Abstract
Both haploidentical hematopoietic stem cell transplantation (HSCT) and donor lymphocyte infusion (DLI) exhibit strong graft-versus-leukemia (GVL) effect. However, the role of prophylactic DLI following haploidentical HSCT remains unclear. Here, 34 patients with high-risk acute leukemia who underwent low-dose anti-T-lymphocyte globulin-Fresenius (ATG-F)-based myeloablative haploidentical HSCT and prophylactic modified DLI (pro-DLI) were well-matched with patients without pro-DLI. The 5-year overall survival (OS) (67.8% versus 41.3%, P < 0.01) and leukemia-free survival (LFS) (64.6% versus 33.9%, P < 0.01) of pro-DLI cohort were superior to the control cohort. A slightly higher GVHD-free/relapse-free survival was found in the pro-DLI cohort (32.8% versus 16.3%, P = 0.32). The 5-year cumulative incidence of relapse of the pro-DLI recipients was significantly lower than that of the control cohort (14.7% versus 49.3%, P = 0.01). The cumulative incidence of grades II-IV and III-IV acute GVHD at 100 days after pro-DLI was 17.6% and 9.1%, respectively. There was no difference between the two cohorts in terms of the cumulative incidence of chronic GVHD and non-relapse mortality. Data from the multivariate analysis demonstrated that pro-DLI was an independent protective variable for LFS (P = 0.01, hazard ratio {HR} = 0.35), OS (P = 0.01, HR = 0.32), and relapse (P = 0.02, HR = 0.33). Taken together, we demonstrate that pro-DLI after ATG-F-based HSCT effectively decreases the risk of relapse and improves long-term survival of patients with high-risk acute leukemia without increasing treatment toxicity.
Similar content being viewed by others
Introduction
Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective antileukemic treatment option, the outcomes of high-risk acute leukemia remain unsatisfying. Previous studies show that acute leukemia with high-risk features, such as unfavorable genetic abnormalities and positive minimal residual disease (MRD) at transplantation, is associated with a high relapse rate ranging from 40 to 60% and a low long-term survival of less than 30% even after allo-HSCT [1,2,3]. Therefore, prophylactic strategies for relapse after allo-HSCT in these patients are urgently required.
Recently, haploidentical-related donor (HRD) has been widely applied and reported to achieve comparable outcomes with human leukocyte antigen (HLA)-matched sibling donor (MSD). We have previously reported that T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin (ATG-F) achieved a significant lower 5-year relapse rate, compared with MSD-HSCT (15.4% versus 49.9%, P = 0.002) in high-risk acute leukemia patients, suggesting a superior graft-versus-leukemia (GVL) effect [4]. Donor lymphocyte infusion (DLI) has been proven to be effective in the management of post-transplantation recurrence, yet the risk of severe acute graft-versus-host disease (aGVHD) and treatment-related-mortality may increased [5,6,7,8]. Prophylactic DLI, a T-cell infusion administered before any signs of relapse or progression, has been preliminarily explored in patients with hematological malignancies. However, there are concerns that prophylactic modified DLI (pro-DLI) after haploidentical HSCT may further aggravate the risk of GVHD, given the higher degree of human HLA disparity between the donor and recipient. The reported rates of aGVHD and non-relapse mortality (NRM) after pro-DLI from HRD range from 4 to 58% and 9 to 38% respectively, with a varied survival rate of 31–76% [9,10,11,12,13]. Without large prospective clinical trials, it remains controversial whether patients with high-risk acute leukemia can benefit from pro-DLI after haploidentical HSCT [9].
Here, we reported the outcomes of pro-DLI after ATG-F-based myeloablative haploidentical HSCT in patients with high-risk acute leukemia. A matched-pair analysis was carried out to further evaluate the safety and efficacy of the pro-DLI.
