Abstract
As maladaptive disgust responses are linked to mental health problems, and cancer patients may experience heightened disgust as a result of treatments they receive, we explored the associations between disgust-related side-effects and symptoms of depression and anxiety in people treated for cancer. One hundred and thirty two (83 women, M age = 57.48 years) participants answered questions about their treatments, side-effects, disgust responding, and mental health. Experiencing bowel and/or bladder problems, sickness and/or nausea (referred to here as “core” disgust-related side-effects) was significantly related to greater symptoms of depression and borderline increased anxiety. Further, these links were explained by a moderated mediation model, whereby the effects of core disgust side-effects on depression and anxiety were mediated by (physical and behavioural) self-directed disgust, and disgust propensity moderated the effect of core disgust side-effects on self-disgust. These findings stress the importance of emotional responses, like disgust, in psychological adaptation to the side-effects of cancer treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major public health problem and the leading cause of death in England and Wales (Office for National Statistics, 2015). The mental health of cancer patients has significant effects on their overall quality of life (e.g., Skarstein et al., 2000), responses to treatment (e.g., DiMatteo et al., 2000), and survival times (e.g., Falagas et al., 2007). While estimates vary, of the most common mental health problems, the prevalence of depression and anxiety in people with cancer has been conservatively estimated to be around twice that of the general population (Hinz et al., 2010; Walker et al., 2014). Common mental health problems, such as depression and anxiety, can be experienced as a consequence of cancer treatment regimens (e.g., Spiegel, 1997), as well as affecting how well cancer patients respond to them (DiMatteo et al., 2000).
Depression and anxiety in cancer patients are associated with decreased treatment adherence (e.g., Arrieta et al., 2013), greater healthcare costs and longer hospital stays (e.g., Hosaka et al., 1999), lower chances of survival (e.g., Falagas et al., 2007), and an increased risk of suicide (e.g., Llorente et al., 2005). Given these and other significant consequences of common mental health problems in people with cancer (which may go unrecognised and untreated; Walker et al., 2014), being able to identify which patients may be particularly vulnerable to developing symptoms of depression and anxiety, and which factors may be important in their antecedence, is increasingly important, as is recognising psychological factors to target during psycho-therapeutic interventions.
Increasingly, one’s emotional reactions are being understood as important, yet historically underexplored, predictors of mental health outcomes (Whelton & Greenberg, 2005). Experiencing cancer, and cancer treatment, can elicit multiple negative emotional responses (e.g., Kennifer et al., 2009). One prominent, yet under-researched, emotion relevant to the experience of cancer is disgust. Individuals with cancer potentially have to confront a range of disgust-inducing stimuli, including, but not being limited to, becoming a “diseased” or “contaminating” object (Neal et al., 2007), sickness and nausea (e.g., Carey & Burish, 1988), bowel and bladder problems (e.g., Bauer et al., 2009), changes to an idealised body envelope (e.g., Bredin, 1999), and the salience of their own mortality and death (e.g., Goldenberg et al., 2008). Moreover, the majority of these may not be caused by the cancer itself, but as side-effects of the treatments patients receive. A study in people with a colostomy, for example, demonstrated significant negative relationships between a bowel-related disgust measure and individuals’ adjustment to colostomy and their overall life satisfaction (Smith et al., 2007). Further experimental work has shown that trait and state disgust indices may interact in deterring help-seeking behaviour for bowel-related symptoms (Reynolds et al., 2014).
Maladaptive disgust responding (e.g., experiencing heightened levels of disgust) has been linked to mental health problems, including anxiety (Cisler et al., 2009) and depression (Alanazi et al., 2015), by a considerable literature (Davey, 2011). Thus, one potential yet underexplored link between the experience of cancer and depressive and/or anxious outcomes is individuals’ maladaptive disgust responses, which may be associated with certain side-effects that are typical of cancer treatments (e.g., problems with body waste products, including incontinence, diarrhoea, and vomiting as a result of chemotherapy, or physical deformities as a consequence of surgery).
Disgust is a universal human emotion (Ekman, 1999), whose primary function is to protect us from the risk of disease (Curtis et al., 2004). Through evolutionary exaptation, disgust has expanded from an oral inhibition response (which has adaptive value in protecting an organism from ingesting harmful substances; Rozin & Fallon, 1987), to an emotion that facilitates the avoidance and rejection of wider pathogenic stimuli, broader threats to our biological fitness (e.g., unfavourable mates), and sociomoral transgressions that desecrate culturally-defined virtues of purity and divinity (e.g., sexual violations; Chapman & Anderson, 2012). The most influential psychological model of disgust to date was proposed by Rozin and Fallon (1987) and distinguishes four categories of disgust elicitors: (1) a “core” set that includes animals, food, and body waste products (e.g., faeces, vomit, urine); (2) an “animal-nature” domain that reminds of us of our base status as animals (e.g., poor hygiene, death, violations of an idealised body envelope); (3) “interpersonal” (or contamination) disgust for contact with other persons; and (4) “moral” disgust elicited by sociomoral violations (Rozin et al., 2008). These theoretical divisions have been supported empirically, by factor analyses differentiating between core, animal-nature, and contamination-based disgusts (e.g., Olatunji et al., 2005; Olatunji et al., 2007), and via unique predictive associations with other related constructs, including mental health outcomes (e.g., Olatunji et al., 2007).
Given its evolutionary grounding as a cornerstone of our “behavioural immune system” (Schaller & Park, 2011), disgust responding has a particular relevance in understanding people’s psychological reactions to disease and its treatment, not least significant threats to health, such as cancer (e.g., Reynolds et al., 2013). Of the disgust categories outlined above, “core” (e.g., sickness, incontinence) and “animal-nature” (e.g., hair loss, scarring) disgust-eliciting side-effects are particularly relevant to cancer treatments. An important mediational pathway through which the disgust-related side-effects of cancer treatments may lead to increased depression and/or anxiety is via affect-congruent negative appraisals of the self. In particular, cancer patients may come to appraise themselves (or certain self-aspects) as disgusting, which may then lead to heightened symptoms of depression and/or anxiety (e.g., Beck, 1967; Powell et al., 2013). Self-disgust has been shown to be a significant temporal antecedent of (i.e., a consistent vulnerability factor for) depressive symptoms (Overton et al., 2008; Powell et al., 2013), and has also been linked to anxious responding (Olatunji et al., 2015). Furthermore, self-directed disgust has a hypothesised role in chronic physical health conditions, including cancer (Reynolds et al., 2015b). Broadly, people can experience enduring self-disgust toward their physical bodies and/or their behavioural acts (Overton et al., 2008), with independent predictive effects on measures of psychopathology (e.g., Powell et al., 2013).
