Abstract
The aim of this study was to explore quantitatively the relationship between disgust responses in cancer patients and their partners, and in turn their relationship to patients’ psychological well-being. We recruited 50 participants with heterogeneous cancer diagnoses and their partners from cancer-related groups (e.g., charities). Patients completed questionnaires to determine levels of disgust propensity, disgust sensitivity, self-disgust, and symptoms of anxiety and depression. Disgust propensity and sensitivity were also assessed in their partners. Partners’ disgust sensitivity was significantly positively correlated with cancer patients’ self-disgust, disgust propensity, and depression. Path analyses suggested that patients’ self-disgust plays a role in mediating the effect of partners’ disgust sensitivity on patients’ psychological well-being. This study provides the first quantitative evidence that psychological well-being in cancer patients is contingent on their partners’ sensitivity to disgust, and that patients’ self-disgust plays a mediating role. Focusing therapeutically on disgust responses could well be beneficial to people with cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is increasingly recognized and conceptualized as a disease that affects the entire family unit, especially the patient’s significant other (Hodges et al. 2005; Baik and Adams 2011; referred to here as their “partner” for brevity). Research indicates that the relationship with their partner plays a critical role in cancer patients’ adaptation to the illness (e.g., Wimberly et al. 2005). When attachment with the partner is less secure, the relationship can lead to the creation, transmission, and maintenance of poor psychological well-being (e.g., Rodin et al. 2007).
One potential means by which partners may influence patients’ well-being is through negative emotions such as disgust, i.e., feelings of revulsion triggered by something offensive or unpleasant, linked to behavioral avoidance and rejection (Rozin et al. 2008). Patients with cancer often experience strong disgust reactions in response to a range of cancer-related stimuli (Powell et al. 2016). With cancer, the disgust emotion is not exclusively experienced by patients, but partners may also experience disgust towards their significant others as a result of symptoms and treatment side effects (e.g., stoma usage; Smith et al. 2002). As well as disgust arising from physical aspects of the disease and cancer care, disgust in the partners of cancer patients may also originate from anxiety concerning infection from (even a non-contagious) disease (e.g., Wortman and Dunkel-Schetter 1979). People naturally avoid individuals who appear to have an infectious disease (Kouznetsova et al. 2012), and also those with non-infectious conditions that mimic disease cues, such as obesity (Park et al. 2007).
Partners of cancer patients, as with all other individuals, will exhibit differences in disgust responding. van Overveld, de Jong, Peters, Cavanagh, & Davey, (2006) make a distinction between “disgust propensity” (an individual’s tendency to experience disgust, i.e., the likelihood that an individual will be disgusted) and “disgust sensitivity” (the degree to which the response is unpleasant or distressing to an individual, i.e., the extent to which the disgust experience is negatively appraised), a distinction validated via the Disgust Propensity and Sensitivity Scale (van Overveld et al. 2006). This instrument measures propensity and sensitivity broadly and has been shown to have a two-factor solution with items separately loading (> 0.3) on the two subscales. Hypervigilance to avoid impurity may be particularly prominent in individuals who have higher disgust propensity, where they may have enhanced sensory sensitivity (e.g., Schäfer et al. 2009), accompanied by a tendency to overestimate threats and the potential risk of infection (e.g., Deacon and Olatunji 2007; Schaller and Park 2011). A similar overstated reaction may also occur in individuals with higher disgust sensitivity, where they may experience difficulties in successfully controlling specific affective experiences (e.g., Cisler et al. 2009), and have a tendency to develop more intense disgust-related evaluations of disgust-relevant stimuli (e.g., Olatunji et al. 2009).
