning and post-transplant cyclophosphamide GVHD prophylaxis. Ann Hematol 102, 613–620 (2023). https://doi.org/10.1007/s00277-022-05077-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-05077-2