In addition, one may expect the psychological impact of these disgust-related physical side-effects to be influenced by an individual’s underlying proneness to disgust (i.e., “disgust propensity”; van Overveld et al., 2006), which is a trait individual difference factor that can be measured reliably by self-report (Olatunji et al., 2007). A recent prospective study by Reynolds et al. (2015a) in patients with anal incontinence, for example, found that proneness to disgust negatively predicted a number of quality of life indices assessed 3 months later. Thus, one’s propensity to be disgusted can be hypothesised to be a critical moderator of the effects of disgust-related side-effects on self-directed disgust, and thus psychological health outcomes. In particular, we would expect the effects of disgust-related side-effects on self-disgust, and thus the mediational pathway described above, to be greater for those who were higher in disgust propensity.
In the present paper we tested the above mediation and moderation hypotheses in a community sample who reported having received treatment(s) for cancer. In particular, we sought to explore: (1) whether experiencing two types of disgust-related physical side-effects (i.e., “core” and “animal-nature”) were positively related to symptoms of depression and anxiety; (2) the degree that physical and/or behavioural self-disgust mediated the link between the presence of a disgust-related side-effect and depressive/anxious symptoms; and (3) whether participants’ underlying propensity to disgust significantly moderated the impact of experiencing a disgust-related side-effect on self-directed disgust, and thus any indirect effect on depression and anxiety. We predicted that:
-
1.
Experiencing a disgust-related physical side-effect (“core” or “animal-nature”) would be positively related to symptoms of depression and anxiety.
-
2.
Physical and/or behavioural self-disgust would positively mediate the effect of having a disgust-related side-effect on depression and anxiety.
-
3.
Trait propensity to disgust would positively moderate the effect of having a disgust-related physical side-effect on self-directed disgust.
Methods
Participants
One hundred and thirty two community volunteers (83 women, M age = 57.48 years, SD age = 14.19 years) who had been treated for a broad range of cancers participated in this study. They were recruited from cancer charities and support groups. Table 1 illustrates study participants’ characteristics. No reimbursement was provided for participation.
Measures
Cancer treatment and side-effects
All participants reported having being treated for cancer. Participants were asked whether they had received a particular treatment (e.g., chemotherapy; coded as 0 = had not received, 1 = had received; see Table 1). They were also asked if their treatment(s) caused any side-effects, and if so to list them as a free-text response. These free-text responses were coded for (1) the number of side-effects reported (continuous scale); and (2) whether the participant reported a “core” or “animal-nature” disgust side-effect (0 = no, 1 = yes). The decision to code a side-effect as related to “core” or “animal-nature” disgust was based on the conceptualisation provided by Rozin et al. (2008), and supported empirically by others (e.g., Olatunji et al., 2007), that core disgust elicitors are related to food/eating and body waste products, and animal-nature disgust elicitors are related to death, hygiene, and body envelope violations. Thus, a participant was coded as having experienced a “core” disgust-related side-effect if they reported any bowel and/or bladder problems or sickness and/or nausea. An “animal-nature” side-effect was noted if participants reported a change to their physical body envelope (i.e., exterior form), including visible infections. Examples of side-effects that were not coded as disgust-related included fatigue, pain, and motor problems.
Participants’ free-text responses were coded independently by two raters, the primary study author and an independent graduate student who was paid for their time. The graduate student was unaffiliated with the current study and blind to its aims, and was directed to code the data using only the definitions given above. Interrater agreement was high, with a two-way absolute average-measures intraclass correlation coefficient (ICC; see Hallgren, 2012) of 1.00, 95 % CI [1.00, 1.00], p < .001 (97 % agreement), for the number of side-effects; Siegel and Castellan’s (1988) kappa (κ) of .98, p < .001 (99 % agreement) for “core” disgust side-effects; and κ = 1.00, p < .001 (100 % agreement) for “animal-nature” side-effects. In the case of discrepancies, the independent secondary coding of the data was used for analyses.
Disgust propensity and sensitivity
Participants’ disgust propensity (how easily one is disgusted) and disgust sensitivity (how negatively these disgust experiences are appraised) were measured using the Disgust Propensity and Sensitivity Scale-Revised (DPSS-R; van Overveld et al., 2006). Participants rated their agreement to 16 items on a 5-point Likert scale (1 = never, 5 = always). An example disgust propensity item is “I experience disgust”, and an example disgust sensitivity item is “It scares me when I feel nauseous”. Disgust sensitivity was included as a control variable in the current analyses. Based on psychometric evaluation of the DPSS-R (Goetz et al., 2013; Olatunji et al., 2007), a recommended 10-item solution (six items for propensity and four for sensitivity) was used for analyses. Both the disgust propensity, α = .78, and disgust sensitivity, α = .83, subscales demonstrated good internal reliability.
Self-disgust
The Self-Disgust Scale (SDS; Overton et al., 2008) was used to measure participants’ trait disgust for the self. For each of 18 items, participants rated their agreement on a 7-point Likert scale (1 = strongly agree, 7 = strongly disagree). The scale contains a number of filler items and two 5-item subscales, one measuring physical self-disgust (e.g., “I find myself repulsive”) and the other behavioural self-disgust (“I often do things I find revolting”). The internal reliabilities of the two subscales in the current sample were excellent, with α = .90 and α = .83, respectively.
Depression and anxiety
Participants’ levels of anxiety and depression were measured using the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983). The scale was developed for use amongst hospital inpatients and has been previously validated in community patients with cancer (e.g., Smith et al., 2002). For 14 items (seven for each subscale) participants rated their current agreement on a 4-point scale (0–3, with reversed labels). An example item from the anxiety subscale is “I get sudden feelings of panic”, and an example from the depression subscale is “I feel as if I am slowed down”. In the current sample, the Cronbach’s alpha coefficients for the anxiety, α = .88, and depression, α = .84, subscales were good.
To maintain consistency with prior research on disgust in the context of depression (e.g., Overton et al., 2008; Powell et al., 2013), and to account for potential criticisms of the sensitivity of the HADS depression subscale (see Luckett et al., 2010), a second measure of depression was included in the surveys, the 7-item depression subscale of the short-form Depression, Anxiety, and Stress Scales (DASS–21; Lovibond & Lovibond, 1995). For each item (e.g., “I felt that life was meaningless”) participants indicated how much it had applied to them over the previous week on a 4-point Likert scale (0 = did not apply to me at all, 3 = applied to me very much, or most of the time). Summative scores were multiplied by two to make them comparable with the extended form of the DASS (Lovibond & Lovibond, 1995). The internal consistency of this scale was excellent, α = .94.
Control variables
In our path analyses we controlled for observed variables that we expected, a priori, to have a significant relationship with symptoms of depression and anxiety, including gender, age, years since cancer diagnosis, number of side-effects reported, disgust sensitivity, and whether the participant reported a current medical diagnosis of depression (0 = no, 1 = yes) or anxiety (0 = no, 1 = yes). Bivariate correlations showed that all control variables were significantly related to at least two of the three outcome variables (rs = .18–.46). Analyses without the inclusion of control variables produced the same (stronger) pattern of results.