Materials and methods
Patients
Consecutive patients with high-risk acute leukemia aged 14 years above who received low-dose ATG-F-based haploidentical HSCT with myeloablative conditioning (MAC) in the First Affiliated Hospital of Zhejiang University School of Medicine from January 1, 2013 to August 30, 2018 were included for initial evaluation. The high-risk features were defined by the following criteria: (i) primary chemoresistance (failure to achieve complete remission {CR} after 2 cycles of induction chemotherapy); (ii) advanced disease at transplantation (beyond second CR or active disease); (iii) MRD positive at transplantation; (iv) acute leukemia with unfavorable cytogenetic abnormalities, such as MLL translocations, or complex karyotype; (vi) acute leukemia with t (9; 22) or FLT3-ITD mutation who could not tolerate prophylactic target therapy after transplantation [14,15,16,17]. All the patients gave their written informed consent in accordance with the Declaration of Helsinki. The ethics review committee of the First Affiliated Hospital of Zhejiang University School of Medicine approved the protocol utilized in this study.
Transplantation procedure and GVHD prophylaxis
All the transplantation procedures were as previously described [4]. Briefly, all patients were given MAC, which consisted of cytarabine (4 g/m2/d IV on days –10 to –9), busulfan (3.2 mg/kg per day IV on days –8 to –6), cyclophosphamide (1.8 g/m2 per day IV on days –5 to –4), methyl-N-(2-chloroethyl)-N-cyclohexyl-N-nitrosourea (250 mg/m2 orally on day –3). Donors were treated with rhG-CSF (Filgrastim; Kirin, Japan; 5 mg/kg per day) injected subcutaneously for 5–6 consecutive days from day –4. Peripheral blood stem cells (PBSCs) were harvested on day −1 and 0 and then infused without ex vivo T-cell depletion. Extra harvested cells were cryopreserved in a nitrogen tank after consent from patients. The GVHD prophylaxis consisted of cyclosporin A (CSA), methotrexate, and low-dose mycophenolate mofetil. ATG-F (Fresenius, Bad Homburg, Germany) was administered 2.5 mg/kg per day on days −5 to −2.
Prophylactic modified DLI strategy
Pro-DLI was suggested to patients with high-risk acute leukemia who fulfilled the following criteria: (i) persistent CR with negative MRD; (ii) full donor chimerism; (iii) without a history of aGVHD greater than grade II; (iv) no active uncontrolled GVHD; (v) no clinical evidence of active infection. Pro-DLI was initially scheduled at +60 days after transplantation (during January 1, 2013 to July 30, 2014) and then adjusted to +90 days after transplantation (since August 1, 2014). In patients with GVHD or infection, DLI would be delayed until the symptoms and signs disappeared. All the patients received modified donor lymphocytes which consisted of G-CSF modified PBSCs instead of steady donor lymphocyte. The cryopreserved GCSF-primed PBSCs at HSCT were revived and infused without further manipulation. The recommended dose of CD3+ cell of DLI was 3 × 107/Kg. Low dose CSA (1–2 mg/kg/d) was given after DLI, which was gradually tapered 3–4 weeks post-DLI and then stopped. Given the lack of donor lymphocytes and refusal to pro-DLI, this subset of high-risk patients who meet the inclusion criteria highlighted in this section but did not receive pro-DLI served as controls.
MRD monitoring and definitions
All the patients received bone marrow examination at 1, 2, 3, 5, 7, 9, 12, 15, 18 months after transplantation and at a 6-month interval thereafter. The specific time intervals were individualized and adjusted by specific circumstance. The MRD was monitored by multiparameter flow cytometry (MFC) and/or polymerase chain reaction (PCR). The threshold to distinguish MRD-positive from MRD-negative patients was 0.1% in acute myeloid leukemia (AML) and 0.01% in acute lymphoblastic leukemia (ALL) [18, 19]. PCR was also used for MRD monitoring in patients with genetic abnormalities at diagnosis, such as a fusion gene, mutated or overexpressed gene, as well as the pan-leukemic marker Wilms’ tumor (WT1) gene [20]. Subjects were MRD-negative only if they were both negative by MFC and PCR.
Matched-pair analysis
A matched-pair was conducted to ensure the comparability and reduce bias between cohorts. For each patient receiving pro-DLI, a control patient was randomly selected from patients without pro-DLI by the basic function of “Case Control Matching” in the SPSS Statistics. The matching factors included diagnosis (AML/ALL), primary chemoresistance or not, genetic risk stratification (good/intermediate/poor), disease status at HSCT (CR1 and CR2/beyond CR2/active disease) or MRD (+/−) at HSCT. To avoid immortal time bias, control patients had to be CR with negative MRD and free of GVHD at least as long as the time interval from transplant to DLI in the respective matched pro-DLI recipient.