The frequency (disgust propensity) and intensity (disgust sensitivity) of disgust reactions in cancer partners may be influential in affecting how patients feel about themselves. It has been suggested that individuals may internalize the revulsion of others directed towards them in the form of “self-disgust” (Powell et al. 2014). Self-disgust has been proposed as an emotion schema consisting of two components, disgust towards the “self” and disgust towards one’s behavior (“disgusting ways”; Powell et al. 2015a). Self-directed disgust has been conceptualized as part of the emotional pantheon centered on bodily characteristics (Fox 2009; Neziroglu et al. 2010; Moncrieff-Boyd et al. 2014). Considerable theoretical interest has been directed towards self-disgust as a pan-diagnostic concept relevant to the development and maintenance of a range of mental health problems including depression (Overton et al. 2008) and anxiety (Azlan, Overton, Simpson, & Powell, 2016). Taken together, the evidence above suggests that disgust propensity and disgust sensitivity in the partners of cancer patients, and the ensuing responses to the patient’s symptoms and side effects of treatments, may influence how disgusted patients feel about themselves and hence their subsequent psychological well-being.
In spite of the potential connection between disgust in cancer patients and partners, work conducted so far on the topic has been largely qualitative and has focused on issues of sexuality (e.g., Hawkins et al. 2009), post-treatment care of colorectal surgery (e.g., Persson et al. 2004), and side effects following therapy (e.g., Navon and Morag 2003). Little is known about the contribution of partners’ disgust responses to patients’ psychological well-being, and no research has yet investigated the relationship quantitatively. In the present study we conducted an initial exploration of the effects of disgust traits in partners on self-disgust and anxious and depressive symptoms in cancer patients. Based on the considerations above, we hypothesized that self-disgust levels (and anxiety/depression) would be heightened in cancer patients and that this would be positively associated with trait disgust propensity and disgust sensitivity in partners.
Methods
Participants and Procedure
Ethical approval was granted by the host research institution prior to data collection. We recruited 50 participants with heterogeneous cancer diagnoses and their partners that had never been diagnosed with cancer. Patients were required to have an active cancer diagnosis (either recently diagnosed, undergoing treatment, or experiencing some degree of persistent or recurrent disease) rather than being in remission. Additionally, participation was only available to those who had a partner.
The cancer sample was recruited from cancer charities, cancer and health forums, cancer care organizations, and mental health organizations for people with cancer, based in English speaking countries. Overall, 1008 organizations were initially approached, and of those, 107 agreed to share our advertisement with their members. The eventual sample came from organizations based in the United Kingdom, United States of America, and Canada.
We conducted recruitment in two phases. In phase 1, the participants were recruited without remuneration (n = 18), and in phase 2 (n = 32), the participants were rewarded with remuneration to boost recruitment (10 US dollars per patient, and 10 US dollars per partner). One British pound was donated to Worldwide Cancer Research for every dyad that took part. Overall, 171 individuals with cancer accessed the study website, but only 131 individuals filled in the measures, with another 40 individuals deciding not to go forward. From the 131 individuals who filled the measures, 78 of their partners initially responded, but only 50 partners finished the measures, the other 28 partners deciding not to go forward.
The data were gathered as part of a larger survey into psychological responses to cancer, examining disgust propensity, sensitivity, and self-disgust in people diagnosed with a broad range of cancers (vs. cancer-free controls), and their association with psychological well-being. In a previous publication based on that survey (Azlan et al., 2016), we published data from 107 individuals with cancer (reduced from the full cohort of 131 by the constraints of matching to a control group). Those included in the present study were the reduced cohort of respondents for whom we had both patient and partner data.
The cancer-related organizations were identified through internet searches. Some of the organizations were contacted through their websites and some were contacted by emailing their staff or coordinators. The contact communication first explained the context of our work (“our group has recently been working on quality of life and mental health in people with cancer and we’d like to extend this work to a cancer-care context”), our current interest (“we would like to evaluate how… feeling states and mental well-being in people with cancer are influenced by their partners’ psychological traits, with a view to ultimately help them to have an improved quality of life.”), and what we needed from them (“[we] were wondering if it might be possible to contact people who have cancer through your organisation, and, if so, what steps would be necessary to make that happen”). If the organization replied and was willing to help, we then forwarded them an advertisement which they could circulate to their members. After introducing the team, the advertisement stated that we were investigating “how partners’ psychological traits and self-conscious emotional factors might impact on how people with cancer feel about themselves”. The study “needs you and your spouse/partner to participate as a pair.” Participants were told that they would receive a full debrief following participation.