Procedure
Ethical approval was granted by the host research institution prior to data collection. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study. As part of a larger survey into psychological responses to cancer, cancer charities and support groups were approached with a link to an online survey. Volunteers completed the measures listed above online in a counterbalanced order and were fully debriefed.
Data analysis
Missing data were minimal, with a single missing value on the depression subscale of the DASS (Lovibond & Lovibond, 1995). To minimise data loss, we imputed this value at the mean of available data. Following descriptive and correlational analyses on SPSS v. 21 (IBM Corp., Armonk, NY, USA), path analysis on AMOS v. 22 (IBM Corp., Armonk, NY, USA) was used to model the hypothesised relationships between the variables. Path analysis has several advantages over standard multiple regression, including the estimation of direct and indirect effects (through mediating variables) simultaneously; the ability to model multiple endogenous (i.e., dependent) variables at the same time, allowing one to account for their interdependence caused by extraneous variables (by correlating their error terms); and the calculation of multiple measures of fit to the data. Further, it is a less resource-intensive technique than structural equation modelling (SEM), which typically requires a larger sample, due to the inclusion of latent variables (Wolf et al., 2013).
As recommended by Hayes (e.g., Hayes, 2009; Hayes & Scharkow, 2013), bias-corrected percentile bootstrapping was used to produce robust confidence intervals and standard errors (and hence probability values) for all estimates, including direct and indirect effects, removing any restrictions on the underlying sampling distribution. Bootstrapping provides a non-parametric robust alternative to traditional parametric estimates, when those estimates may be biased due, for example, to the violation of parametric assumptions and/or a restricted sample size (Fox, 2008). Ten thousand resamples were used for the bootstrapped estimates (Mallinckrodt et al., 2006). The Bollen and Stine (1992) bootstrap adjusted p value was interpreted to assess model fit based on the Chi-square statistic, along with the CFI and RMSEA. After fitting the model, to obtain separate bootstrapped estimates of specific indirect effects through a single mediator (not provided by default in AMOS), the path from the predictor to the alternative mediator was constrained to zero, as was the correlation between the two mediators (MacKinnon, 2008). To reduce potential multicollinearity associated with the inclusion of an interaction term, all continuous predictors (and mediators) were centred prior to analysis.
Results
Descriptives and correlations
Initial bivariate correlations and descriptive data for the primary study variables are presented in Table 2. One hundred and nineteen participants (90 %) reported at least one side-effect from their treatment(s). The average number of side-effects reported per participant was 3.36 (SD = 2.60). Forty-five (34.1 %) participants reported a “core” disgust side-effect, and 55 (41.7 %) an “animal-nature” disgust side-effect, from their treatment(s). The results from the HADS are broadly similar to those reported for other heterogeneous cancer samples (e.g., Smith et al., 2002), with higher scores for anxiety than depression. In a large varied cancer sample, Smith et al. (2002) reported a mean of 4.38 for depression and 6.05 for anxiety, with 33.3 % of participants presenting with clinically-relevant symptoms of anxiety and 19.8 % with clinically-relevant symptoms of depression (Zigmond & Snaith, 1983). Using the same criteria (scores ≥ 8 indicating potentially clinically-relevant symptoms; Zigmond & Snaith, 1983), 19.7 % of our sample presented with current indicative symptoms of depression, but approximately 50.0 % presented with indicative symptoms of anxiety. The DASS has been used less in cancer samples (Luckett et al., 2010), but indicated a potentially higher level of depressive symptoms in the participants over the previous week. Using the criteria of Lovibond and Lovibond (1995), 37.9 % presented with mild-to-severe symptoms of depression using the DASS (scores ≥ 10), suggesting it may be a more sensitive measure of, at least, mild depressive states (Luckett et al., 2010).
Partial support was found for prediction (1); total number of side-effects reported was significantly bivariately-related to symptoms of depression (HADS), r = .19, p < .05, and depression (DASS), r = .18, p < .05, but not anxiety, r = .14, p = .111. Reporting a core disgust side-effect was significantly positively related to symptoms of depression (HADS), r pb = .23, p < .01, and depression (DASS), r pb = .27, p < .01, and had a borderline significant relationship with anxiety, r pb = .16, p < .10. However, reporting an animal-nature side-effect was not related to depression (HADS), r pb = −.03, p = .716, depression (DASS), r pb = −.11, p = .219, or anxiety, r pb = −.06, p = .532, nor was it significantly related to any of the measured disgust traits. Accordingly, only the core disgust-related side-effect variable was included in the path analyses below. Experiencing a core disgust side-effect was significantly positively related to physical, r pb = .20, p < .05, and behavioural, r pb = .22, p < .05, self-disgust, and borderline significantly related to disgust sensitivity, r pb = .16, p < .10. All the proposed mediating and outcome variables were significantly related (p < .001).
Path models
Model 1 (mediation)
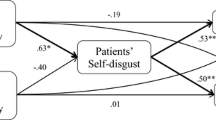
We estimated two path models to test predictions (2) and (3). The first was a mediation model, with core disgust side-effects and disgust propensity as the exogenous predictors, physical and behavioural self-disgust as hypothesised mediators, and depression (HADS), depression (DASS), and anxiety as outcomes (Fig. 1). In this model, regression weights on the disgust propensity*core side-effect interaction term were constrained to zero. The model fit the data reasonably well, χ2(5) = 9.45, p = .174; CFI = 0.99; RMSEA = 0.08, 90 % CI [0.00, 0.16], p = .210. All path estimates and associated maximum likelihood and bootstrap SEs/CIs are presented in Table 3. Reporting a core disgust side-effect significantly predicted behavioural, \(\upbeta_{{{\text{a}}_{2} }}\) = .17, p < .05, but not physical, \(\upbeta_{{{\text{a}}_{1} }}\) = .14, p = .119, self-disgust. Physical self-disgust significantly predicted depression (HADS), \(\upbeta_{{{\text{b}}_{1} }}\) = .57, p < .001, depression (DASS), \(\upbeta_{{{\text{b}}_{2} }}\) = .42, p < .01, and anxiety, \(\upbeta_{{{\text{b}}_{3} }}\) = .27, p < .05. Similarly, behavioural self-disgust significantly predicted depression (DASS), \(\upbeta_{{{\text{b}}_{5} }}\) = .27, p < .05, and anxiety, \(\upbeta_{{{\text{b}}_{6} }}\) = .23, p < .05, but not current depression as measured by the HADS, \(\upbeta_{{{\text{b}}_{4} }}\) = .06, p = .566.