Endpoint definitions and statistical analysis
Differences between cohorts were examined using Pearson’s chi-square test and Fisher’s exact test for categorical variables while the Mann–Whitney U test was used for continuous variables. GVHD-free and relapse-free survival (GRFS) was defined as the time between transplantation and the development of GVHD (grades III-IV aGVHD) or chronic GVHD (cGVHD) requiring systemic immunosuppressants, disease recurrence, or death [21]. Overall survival (OS), leukemia-free-survival (LFS) and GRFS were estimated using the Kaplan-Meier method and compared using the log-rank test. Patients who survived ≥100 days were analyzed for chronic GVHD. Cumulative incidence of GVHD, relapse or NRM were calculated by the competing risk method [22]. Multivariate Cox regression models were applied to analyze the effects of variables with a P value < 0.2 in the univariate analysis and other known clinical or biological factors on the HSCT outcomes. A two-sided P value < 0.05 was considered statistically significant. Statistical analyses were done with SPSS Statistics, version 22.0 (SPSS, Chicago, IL, USA) and R (http://www.r-project.org).
Results
Patient characteristics
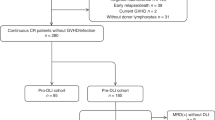
A total of 405 patients with acute leukemia received low dose ATG-F-based myeloablative haploidentical HSCT in our center from January 1, 2013 to August 30, 2018. Among 228 patients with high-risk features, 53 patients were excluded for pro-DLI because of early disease progression, history of severe aGVHD, active GVHD or infection (Fig. 1). Finally, of the 175 remaining patients, 54 patients received DLI prophylactically as planned, whereas the remaining 121 patients did not receive pro-DLI due to the lack of donor lymphocytes (n = 45) or unwillingness (n = 76). The comparison of baseline characteristics of patients with/without pro-DLI indicated that more patients who received pro-DLI underwent HSCT in more advanced status (≥CR3 or NCR) (P = 0.03) and earlier year (P < 0.001). To further explore the role of pro-DLI, thirty-four matched pairs were identified. Table 1 shows the general characteristics of two cohorts. Patients in the pro-DLI cohort received hematopoietic stem cells from an older donor (42 versus 29.5 years old, P = 0.02) compared with the patients in the control cohort. Meanwhile, no significant differences in terms of gender, age, hematopoietic cell transplantation comorbidity index (HCT-CI) scores and donor gender was identified between the two cohorts.
Prophylactic modified DLI and GVHD
Pro-DLI was administered at a median interval of 117.5 days (range, 63–255 days) from transplantation in the pro-DLI group. The median interval between allo-HSCT and pro-DLI were 91 days (range,63–183 days) from January 1, 2013 to July 30, 2014 and 125 days (range,86–255 days) from August 1, 2014 to December 31, 2018. The median dose of CD3+ cells was 3.8 (range, 0.73–7.5) × 107/Kg. No onset of DLI-associated pancytopenia was documented in these patients.
After pro-DLI, sixteen patients developed aGVHD (grades I, n = 10; grades II, n = 3; grades III, n = 1; grades IV, n = 2). The median interval time from pro-DLI to the onset of aGVHD was 26 days (range, 6–65 days). Eighteen patients didn’t develop any grades of aGVHD. The cumulative incidence of grades II-IV aGVHD at 100 days after pro-DLI was 17.6% (Fig. 2a), and that of grades III-IV was 9.1% (Fig. 2b).
Sixteen patients (mild, n = 4; moderate, n = 5; severe, n = 7) developed cGVHD after pro-DLI. The cGVHD occurred at a median of 120 days (range, 31–739 days) after pro-DLI administration and a median of 226.5 days (range,134–813 days) after HSCT. Among patients with moderate to severe cGVHD, two patients died (cGVHD, n = 1; relapse, n = 1), three patients receive low dose immunosuppressive therapy while the remaining seven patients responded to treatment and were free of anti-GVHD therapy. In the control cohort, eleven patients developed cGVHD (mild, n = 6; moderate, n = 3; severe, n = 2) at a median time of 170 days (range,103–899 days) after HSCT. The 5-year cumulative incidence of moderate to severe cGVHD in the two cohorts was 35.8% and 16.7%, respectively (P = 0.05) (Fig. 2c). There was one patient in each cohort developed severe bronchiolitis obliterans and died.