On the study website to which potential participants were directed, patients were reminded that the study aimed to explore what impact “your partners’ psychological traits and self-conscious emotional factors have on your emotional responses,” and that the study “needs you and your spouse/partner to participate as a pair, but for the study to be valid and produce meaningful results you must complete the survey separately.” In the informed consent, patients were told “If you agree to participate in this survey, please leave your and your partners’ email address in the space provided.” Furthermore, in the informed consent, patients declared “I agree to complete the survey separately to my partner, in confidence, and we will not actively try to influence each other’s responses.” Participants completed the measures listed below in a counterbalanced order and were then fully debriefed. In the debrief, participants were told that the study was “concerned with how partners’ psychological traits influence emotional responses and psychological well-being (i.e., depression and anxiety) in cancer patients.” Furthermore, “it was hypothesised that those who have partners with lower levels of such emotions would report lower levels of negative self-directed emotions (and hence better well-being on average) than those who have partners with higher levels of negative, externally directed emotions.”
The partners of cancer patients were contacted using the email addresses the patients had provided. In the distribution email for the partners, the partners were informed that the cancer patient has participated in a survey. The partner was told that the patient “has participated in a survey that needs you to participate as a pair, but for the study to be valid and produce meaningful results you must complete the survey separately” and that the research is “looking at the relationship between your psychological traits and your partner’s [i.e., the patient’s] emotional responses.” In the informed consent, the partner was told that: “If you decide to take part you will be asked to fill-out a series of questionnaires about yourself, your background and your psychological traits… you are asked to participate regardless of the nature (e.g., negative, neutral or positive) of your cancer care experience.” We also emphasized that “it is very important that you and your partner do not actively try to influence each other’s responses.” Furthermore “your partner will not see your responses.” Partners were then directed to a separate link that presented a modified online survey. The measures they completed are listed below. They were debriefed after completing the survey.
Patients had a mean age of 49.16 years (SD = 14.20) and partners a mean age of 49.70 years (SD = 12.80). Nine of the couples were same sex, and of the remaining 41, the patient was male in 15 couples and female in 26. Ethnicity was assessed by a question that asked “How would you describe your ethnicity?” with a range of response options (White British, Asian British, Asian Other, Black Other, White Irish, Indian, Black British, Chinese, White European, Pakistani, Black Caribbean, Other ethnic group, White Other, Bangladeshi, and Black African; “white” here is used to mean people of native British, Irish, and European origin). The majority of couples, 38 of 50, had the same ethnicity. Regarding patient ethnicity, 36 of 50 were non-White British (most frequently ‘White Other,’ n = 17, or ‘White European,’ n = 10). Of the partners, 34 of 50 partners were non-white British (most frequently ‘White European,’ n = 14, or ‘White Other,’ n = 13), the remainder of each group being White British.
Survey questions in the cancer patients’ survey requested information about medical history and status. The survey asked “What type of primary cancer have you been diagnosed with? What stage is your cancer at now? Have you received treatment for your cancer? Which form of treatment have you received?” Responses indicated that participants had various types of primary cancer, the most common being gastrointestinal stromal tumor (14%), gynecological (10%), breast (8%), colon (8%), and Hodgkin lymphoma (8%). One participant reported more than one type of primary cancer. Of those who chose to declare, the modal Stage (12/40) was II in terms of progression. The majority of participants had received multiple treatments for their cancer, with chemotherapy (60%), surgery (44%), and radiotherapy (42%) being the most common. Only two participants had not had treatment for their cancer.
Measures
Patients provided demographic information and completed measures of trait self-disgust, disgust propensity, disgust sensitivity, and anxiety and depression, whereas their partners only completed demographics and measures of disgust propensity and disgust sensitivity.
Self-disgust
Participants’ trait self-disgust was measured using the Self-Disgust Scale (Overton et al. 2008). For each of 18 items, participants rate how much they agree it is descriptive of them on a 7-point Likert scale (1 = strongly agree, 7 = strongly disagree). The scale contains a number of filler items and two 5-item subscales, one measuring physical self-disgust (an example item from the physical self-disgust subscale is “I find myself repulsive”), and the other behavioral self-disgust (an example item from the behavioral subscale is “I often do things I find revolting”). Hence the lowest score for the full scale (used here) was 10 and the highest—indicating the highest level of self-disgust—was 70. In the cancer patient sample, the Cronbach’s alpha for self-disgust was .93.