Mediation model explaining the effect of “core” disgust side-effects on symptoms of depression and anxiety in people treated for cancer. Self-disgust significantly mediated the effect of experiencing a core disgust side-effect on symptoms of mental health, with significant indirect effects through behavioural self-disgust on depression (DASS), \(\upbeta_{{{\text{a}}_{2} {\text{b}}_{5} }}\) = .05, p < .05, and anxiety, \(\upbeta_{{{\text{a}}_{2} {\text{b}}_{6} }}\) = .04, p < .05; and borderline-significant indirect effects through physical self-disgust on depression (DASS), \(\upbeta_{{{\text{a}}_{1} {\text{b}}_{2} }}\) = .06, p < .10, and anxiety, \(\upbeta_{{{\text{a}}_{1} {\text{b}}_{3} }}\) = .04, p < .10. Regression coefficients associated with the interaction term (a5–6) were constrained to zero. Control variables and error terms are omitted for clarity. Estimates on the endogenous variables were conditioned on: gender, age, years since diagnosis, number of side-effects reported, disgust sensitivity, and medical diagnoses of depression or anxiety. Error terms for the pair of mediators (physical and behavioural self-disgust) were correlated, as were the error terms for the three outcome variables (depression and anxiety). All estimates are standardised betas (β). Significance levels were determined based on bootstrapped CIs (10,000 resamples). A Chi square test with Bollen–Stine bootstrap indicated adequate model fit, χ2(5) = 9.45, p = .174; CFI = 0.99; RMSEA = 0.08, p = .210. *p < .05; **p < .01; ***p < .001
The direct effects of experiencing a core disgust side-effect on depression (HADS), \(\upbeta_{{{\text{c}}_{1} }}\) = .07, p = .287, depression (DASS), \(\upbeta_{{{\text{c}}_{2} }}\) = .11, p = .129, and anxiety, \(\upbeta_{{{\text{c}}_{3} }}\) = −.01, p = .997, were not significant. The indirect effect of core disgust side-effects through physical self-disgust was borderline significant for depression (DASS), \(\upbeta_{{{\text{a}}_{1} {\text{b}}_{2} }}\) = .06, p < .10, and anxiety, \(\upbeta_{{{\text{a}}_{1} {\text{b}}_{3} }}\) = .04, p < .10, but not significant for depression (HADS), \(\upbeta_{{{\text{a}}_{1} {\text{b}}_{1} }}\) = .08, p = .105, while the indirect effect of core disgust side-effects via behavioural self-disgust was statistically significant for depression (DASS), \(\upbeta_{{{\text{a}}_{2} {\text{b}}_{5} }}\) = .05, p < .05, and anxiety, \(\upbeta_{{{\text{a}}_{2} {\text{b}}_{6} }}\) = .04, p < .05, but not depression (HADS), \(\upbeta_{{{\text{a}}_{2} {\text{b}}_{4} }}\) = .01, p = .383. The total indirect effect of core disgust side-effects through self-disgust was borderline significant for depression (HADS), \(\upbeta_{{{\text{a}}_{1, 2} {\text{b}}_{1, 4} }}\) = .09, p < .10, depression (DASS), \(\upbeta_{{{\text{a}}_{1, 2} {\text{b}}_{2, 5} }}\) = .11, p < .10, and anxiety, \(\upbeta_{{{\text{a}}_{1, 2} {\text{b}}_{3, 6} }}\) = .08, p < .10.
Model 2 (moderated mediation)
Second, we estimated a moderated mediation model, by removing the parameter constraints on the disgust propensity*core disgust-related side-effect interaction term (Fig. 2). This model fit the data very well, χ2(3) = 2.82, p = .530, CFI = 1.00, RMSEA = 0.00, 90 % CI [0.00, 0.14], p = .555, showing a significant improvement in overall model fit over the constrained model, Δχ2(2) = 6.63, p < .05. The interaction term significantly positively predicted behavioural self-disgust, \(\upbeta_{{{\text{a}}_{6} }}\) = .25, p < .05, and borderline-significantly predicted physical self-disgust, \(\upbeta_{{{\text{a}}_{5} }}\) = .18, p < .10. To clarify the nature of the moderating effect, the effects of experiencing a core disgust-related side-effect on (physical and behavioural) self-disgust were estimated at three levels of disgust propensity (−1 SD, M, +1 SD). As shown in Fig. 3, having a core disgust side-effect significantly predicted behavioural self-disgust at high, \(\upbeta_{{{\text{a}}_{2} }}\) = .36, p < .05, but not low or moderate levels of disgust propensity. This pattern was the same for physical self-disgust. Tests of mediation (model 1) at differing levels of disgust propensity reflected the above; self-disgust significantly mediated the relationship between core disgust side-effects and depression and anxiety only when disgust propensity was high, providing evidence of moderated mediation (see Table 4).
Moderated mediation model explaining the effect of “core” disgust side-effects on symptoms of depression and anxiety in people treated for cancer. Propensity to disgust significantly positively moderated the effect of experiencing a core disgust side-effect on behavioural self-disgust, \(\upbeta_{{{\text{a}}_{6} }}\) = .25, p < .05, and had a borderline-significant moderation effect on physical self-disgust, \(\upbeta_{{{\text{a}}_{5} }}\) = .18, p < .10. Control variables and error terms are omitted for clarity. Estimates on the endogenous variables were conditioned on: gender, age, years since diagnosis, number of side-effects reported, disgust sensitivity, and medical diagnoses of depression or anxiety. Error terms for the pair of mediators (physical and behavioural self-disgust) were correlated, as were the error terms for the three outcome variables (depression and anxiety). All estimates are standardised betas (β). Significance levels were determined based on bootstrapped CIs (10,000 resamples). A Chi square test with Bollen–Stine bootstrap indicated excellent model fit, χ2(3) = 2.82, p = .530, CFI = 1.00, RMSEA = 0.00, 90 % CI [0.00, 0.14], p = .555. † p < .10; *p < .05; **p < .01; ***p < .001
Simple slopes analysis explaining the effect of experiencing a “core” disgust-related side-effect on levels of behavioural self-disgust at three levels of underlying disgust propensity (−1 SD = “low”; M = “mean”; and +1 SD = “high”). Experiencing a core disgust side-effect significantly predicted greater behavioural self-disgust only when disgust propensity was high. This pattern of results is the same for physical self-disgust (not shown)
Discussion
In this paper we explored the relationship between disgust-related physical side-effects of cancer treatments and individuals’ symptoms of depression and anxiety. Our first prediction (1), that disgust-related side-effects would be positively related to mental health symptoms, received partial support. Reporting a “core” (e.g., incontinence), but not “animal-nature” (e.g., hair loss) disgust side-effect (vs. having no or any other type of side-effect) was positively related to symptoms of depression and anxiety. There are at least three reasons why disgust-related side-effects may be especially associated with states of depression and/or anxiety. First, disgust has been implicated in the phenomenology of depressed mood, which is theorised to be a combination of sadness and self-disgust (Alanazi et al., 2015; Power & Dalgleish, 2008). Second, disgust is socioculturally-determined (Rozin et al., 2008), and in experiencing disgust there is an implicit appraisal that the same stimulus would be disgusting to others. Individuals experiencing self-disgust think that they are repulsive to other people (Powell et al., 2014). Disgust causes a negative interpretation bias (Davey et al., 2006), motivates avoidance (Rozin & Fallon, 1987), and is associated with stigma (Park et al., 2007). Vartanian (2010), for example, found disgust to be a stronger predictor of obesity stigma in undergraduate students than attributions of control or demographic factors (e.g., body mass index). Thus, disgust-related side-effects may increase the perceived (and actual) prejudicial discrimination patients receive from others, and/or increase social isolation (Powell et al., 2014), leading to heightened anxiety and/or depression (e.g., Else-Quest et al., 2009). Third, disgust is linked to other negative self-conscious emotional states (e.g., shame; Alanazi et al., 2015; Powell et al., 2014), and dysfunctional thought processes (Overton et al., 2008), which are linked to depression and anxiety (e.g., Gilbert, 2000).