Relapse
Eight and fifteen patients relapsed in the pro-DLI and control cohorts, respectively. In the pro-DLI cohort, the median time of relapse were 207 days (range,112–2195 days) after transplantation and 110.5 days (range, 25–2104 days) after pro-DLI. The median time from transplantation to the onset of relapse was 292 days (range, 167–1012 days) in the control cohort. The 5-year cumulative incidence of relapse (CIR) was significantly lower in patients who received pro-DLI compared with patients in the control cohort (20.5% versus 49.3%, P < 0.01) (Fig. 3a). In the multivariate analysis of relapse rate (Table 2), pro-DLI (P = 0.02, HR = 0.33) and cGVHD (P = 0.01, HR = 0.26) protected the patients from relapse.
Long-term follow-up and survival
After a median follow-up of 1045 (174–2296) days after transplantation, 5-year LFS and OS were 66.5% and 69.9%, respectively for all 54 pro-DLI recipients. The 5-year LFS and OS were 45.2% and 51.4% for 121 patients who didn’t receive pro-DLI with a median follow-up of 645 (127–2429) days. Pro-DLI recipients achieved higher LFS (P = 0.003) and OS (P = 0.002) compared with 121 patients who did not receive pro-DLI.
In the matched pro-DLI and control cohorts, the median follow-up days after allo-HSCT was 1293.5 days (range, 174–2296 days) and 704.5 days (range, 146–1607 days) respectively. A total of 28 patients died in the two cohorts and the causes of death were relapse (n = 17), infection (n = 7), aGVHD (n = 1), cGVHD (n = 2) and unknown cause (n = 1) (Table 3). The 5-year incidence of NRM of two cohorts were comparable (14.8% versus 16.7%, P = 0.44) (Fig. 3b). The 5-year probability of OS was higher in patients who received pro-DLI (67.8%) than patients who did not (41.3%) (P < 0.01) (Fig. 4a). The probability of 5-year LFS was also superior in patients who received pro-DLI (64.6% vs 33.9%, P < 0.01) (Fig. 4b). The patients in the pro-DLI cohort had a 5-year GRFS of 32.8% compared with 16.3% in the control cohort, although it failed to reach significance (P = 0.32) (Fig. 4c).
In multivariate analysis, pro-DLI and development of cGVHD were independently associated with better LFS and OS (Table 2). No statistically significant factors were found in the multivariate analysis of NRM and GRFS.
Discussion
To our knowledge, this is the first case control study comparing the outcomes of patients receiving pro-DLI with a well-matched control cohort after ATG-F-based haploidentical HSCT with MAC. Our data demonstrated that pro-DLI effectively prevents disease relapse and improves survival in patients with high-risk acute leukemia.
The efficacy of DLI was directly related to the disease burden of patients. The response rates after DLI in patients with hematologic relapse was low and survival was unsatisfying [23, 24]. To further improve the outcome of patients with advanced diseases, some researchers attempted to perform DLI prophylactically after allo-HSCT. Some studies have shown promising clinical outcomes of pro-DLI after MSD/unrelated donor (URD) allo-HSCT, with 36–80% of the patients with acute leukemia experiencing long-term survival [25,26,27]. In the setting of haploidentical transplantation, Huang et al. at Peking University developed an approach of MRD- and GVHD-guided multiple pro-DLI strategy and reported the 3-year CIR, PFS, and OS of 32.4%, 50.3%, and 51.4%, respectively [28]. A retrospective study in Europe investigating the use of pro-DLI following post-transplantation cyclophosphamide (PTCy)-based haploidentical-HSCT showed a 1-year CIR and OS of 16% and 83%, respectively [12]. However, the published literatures were quite heterogeneous and a randomized comparative clinical trial was lacking. Recently, the Acute Leukemia Working Party of EBMT enrolled pro-DLI recipients and controls who were pair-matched for age, diagnosis, cytogenetics, stage, donor, gender, conditioning and T-cell depletion. The results of this matched-pair analysis strongly demonstrated the survival benefit of pro-DLI after MSD/matched unrelated donor allo-HSCT in patients with high-risk AML (5-year OS: 69.8% vs. 40.2%, P = 0.03) [29]. However, the experience in haploidentical pro-DLI is much limited, leading to no consistent conclusions. A prospective study on refractory/relapsed AML was conducted to explore the influence of different intensity of conditioning regimen and pro-DLI after PTCy-based haploidentical HSCT. In the initial protocol of nonmyeloablative regimen and early pro-DLI, 90% of patients had early disease progression. Subsequently, 21 patients received MAC and pro-DLI achieved significant improved LFS (61.9% vs 25%) and lower relapse rates (21.4% vs 66%) (P = 0.01) compared with patients without DLI [30]. In our study, patients with high-risk acute leukemia received MAC followed by ATG-F-based haploidentical HSCT and pro-DLI. The matched-pair analysis demonstrated that the patients in the pro-DLI cohort achieved significant superior OS (67.8% versus 41.3%, P < 0.01) and LFS (64.6% versus 33.9%, P < 0.01), which based on a reduced relapse rate (20.5% versus 49.3%, P = 0.01) and a comparable NRM rate (14.8% versus 16.7%) (P = 0.44). Furthermore, the multivariate analysis results showed that pro-DLI is an independent protective factor of relapse and survival. To our knowledge, this is the first study showing the efficiency of pro-DLI in decreasing relapse and improving survival after ATG-F-based haploidentical HSCT.