Disgust Propensity and Sensitivity
Participants’ disgust propensity and disgust sensitivity were measured using a version of the 12-item Disgust Propensity and Sensitivity Scale-Revised (DPSS-R; Olatunji et al. 2007). Participants read 12 statements and chose the answer which is most appropriate to them, on a 5-point scale (1 = never, 5 = always). Examples of disgust propensity items are “I experience disgust” and “I feel repulsed,” and examples disgust sensitivity items are “It scares me when I feel nauseous” and “I think disgusting items could cause me illness/infection.” Based on psychometric evaluations of the DPSS-R (Goetz et al. 2013), a recommended ten-item solution (six items for disgust propensity and four for disgust sensitivity) was used for analyses, with potential scores ranging from 6 to 30 on the propensity subscale and 4 to 20 on the sensitivity subscale, with higher scores indicating higher levels of disgust propensity and sensitivity (respectively). The ten-item solution proposed by Goetz et al., (2013) involves removing items that loaded onto a third factor in their study (i.e., neither propensity nor sensitivity), that factor concerning negative appraisals of oneself in response to feeling disgusted—“It embarrasses me when I feel disgusted,” and “I think feeling disgusted is bad for me.” For the ten-item solution in the cancer sample, the Cronbach’s alpha for disgust propensity was 0.79 and 0.69 for disgust sensitivity. In the partner sample, alphas were 0.83 for disgust propensity and 0.77 for disgust sensitivity.
Anxiety and Depression
Levels of anxiety and depression in participants were measured using the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith 1983). The scale was developed for use amongst hospital inpatients and has been previously validated in patients with cancer (e.g., Smith et al. 2002). The HADS also has been used in control samples (e.g., Azlan et al., 2016). The scale consists of 14 items with seven items measuring anxiety and another seven items measuring depressive symptoms. Each item is rated on a 4-point scale (0–3 with varying labels) according to the severity of difficulties experienced, hence scores range from 0 to 21 on each subscale, with higher scores indicating higher levels of anxiety and/or depression. Example items from the anxiety subscale are “I get sudden feelings of panic” and “I feel tense and wound up,” and example items from the depression subscale are “I feel as if I am slowed down” and “I have lost interest in my appearance.” In our cancer sample, the alpha coefficients for HADS were 0.82 (anxiety) and 0.81 (depression).
Data Analysis Plan
Following descriptive and correlational analyses on SPSS v. 22 (IBM Corp., Armonk, NY, US), a path model was developed using AMOS version 22 (IBM Corp.) to examine the relationship between partners’ disgust traits and patients’ psychological well-being. Path analysis has several advantages over standard multiple regression, including the estimation of direct and indirect effects (through mediating variables) simultaneously; the ability to model multiple endogenous (i.e., dependent) variables at the same time, allowing one to account for their interdependence caused by extraneous variables (by correlating their error terms); and the calculation of multiple measures of fit to the data (see e.g., Powell et al. 2016).
As recommended by Hayes (2009), bias-corrected bootstrapping was used to produce robust confidence intervals and standard errors (and hence probability values) for all estimates, including direct and indirect effects, removing any restrictions on the nature of the underlying sampling distribution. Ten thousand resamples were used for the bootstrapped estimates (Mallinckrodt et al. 2006). The bootstrap adjusted p-value was interpreted to assess model fit based on the Chi-square statistic (χ 2), along with the Comparative Fit Index (CFI) and the Root Mean Square Error of Approximation (RMSEA).
One note of caution needs to be mentioned here, namely that the statistical analyses include 5 predictor variables and a number of control variables (see below), hence with 100 participants, the subject/predictor ratio falls below the criteria suggested for regression-based models (for example, Green 1991, suggests n > 50 + 8 m, where n is the number of participants and m is the number of predictors), with a consequent increase in the likelihood of Type 2 errors.