It is unclear, however, why the animal-nature disgust side-effects did not show the same pattern of results as core disgust side-effects in this sample. It may be that there is a greater range of side-effects in this grouping (e.g., “ulceration” to “facial reconstruction”), than in core disgust side-effects, which constitute a more narrowly-defined class of elicitors (i.e., bodily waste products), thus obscuring any effects at the aggregate level. Further work with larger samples may be able to explore this heterogeneity in greater detail. Further, it may be that the animal-nature category suffered from a lack of validity (i.e., our sample of cancer patients did not actually find these side-effects disgusting). Certain animal-nature side-effects (e.g., hair loss) may be more accepted socially, particularly in the context of cancer, than core disgust side-effects (e.g., incontinence; Reeve, 2015). Furthermore, disgust is heterogeneous (Simpson et al., 2006); core disgust stimuli (e.g., faeces) are considered some of the most salient and universal disgust-provoking objects in existence (Rozin et al., 2008). They elicit disgust in almost all adults, while animal-nature disgust shows greater variability (Haidt, 2015). This factor too may contribute to the stronger impact of core (vs. animal-nature) disgust side-effects on mental health.
In general, predictions (2) and (3) were supported through a moderated mediation model (Fig. 2). Regarding (2), the impact of core disgust side-effects on depression and anxiety were fully explained by indirect effects through self-directed disgust, significantly predicting two out of three outcome variables (DASS depression and HADS anxiety). The specific indirect effects were larger through behavioural than physical self-disgust, presumably representing the nature of the core disgust category as measured in this study (i.e., problems with bodily waste products, including incontinence and sickness, typically constitute disgusting behaviours rather than necessitate alterations to the physical self). That a significant mediation effect was observed for only one of the depression indices (DASS) included in the study, and not the HADS depression subscale (although this effect was borderline), raises questions about measurement. First, the HADS asked about current (i.e., “in-the-moment”) symptoms of depression, while the DASS measures symptoms over the previous week, which may help to explain this discrepancy. Second, the HADS has been criticised as having a reduced sensitivity to minor depression (Luckett et al., 2010). Thus, the DASS may be a more sensitive instrument than the HADS, yet its use in cancer samples remains limited. Of course, the way self-report measures such as this may translate to clinical outcomes should be interpreted with caution.
Supporting prediction (3), disgust proneness positively moderated the effect of experiencing core disgust side-effects on self-disgust. This is consistent with prior work in the field, showing a potentially detrimental interactive effect between individuals’ disgust proneness and exposure to one’s core bodily disgust elicitors on psychological well-being (Reynolds et al., 2015a; Smith et al., 2007). This significant interaction also supports the contention that it is the disgust(ing) aspect of these physical side-effects that contributes to the development of trait self-disgust, and thereby symptoms of depression and anxiety.
As well as depression (Overton et al., 2008) and anxiety (Olatunji et al., 2015), self-disgust has been shown to be involved in a number of, often comorbid, mental health issues, including eating (Moncrieff-Boyd et al., 2014) and personality (Abdul-Hamid et al., 2014) disorders. A model of enduring, problematic self-disgust as an “emotion schema” was outlined by Powell et al. (2015a). Powell et al. (2015a) described self-disgust as a lasting disgust-based cognitive-affective orientation toward (an aspect of) the self, composed of interacting state and higher-order trait components, the latter of which are largely cognitive and persist over time (i.e., “my body is revolting”). Importantly, self-disgust has been shown to be relatively temporally stable (Powell et al., 2013), and thus is likely to be particularly detrimental based on its schematic, top-down influence on information processing (Powell et al., 2015a). Self-disgust is formed either during the acquisition of disgust, as one learns that certain self-aspects fit within an emerging repertoire of disgust elicitors, or through a dramatic change in the self (e.g., via physical trauma) that is then appraised as disgusting (Powell et al., 2015a). The latter would explain self-disgust as a consequence of disgust-related side-effects of cancer treatment.
The findings from this study have implications for understanding patients’ psychological adaptation to cancer treatments, and they add to a literature on the importance of addressing emotional factors in the aetiology of mental health problems (Greenberg, 2008). This work suggests that the core disgust side-effects possible in cancer treatments may be particularly deleterious to psychological wellbeing through increases in (behavioural) self-directed disgust. There are two potential points for intervention. First, it is possible to identify, by measuring disgust proneness, which patients may particularly suffer as a result of these side-effects, and to monitor and treat them accordingly (Reynolds et al., 2015a). There is evidence that cognitive reappraisal (vs. e.g., affective suppression) may be a useful strategy for the psychological regulation of disgust, and that it can be primed in the laboratory (e.g., Goldin et al., 2008; Gross, 1998). Second, reducing the enduring self-disgust associated with core disgust side-effects may disrupt the link with depressive and anxious outcomes. Recent experimental work has shown that the self-affirmation of valued character traits may be a promising tool for reducing in-the-moment feelings of self-directed disgust (Powell et al., 2015b). Paul Gilbert’s “compassion-focused therapy” is another approach formulated to work with people with high levels of self-criticism, hatred, and disgust (Gilbert, 2015). Nevertheless, research on the effective regulation and treatment of disgust is in its infancy and there are plentiful opportunities for future work in this area; what is critical, in this context, is that the emotional (i.e., disgust) component forms the primary target for clinical intervention (Whelton & Greenberg, 2005).