GVHD has remained to be the most concern after DLI and offsets the GVL effect by varying degrees. Multiple biological effects of G-CSF had been reported. Huang et al. indicated that G-CSF-primed DLI exhibits a comparable GVL effect and a lower incidence of GVHD compared with traditional DLI [31]. Therefore, G-CSF-primed DLI was preferred by many centers and gained increasing application worldwide. Besides the methods of cell collection, DLI-related GVHD was closely related to the time interval between transplantation and DLI, the dose of CD3+ cells, donor type and immunosuppressant. It was accepted that the earlier that DLIs are given, the higher risk for the development of GVHD. Yet, the optimal timing for pro-DLI to be performed was not defined. Jedlickova et al. performed pro-DLI at least 120 days after MSD/URD allo-HSCT in 34 patients who had been off immunosuppressant for 30 days and free of GVHD. Four patients (11.8%) developed grades II/III aGVHD and eight patients (23.5%) developed cGVHD [32]. Gao et al. conducted relative early pro-DLI at days 60 to 90 with concurrent short-term immunosuppressant after haploidentical HSCT. The incidences of aGVHD grades II-IV (55.3%) and cGVHD (52.0%) were much higher [9]. Maria Liga et al. conducted pro-DLI after Allo-HSCT with an Alemtuzumab-Containing Conditioning Regimen and recommended withholding pro-DLI beyond day +100 [33]. At the beginning stage of our study, pro-DLI was scheduled to be performed at +60 days after allo-HSCT in ten patients. Among the five patients who received pro-DLI at a median of 71 days (range, 63–78 days) post-HSCT, two patients developed grade IV aGVHD and one had grade I aGVHD. Meanwhile, there was only one patient developed grades II aGVHD in the other five patients who received pro-DLI after +90d due to various reasons. Considering the high rates of aGVHD after early pro-DLI in reported studies and our experience, we postponed pro-DLI to +90 days since August 1, 2014 to avoid severe aGVHD. Compared with early DLI before August 1, 2014, postponed pro-DLI at median 125 days (range, 86–255 days) achieved lower cumulative incidence of grades II-IV aGVHD (30.0% versus 12.5%, P = 0.25) and grades III-IV (20.0% versus 4.3%, P = 0.15) but without statistical significance. Late pro-DLI and concurrent immunosuppressant also attributed to the quite low and acceptable incidence of GVHD. The significantly lower relapse rate in the pro-DLI cohort (20.5% versus 49.3%, P = 0.01) could eliminate the concern of the potential impairment on GVL effect by short-term CSA. Therefore, it is feasible to perform haploidentical DLI prophylactically at days +90 after HSCT along with short-term immunosuppressive treatment.