Results
Bivariate Associations and Other Comparisons
Disgust sensitivity was higher in cancer patients (M = 9.60, SD = 3.23) than in their partners (M = 9.16, SD = 3.27), while disgust propensity was lower in cancer patients (M = 14.44, SD = 3.83) than their partners (M = 15.80, SD = 3.86; as in Azlan et al., 2016), although in neither case were these differences significant, although in the case of disgust propensity, there was trend, t(49) = − 1.83, p < .01, d = 0.38.
Bivariate correlational analyses between partner and patient variables are presented in Table 1. There were significant positive correlations between partners’ disgust sensitivity and two of three disgust traits in the cancer patients: self-disgust and disgust propensity, but not disgust sensitivity. There was also a significant positive correlation between partners’ disgust sensitivity and patients’ depression. However, there were no significant correlations between disgust propensity in partners and any of the cancer patients’ disgust traits or measures of their psychological well-being.
Mediation Analyses
In our path analyses we controlled for the patient’s gender, age of patients and partners, ethnicity (1 = White British, 0 = non-White British), the ethnic match within the couples (1 = same ethnicity, 0 = different ethnicity), and sexuality of the couples (1 = heterosexual, 0 = homosexual). Gender (e.g., Rohrmann et al. 2008), age (Curtis et al. 2004), and cultural background (Moretz et al. 2009) have all been shown to influence disgust responding. Furthermore, given that attitudes to same sex and heterosexual couples differ (Inbar, Pizarro, Knobe, & Bloom, 2009), insofar as self-disgust is constructed in part from the attitudes of others towards us (Powell et al. 2015a), this may in turn influence self-disgust levels in these two groups.
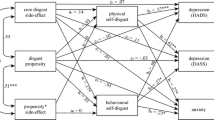
The results of the path analyses are presented in Table 2. The first analysis, without patients’ disgust propensity and disgust sensitivity (see Model 1 in Fig. 1), yielded a reasonable fit to the data: χ 2 (6) = 15.45, p = .02; CFI = 0.94, RMSEA = 0.18, 90% CI [0.07, 0.29], p = .03. Results revealed a positive relationship between partners’ disgust sensitivity and patients’ self-disgust, which in turn had a positive relationship with patients’ anxiety and depression. Patients’ self-disgust fully mediated the association between partners’ disgust sensitivity and levels of anxiety and depression, controlling for patients’ gender, sexuality, and the age of both partners and patients. Partners’ disgust propensity also exerted a significant indirect effect on patients’ anxiety and depression via patients’ self-disgust, but the effect was in the opposite direction to that of disgust sensitivity (i.e., partners’ disgust propensity was related to anxious and depressive symptoms via reduced self-disgust in patients).
Mediation model 1—effect of partners’ disgust sensitivity and disgust propensity on anxiety and depression in people with cancer through patients’ self-disgust. Control variables and error terms are omitted for clarity. Error terms for the two outcome variables (anxiety and depression) were correlated. All estimates are standardized betas (β). Significance levels were determined based on bootstrapped CIs (10,000 resamples). Paths in bold represent significant path estimates. Asterisked coefficients are significant at *p < .05 and **p < .01
When patients’ disgust propensity and disgust sensitivity were also included in the model (see Model 2 in Fig. 2), this necessarily yielded a perfect fit to the data, χ 2 = 0.00. The indirect effects of partners’ disgust sensitivity on patients’ anxiety, β = 0.15, 95% CI [0.01, 0.48], p = .07, and depression, β = 0.17, 95% CI [0.01, 0.50], p = .07, via patients’ self-disgust, were still borderline significant. However, the indirect effects of partners’ disgust propensity on patients’ anxiety, β = −.10, 95% CI [− .36, 0.01], p = .13, and depression, β = −0.11, 95%, CI [− 0.39, .01], p = .13, via the patients’ self-disgust, were no longer significant. The results suggest that the effect of partners’ disgust traits on patients’ anxiety and depression is partly driven by the shared variance they have with the patients’ disgust traits.