This study is limited by its cross-sectional design. While mediation normally requires a temporal lag between observed variables, a strong theoretical justification can be made for the proposed directionality of the current model. In particular, treatment side-effects can be viewed as primarily exogenous to self-disgust (i.e., they are more likely to cause self-disgust than the reverse; a pathway that is moderated by disgust proneness). In turn, previous longitudinal work has shown that self-disgust (as measured by the SDS; Overton et al., 2008) significantly predicts depressive symptoms (as measured by the DASS; Lovibond & Lovibond, 1995)—and not the reverse—over 6- and 12-month periods (Powell et al., 2013). Given the strong relationship observed between symptoms of depression (DASS) and anxiety, r = .62, p < .001, we assume this directional pattern to be analogous for anxiety. Nevertheless, reverse effects to those hypothesised are possible; the side-effects measure is subjective and limited by recollection, so it is plausible that people who had higher levels of self-disgust at data collection were more likely to recall disgust-related side-effects. Further, disgust propensity may be affected by self-disgust (and mental health) as much as the reverse. Experimental designs (i.e., focusing on reducing disgust) are necessary to establish causation and test between the above alternative explanations.
This study is also limited by its modest sample size. However, this sample exhibited very similar levels of depressive symptoms (HADS) to a larger heterogeneous cancer sample (e.g., Smith et al., 2002), suggesting a degree of representativeness. While all cell sizes for the dummy side-effect variables were above a commonly-accepted minimum (30 cases), the reduction in cell sizes that would occur by further subsampling by side-effect type restricts the utility of such an analysis (Gordon, 2010). The study is also limited by our aggregated, “top-level” analysis of physical side-effects. While we control for some important factors (e.g., years since cancer diagnosis), we do not have available deeper information on participants’ side-effects, such as their temporal permanency or recency, which may be key moderators of the effects observed. Nevertheless, the fact that an effect is obtained at the “top-level” of these events (i.e., experiencing a core disgust side-effect or not) is impressive and speaks to the enduring power of acquired disgust, which is particularly resistant to temporal extinction (Olatunji et al., 2007). Future work may explore how the above (and other) conditioning variables influence the impact of core disgust side-effects and how patients’ disgust appraisals change as a result.
This study was the first to explore quantitatively the effects of disgust-related physical side-effects of cancer treatment on mental health outcomes. The findings suggest that disgust matters; people who had experienced a core disgust side-effect (vs. no or any other kind of side-effect) exhibited higher levels of depression and anxiety. Furthermore, this association was explained entirely by increased self-directed disgust, as moderated by trait disgust proneness. Taken together, these findings stress the importance of emotional factors (i.e., disgust) in psychological adaptation to the side-effects of cancer treatment. They suggest that disgust-targeted interventions may be useful in reducing self-disgust and/or disgust proneness in individuals exposed to relevant side-effects in order to improve mental health outcomes.
References
Abdul-Hamid, S., Denman, C., & Dudas, R. B. (2014). Self-relevant disgust and self-harm urges in patients with borderline personality disorder and depression: A pilot study with a newly designed psychological challenge. PLoS ONE, 9, e99696. doi:10.1371/journal.pone.0099696
Alanazi, F. S. M., Powell, P. A., & Power, M. (2015). Depression as a disorder of disgust. In P. A. Powell, P. G. Overton, & J. Simpson (Eds.), The revolting self: Perspectives on the psychological, social, and clinical implications of self-directed disgust (pp. 151–165). London: Karnac.
Arrieta, O., Angulo, L. P., Núñez-valencia, C., Dorantes-gallareta, Y., Macedo, E. O., Martínez-López, D., et al. (2013). Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Annals of Surgical Oncology, 20, 1941–1948. doi:10.1245/s10434-012-2793-5
Bauer, R. M., Bastian, P. J., Gozzi, C., & Stief, C. G. (2009). Postprostatectomy incontinence: All about diagnosis and management. European Urology, 55, 322–333. doi:10.1016/j.eururo.2008.10.029
Beck, A. T. (1967). Depression: Clinical, experimental, and theoretical aspects. New York, NY: Harper Row.
Bollen, K. A., & Stine, R. A. (1992). Bootstrapping goodness-of-fit measures in structural equation models. Sociological Methods and Research, 21, 205–229. doi:10.1177/0049124192021002004
Bredin, M. (1999). Mastectomy, body image and therapeutic massage: A qualitative study of women’s experience. Journal of Advanced Nursing, 29, 1113–1120. doi:10.1046/j.1365-2648.1999.00989.x
Carey, M. P., & Burish, T. G. (1988). Eitology and treatment of the psychological side effects associated with cancer chemotherapy: A critical review and discussion. Psychological Bulletin, 104, 307–325. doi:10.1037/0033-2909.104.3.307
Chapman, H. A., & Anderson, A. K. (2012). Understanding disgust. Annals of the New York Academy of Sciences, 1251, 62–76. doi:10.1111/j.1749-6632.2011.06369.x
Cisler, J. M., Olatunji, B. O., & Lohr, J. M. (2009). Disgust, fear, and the anxiety disorders: A critical review. Clinical Psychology Review, 29, 34–46. doi:10.1016/j.cpr.2008.09.007
Curtis, V., Aunger, R., & Rabie, T. (2004). Evidence that disgust evolved to protect from risk of disease. Proceedings of the Royal Society B: Biological Sciences, 271, S131–S133. doi:10.1098/rsbl.2003.0144
Davey, G. C. L. (2011). Disgust: The disease-avoidant emotion and its dysfunctions. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 366, 3453–3465. doi:10.1098/rstb.2011.0039
Davey, G. C. L., Bickerstaffe, S., & MacDonald, B. A. (2006). Experienced disgust causes a negative interpretation bias: A causal role for disgust in anxious psychopathology. Behaviour Research and Therapy, 44, 1375–1384. doi:10.1016/j.brat.2005.10.006
DiMatteo, M. R., Lepper, H. S., & Croghan, T. W. (2000). Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine, 160, 2101–2107. doi:10.1001/archinte.160.14.2101
Ekman, P. (1999). Basic emotions. In T. Dalgleish & M. J. Power (Eds.), Handbook of cognition and emotion (pp. 45–60). New York, NY: Wiley.
Else-Quest, N. M., LoConte, N. K., Schiller, J. H., & Hyde, J. S. (2009). Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychology & Health, 24, 949–964. doi:10.1080/08870440802074664
Falagas, M. E., Zarkadoulia, E. A., Ioannidou, E. N., Peppas, G., Christodoulou, C., & Rafailidis, P. I. (2007). The effect of psychosocial factors on breast cancer outcome: A systematic review. Breast Cancer Research, 9, 1–23. doi:10.1186/bcr1744
Fox, J. (2008). Applied regression analysis and generalized linear models (2nd ed.). London: Sage.