The doses of infused CD3+ cells were thought to be crucial in the development of GVHD. A retrospective comparable study that included 225 patients with relapsed hematological malignancies showed that an initial DLI CD3+ cell dose of 108/Kg or higher was associated with an increased risk of GVHD, without decreasing the risk of relapse and improving survival [34]. There are no available data on the optimal cell dose of pro-DLI. Except the dose of CD3+ cells, the course and intervals of donor lymphocytes infusions seemed more critical to the DLI-related GVHD. A previous comparative study of therapeutic DLI in chronic myeloid leukemia showed that an escalating cell dose regimen led to less GVHD while equal effectiveness versus a single bulk infusion [35]. The escalating dose approach of pro-DLI from haploidentical donor usually started from a CD3+ cell dose of 105–106/Kg and escalated at regular intervals with 5- to 10-fold increase. Jaiswal et al. performed haploidentical DLI with escalating dose in 21 patients and the cumulative incidences of aGVHD was 31% [30]. Similarly, the incidence of DLI-related GVHD observed by Cauchois et al. in 36 prophylactic recipients was 33% [12]. Here, we performed one course of haploidentical DLI at a median dose of 3.8 × 107/Kg CD3+ cells without repeated dose escalation and recorded a cumulative incidence of 17.6% and 9.1% for grades II-IV and grades III-IV aGVHD, which seemed lower than data published. The frequency of DLI and optimal time intervals of administration of escalating dose regimen yet to be determined. Besides, it is much more difficult to evaluate the effect of pro-DLI and to decide the endpoint of infusion without decreasing donor chimerism or positive MRD. Therefore, given these unsolved issues, single bulk lymphocytes infusion presents a more practical option for pro-DLI. However, further studies are needed to confirm the superiority between single dose and escalating dose regimen.
As a retrospective study, the major limitation of our current study is the nature of the retrospective research and the lack of randomization. With large prospective trial unaccessible, a well-matched-pair analysis was introduced to minimize the imbalance and select bias between the two cohorts. Second, the number of patients was limited due to strict matching of the clinical characteristics and therapeutic strategies. Despite these limitations, the survival advantages associated with the pro-DLI after haploidentical HSCT was effectively demonstrated in the current study. Certainly, our findings should to be further confirmed.
In summary, our protocol of pro-DLI after low dose ATG-F-based haploidentical HSCT with MAC is safe and feasible. Furthermore, the results of this matched-pair analysis strongly indicate that pro-DLI significantly reduces the risk of relapse and improves long-term survival in patients with high-risk acute leukemia.
References
Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2007;13:1160–8.
Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104:524–32.
Tachibana T, Kanda J, Ishizaki T, Najima Y, Tanaka M, Doki N et al. Outcomes and prognostic factors for patients with relapsed or refractory acute lymphoblastic leukemia who underwent allogeneic hematopoietic cell transplantation: a KSGCT multicenter analysis. Biol Blood Marrow Transpl. 2020; e-pub ahead of print 18 January 2020; https://doi.org/10.1016/j.bbmt.2020.01.007.
Luo Y, Xiao HW, Lai XY, Shi JM, Tan YM, He JS, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007;92:414–7.
Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transpl. 2014;20:1785–90.
Zeidan AM, Forde PM, Symons H, Chen A, Smith BD, Pratz K, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transpl. 2014;20:314–8.
Miyamoto T, Fukuda T, Nakashima M, Henzan T, Kusakabe S, Kobayashi N, et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Unrelated Allogeneic Bone Marrow Transplantation Facilitated by the Japan Marrow Donor Program. Biol Blood Marrow Transpl. 2017;23:938–44.
Gao XN, Lin J, Wang SH, Huang WR, Li F, Li HH, et al. Donor lymphocyte infusion for prevention of relapse after unmanipulated haploidentical PBSCT for very high-risk hematologic malignancies. Ann Hematol. 2019;98:185–93.
Gilman AL, Leung W, Cowan MJ, Cannon M, Epstein S, Barnhart C, et al. Donor lymphocyte infusion and methotrexate for immune recovery after T-cell depleted haploidentical transplantation. Am J Hematol. 2018;93:169–78.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Zhang XH, et al. Prevention of relapse using granulocyte CSF-primed PBPCs following HLA-mismatched/haploidentical, T-cell-replete hematopoietic SCT in patients with advanced-stage acute leukemia: a retrospective risk-factor analysis. Bone Marrow Transpl. 2012;47:1099–104.
Cauchois R, Castagna L, Pagliardini T, Harbi S, Calmels B, Bramanti S, et al. Prophylactic donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation for high risk haematological malignancies: a retrospective bicentric analysis of serial infusions of increasing doses of CD3(+) cells. Br J Haematol 2019;185:570–3.