Gilbert, P. (2000). The relationship of shame, social anxiety and depression: The role of the evaluation of social rank. Clinical Psychology and Psychotherapy, 7, 174–189. doi:10.1002/1099-0879(200007)7:3<174::aid-cpp236>3.0.co;2-u
Gilbert, P. (2015). Self-disgust, self-hatred, and compassion-focused therapy. In P. A. Powell, P. G. Overton, & J. Simpson (Eds.), The revolting self: Perspectives on the psychological, social, and clinical implications of self-directed disgust (pp. 223–242). London: Karnac.
Goetz, A. R., Cougle, J. R., & Lee, H.-J. (2013). Revisiting the factor structure of the 12-item Disgust Propensity and Sensitivity Scale—Revised: Evidence for a third component. Personality and Individual Differences, 55, 579–584. doi:10.1016/j.paid.2013.04.029
Goldenberg, J. L., Arndt, J., Hart, J., & Routledge, C. (2008). Uncovering an existential barrier to breast self-exam behavior. Journal of Experimental Social Psychology, 44, 260–274. doi:10.1016/j.jesp.2007.05.002
Goldin, P. R., McRae, K., Ramel, W., & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–586. doi:10.1016/j.biopsych.2007.05.031
Gordon, R. A. (2010). Regression analysis for the social sciences. New York, NY: Routledge.
Greenberg, L. (2008). Emotion and cognition in psychotherapy: The transforming power of affect. Canadian Psychology, 49, 49–59. doi:10.1037/0708-5591.49.1.49
Gross, J. J. (1998). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–237. doi:10.1037/0022-3514.74.1.224
Haidt, J. (2015). Means for the DS-R. Unpublished aggregated data. Retrieved from http://people.stern.nyu.edu/jhaidt/DSR-means.docx
Hallgren, K. A. (2012). Computing inter-rater reliability for observational data: An overview and tutorial. Tutorials in Quantitative Methods for Psychology, 8, 23–24.
Hayes, A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. doi:10.1080/03637750903310360
Hayes, A. F., & Scharkow, M. (2013). The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science, 24, 1918–1927. doi:10.1177/0956797613480187
Hinz, A., Krauss, O., Hauss, J. P., Höckel, M., Kortmann, R. D., Stolzenburg, J. U., & Schwarz, R. (2010). Anxiety and depression in cancer patients compared with the general population. European Journal of Cancer Care, 19, 522–529. doi:10.1111/j.1365-2354.2009.01088.x
Hosaka, T., Aoki, T., Watanabe, T., Okuyama, T., & Kurosawa, H. (1999). Comorbidity of depression among physically ill patients and its effect on the length of hospital stay. Psychiatry and Clinical Neurosciences, 53, 491–495. doi:10.1046/j.1440-1819.1999.00580.x
Kennifer, S. L., Alexander, S. C., Pollak, K. I., Jeffreys, A. S., Olsen, M. K., Rodriguez, K. L., et al. (2009). Negative emotions in cancer care: Do oncologists’ responses depend on severity and type of emotion? Patient Education and Counseling, 76, 51–56. doi:10.1016/j.pec.2008.10.003
Llorente, M. D., Burke, M., Gregory, G. R., Bosworth, H. B., Grambow, S. C., Horner, R. D., et al. (2005). Prostate cancer: A significant risk factor for late-life suicide. The American Journal of Geriatric Psychiatry, 13, 195–201. doi:10.1097/00019442-200503000-00004
Lovibond, S. H., & Lovibond, P. F. (1995). Manual for the depression, anxiety and stress scales (2nd ed.). Sydney: Psychology Foundation.
Luckett, T., Butow, P. N., King, M. T., Oguchi, M., Heading, G., Hackl, N. A., et al. (2010). A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Supportive Care in Cancer, 18, 1241–1262. doi:10.1007/s00520-010-0932-8
MacKinnon, D. P. (2008). Introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum.
Mallinckrodt, B., Abraham, W. T., Wei, M., & Russell, D. W. (2006). Advances in testing the statistical significance of mediation effects. Journal of Counseling Psychology, 53, 372–378. doi:10.1037/0022-0167.53.3.372
Moncrieff-Boyd, J., Allen, K., Byrne, S., & Nunn, K. (2014). The Self-Disgust Scale revised version: Validation and relationships with eating disorder symptomatology. Journal of Eating Disorders, 2, O48. doi:10.1186/2050-2974-2-S1-O48
Neal, C., Beckjord, E. B., Rechis, R., Schaeffer, J., Berno, D., & Duchover, Y. (2007). Cancer stigma and silence around the world. Austin, TX: LIVESTRONG Foundation.
Office for National Statistics. (2015). Mortality statistics: Deaths registered in England and Wales, 2014. Swansea: Office for National Statistics.
Olatunji, B. O., Cisler, J. M., Deacon, B. J., Connolly, K., & Lohr, J. M. (2007a). The Disgust Propensity and Sensitivity Scale-Revised: Psychometric properties and specificity in relation to anxiety disorder symptoms. Journal of Anxiety Disorders, 21, 918–930. doi:10.1016/j.anxdis.2006.12.005
Olatunji, B. O., Cox, R., & Kim, E. H. (2015). Self-disgust mediates the associations between shame and symptoms of bulimia and obsessive-compulsive disorder. Journal of Social and Clinical Psychology, 34, 239–258. doi:10.1521/jscp.2015.34.3.239
Olatunji, B. O., Forsyth, J. P., & Cherian, A. (2007b). Evaluative differential conditioning of disgust: A sticky form of relational learning that is resistant to extinction. Journal of Anxiety Disorders, 21, 820–834. doi:10.1016/j.anxdis.2006.11.004
Olatunji, B. O., Williams, N. L., Lohr, J. M., & Sawchuk, C. N. (2005). The structure of disgust: Domain specificity in relation to contamination ideation and excessive washing. Behaviour Research and Therapy, 43, 1069–1086. doi:10.1016/j.brat.2004.08.002
Olatunji, B. O., Williams, N. L., Tolin, D. F., Abramowitz, J. S., Sawchuk, C. N., Lohr, J. M., & Elwood, L. S. (2007c). The Disgust Scale: Item analysis, factor structure, and suggestions for refinement. Psychological Assessment, 19, 281–297. doi:10.1037/1040-3590.19.3.281
Overton, P. G., Markland, F. E., Taggart, H. S., Bagshaw, G. L., & Simpson, J. (2008). Self-disgust mediates the relationship between dysfunctional cognitions and depressive symptomatology. Emotion, 8, 379–385. doi:10.1037/1528-3542.8.3.379
Park, J. H., Schaller, M., & Crandall, C. S. (2007). Pathogen-avoidance mechanisms and the stigmatization of obese people. Evolution and Human Behavior, 28, 410–414. doi:10.1016/j.evolhumbehav.2007.05.008
Powell, P. A., Overton, P. G., & Simpson, J. (2014). The revolting self: An interpretative phenomenological analysis of the experience of self-disgust in females with depressive symptoms. Journal of Clinical Psychology, 70, 562–578. doi:10.1002/jclp.22049
Powell, P. A., Simpson, J., & Overton, P. G. (2013). When disgust leads to dysphoria: A three-wave longitudinal study assessing the temporal relationship between self-disgust and depressive symptoms. Cognition and Emotion, 27, 900–913. doi:10.1080/02699931.2013.767223
Powell, P. A., Simpson, J., & Overton, P. G. (2015a). An introduction to the revolting self: Self-disgust as an emotion schema. In P. A. Powell, P. G. Overton, & J. Simpson (Eds.), The revolting self: Perspectives on the psychological, social, and clinical implications of self-directed disgust (pp. 1–24). London: Karnac.
Powell, P. A., Simpson, J., & Overton, P. G. (2015b). Self-affirming trait kindness regulates disgust toward one’s physical appearance. Body Image, 12, 98–107. doi:10.1016/j.bodyim.2014.10.006
Power, M. J., & Dalgleish, T. (2008). Cognition and emotion: From order to disorder (2nd ed.). Hove: Psychology Press.
Reeve, D. (2015). Disgust and self-disgust: A disability studies perspective. In P. A. Powell, P. G. Overton, & J. Simpson (Eds.), The revolting self: Perspectives on the psychological, social, and clinical implications of self-directed disgust (pp. 53–74). London: Karnac.
Reynolds, L. M., Bissett, I. P., & Consedine, N. S. (2015a). Predicting the patients who will struggle with anal incontinence: Sensitivity to disgust matters. Colorectal Disease, 17, 73–80. doi:10.1111/codi.12781
Reynolds, L. M., Consedine, N. S., Pizarro, D. A., & Bissett, I. P. (2013). Disgust and behavioral avoidance in colorectal cancer screening and treatment: A systematic review and research agenda. Cancer Nursing, 36, 122–130. doi:10.1097/NCC.0b013e31826a4b1b
Reynolds, L. M., McCambridge, S. A., Bissett, I. P., & Consedine, N. S. (2014). Trait and state disgust: An experimental investigation of disgust and avoidance in colorectal cancer decision scenarios. Health Psychology, 33, 1495–1506. doi:10.1037/hea0000023
Reynolds, L. M., McCambridge, S. A., & Consedine, N. S. (2015b). Self-disgust and adaptation to chronic physical health conditions: Implications for avoidance and withdrawal. In P. A. Powell, P. G. Overton, & J. Simpson (Eds.), The revolting self: Perspectives on the psychological, social, and clinical implications of self-directed disgust (pp. 75–88). London: Karnac.
Rozin, P., & Fallon, A. E. (1987). A perspective on disgust. Psychological Review, 94, 23–41. doi:10.1037/0033-295X.94.1.23
Rozin, P., Haidt, J., & McCauley, C. R. (2008). Disgust. In M. Lewis, J. M. Haviland-Jones, & L. F. Barrett (Eds.), Handbook of emotions (3rd ed., pp. 757–776). New York, NY: Guilford Press.
Schaller, M., & Park, J. H. (2011). The behavioral immune system (and why it matters). Current Directions in Psychological Science, 20, 99–103. doi:10.1177/0963721411402596
Siegel, S., & Castellan, N. J. (1988). Nonparametric statistics for the behavioral sciences 2. New York: McGraw-Hill.
Simpson, J., Carter, S., Anthony, S. H., & Overton, P. G. (2006). Is disgust a homogeneous emotion? Motivation and Emotion, 30, 31–41. doi:10.1007/s11031006-9005-1
Skarstein, J., Aass, N., Fosså, S. D., Skovlund, E., & Dahl, A. A. (2000). Anxiety and depression in cancer patients: Relation between the Hospital Anxiety and Depression Scale and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire. Journal of Psychosomatic Research, 49, 27–34. doi:10.1016/S0022-3999(00)00080-5
Smith, D. M., Loewenstein, G., Rozin, P., Sherriff, R. L., & Ubel, P. A. (2007). Sensitivity to disgust, stigma, and adjustment to life with a colostomy. Journal of Research in Personality, 41, 787–803. doi:10.1016/j.jrp.2006.09.006
Smith, A. B., Selby, P. J., Velikova, G., Stark, D., Wright, E. P., Gould, A., & Cull, A. (2002). Factor analysis of the Hospital Anxiety and Depression Scale from a large cancer population. Psychology and Psychotherapy: Theory, Research and Practice, 75, 165–176. doi:10.1348/147608302169625
Spiegel, D. (1997). Psychosocial aspects of breast cancer treatment. Seminars in Oncology, 24, S36–S47.
Van Overveld, M., de Jong, P. J., Peters, M. L., Cavanagh, K., & Davey, G. C. L. (2006). Disgust propensity and disgust sensitivity: Separate constructs that are differentially related to specific fears. Personality and Individual Differences, 41, 1241–1252. doi:10.1016/j.paid.2006.04.021
Vartanian, L. R. (2010). Disgust and perceived control in attitudes toward obese people. International Journal of Obesity, 34, 1302–1307. doi:10.1038/ijo.2010.45
Walker, J., Hansen, C. H., Martin, P., Symeonides, S., Ramessur, R., Murray, G., & Sharpe, M. (2014). Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. The Lancet Psychiatry, 1, 343–350. doi:10.1016/S2215-0366(14)70313-X
Whelton, W. J., & Greenberg, L. S. (2005). Emotion in self-criticism. Personality and Individual Differences, 38, 1583–1595. doi:10.1016/j.paid.2004.09.024
Wolf, E. J., Harrington, K. M., Clark, S. L., & Miller, M. W. (2013). Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educational and Psychological Measurement, 76, 913–934. doi:10.1177/0013164413495237
Zigmond, A. S., & Snaith, R. P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67, 361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
Acknowledgments
This research was supported by a postgraduate studentship grant to Haffiezhah Azlan from MARA Education Sponsorship Division, Malay for Indigenous People’s Trust Council (MARA), MARA Head Office 21, Jalan Raja Laut 50609 Kuala Lumpur: 330408224812. We thank Kate Adkins for her help with coding the data for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Philip A. Powell, Haffiezhah A. Azlan, Jane Simpson and Paul G. Overton declare that they have no conflict of interest.
Human and animal rights and Informed consent
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. Provided Funding information has to be tagged.
Rights and permissions
About this article
Cite this article
Powell, P.A., Azlan, H.A., Simpson, J. et al. The effect of disgust-related side-effects on symptoms of depression and anxiety in people treated for cancer: a moderated mediation model. J Behav Med 39, 560–573 (2016). https://doi.org/10.1007/s10865-016-9731-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-016-9731-0