Dholaria B, Savani BN, Labopin M, Luznik L, Ruggeri A, Mielke S, et al. Clinical applications of donor lymphocyte infusion from an HLA-haploidentical donor: consensus recommendations from the Acute Leukemia Working Party of the EBMT. Haematologica. 2020;105:47–58.
Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: the value of complete remission. Cancer. 2017;123:2025–34.
Middeke JM, Herold S, Rucker-Braun E, Berdel WE, Stelljes M, Kaufmann M, et al. TP53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol 2016;172:914–22.
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30:1456–64.
Ciurea SO, Labopin M, Socie G, Volin L, Passweg J, Chevallier P, et al. Relapse and survival after transplantation for complex karyotype acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation and the University of Texas MD Anderson Cancer Center. Cancer. 2018;124:2134–41.
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91.
Bruggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1:2456–66.
Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett 2018;438:63–75.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl. 2007;40:381–7.
Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27:1229–35.
Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 2009;27:256–63.
Wang Y, Liu DH, Fan ZP, Sun J, Wu XJ, Ma X, et al. Prevention of relapse using DLI can increase survival following HLA-identical transplantation in patients with advanced-stage acute leukemia: a multi-center study. Clin Transplant 2012;26:635–43.
Eefting M, Halkes CJ, de Wreede LC, van Pelt CM, Kersting S, Marijt EW, et al. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transpl. 2014;49:287–91.
Legrand F, Le Floch AC, Granata A, Furst S, Faucher C, Lemarie C, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation for high-risk AML. Bone Marrow Transpl. 2017;52:620–1.
Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, et al. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-versus-Host Disease-Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transpl. 2017;23:1311–9.
Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia - a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol 2019;184:782–7.
Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, et al. Improved Outcome of Refractory/Relapsed Acute Myeloid Leukemia after Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation with Myeloablative Conditioning and Early Prophylactic Granulocyte Colony-Stimulating Factor-Mobilized Donor Lymphocyte Infusions. Biol Blood Marrow Transpl. 2016;22:1867–73.
Huang XJ, Guo NL, Ren HY, Zhang YC, Gao ZY, Lu DP. An improved anti-leukemic effect achieved with donor progenitor cell infusion for relapse patients after allogeneic bone marrow transplantation. Chin Med J-Peking. 2003;116:736–41.
Jedlickova Z, Schmid C, Koenecke C, Hertenstein B, Baurmann H, Schwerdtfeger R, et al. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transpl. 2016;51:663–7.
Liga M, Triantafyllou E, Tiniakou M, Lambropoulou P, Karakantza M, Zoumbos NC, et al. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol Blood Marrow Transpl. 2013;19:75–81.
Bar M, Sandmaier BM, Inamoto Y, Bruno B, Hari P, Chauncey T, et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transpl. 2013;19:949–57.
Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood. 2000;95:67–71.
Acknowledgements
This work was supported by grants from The National Key Research and Development Program of China [grant number 2018YFA0107804], The National Natural Science Foundation of China [grant numbers 81970158] and Zhejiang Natural Science Foundation [grant numbers LY18H080001].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, L., Tan, Y., Shi, J. et al. Prophylactic modified donor lymphocyte infusion after low-dose ATG-F-based haploidentical HSCT with myeloablative conditioning in high-risk acute leukemia: a matched-pair analysis. Bone Marrow Transplant 56, 664–672 (2021). https://doi.org/10.1038/s41409-020-01088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01088-7
- Springer Nature Limited
This article is cited by
-
Prophylactic versus Preemptive modified donor lymphocyte infusion for high-risk acute leukemia after allogeneic hematopoietic stem cell transplantation: a multicenter retrospective study
Bone Marrow Transplantation (2024)
-
A novel risk model for predicting early relapse in acute myeloid leukemia patients undergoing allogeneic hematopoietic stem-cell transplantation
Bone Marrow Transplantation (2023)
-
Donor lymphocyte infusions after haploidentical stem cell transplantation with PTCY: A study on behalf of the EBMT cellular therapy & immunobiology working party
Bone Marrow Transplantation (2023)
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)
-
Comparison of non-first-degree related donors and first-degree related donors in haploidentical HSCT: a multi-centre retrospective analysis
Bone Marrow Transplantation (2021)