Mediation model 2—effect of partners’ disgust sensitivity and disgust propensity on anxiety and depression in people with cancer through patients’ self-disgust, controlling for patients’ disgust traits. Control variables and error terms are omitted for clarity. Error terms for the two outcome variables (anxiety and depression) were correlated. All estimates are standardized betas (β). Significance levels were determined based on bootstrapped CIs (10,000 resamples). Paths in bold represent significant path estimates. Asterisked coefficients are significant at *p < .05
Discussion
The main purpose of this study was to explore how partners’ disgust traits affect psychological well-being in cancer patients. The strongest finding from the study—in line with our original hypothesis—was a positive relationship between partners’ disgust sensitivity and patients’ self-disgust, and between patients’ self-disgust and patients’ anxiety and depression, that is, the more intense the disgust sensitivity in partners, the poorer the psychological well-being in patients, a relationship in which patient’s self-disgust plays a mediating role. Existing studies acknowledge that partners experience disgust towards cancer patients (e.g., Hawkins et al. 2009; Persson et al. 2004; Wortman and Dunkel-Schetter 1979), and aversion towards cancer patients generally stems from changes in the appearance of the patient and fears that the disease is contagious, which has been documented as a major cause of rejection of the patient (Crowther 2010). Patients are explicitly aware of the rejection, some of them saying that their partners refuse to have any physical contact with them, due to the disgust evoked by the sight of their bodies (Navon and Morag 2003).
The features of the facial disgust reaction are essentially defensive, with the narrowing of the nostrils and movements of the mouth region suggestive of expulsion and the prevention of penetration (Angyal 1941). Disgust-related avoidance in cancer can take many forms (Reynolds et al. 2016), and partners’ heightened disgust sensitivity may serve as an instinctive response to protect them from infection and contamination (e.g., Curtis et al. 2004), possibly arising from a failure of emotion regulation and impulse control (e.g., Cisler et al. 2009). This is consistent with evidence elsewhere that disgust levels increase when the threat of infection (Fessler et al. 2005), or even the perceived threat of infection is high (Prokop and Fančovičová 2013).
Behaviors engendered by the heightened disgust sensitivity in partners might be perceived as indicating rejection or disapproval by patients. For example, partners may engage in “neutralizing” behaviors such as wiping their hands, or showering immediately after contact with the patients, which might be interpreted by patients as evidence for them being appraised as repulsive, leading to heightened self-disgust (e.g., de Jong and Borg 2015). Consequently, if partners experience a greater intensity of disgust and are not effective in hiding their disgust, it might intensify self-disgust in patients via internalization of the partners’ expression of disgust (Powell et al. 2014; de Jong and Borg 2015), which in turn may result in patients’ mental health problems (e.g., Azlan et al., 2016; Powell et al. 2016).
Although there was a relationship between partners’ disgust sensitivity and patients’ self-disgust, contrary to our original hypothesis, the same was not true for partners’ disgust propensity and patients’ self-disgust. While it might be anticipated that partners’ disgust propensity—their tendency to experience disgust, or how readily they respond with disgust—would influence patients’ self-disgust in the same way as partners’ disgust sensitivity, disgust propensity appears to be relatively malleable, being influenced (for example) by context (Viar-Paxton and Olatunji 2012), emotion regulation (Cisler et al. 2009), and habituation (Azlan et al., 2016). That may make disgust propensity (vs. disgust sensitivity) a fluctuating, “noisy” source of information about the partners’ emotional state, adding little to the information provided by disgust sensitivity, which appears to be more stable over time (cf. test–retest reliability; van Overveld et al. 2006; Olatunji et al. 2007).
In the context of cancer, therapy for couples has tended to focus almost exclusively on protecting and rebuilding their sexual relationship (e.g., Grayer 2016). However, findings from the present research suggest that focusing on disgust responses, particularly self-disgust, could well be beneficial therapeutically to people with cancer. The development of depression and anxiety might be diminished by attention to the degree of self-disgust experienced by cancer patients, and interventions intended to reduce levels of these maladaptive responses (Azlan et al., 2016). Recent experimental work has shown that the self-affirmation of valued character traits may be a promising tool for reducing in-the-moment feelings of self-directed disgust (Powell et al. 2015b).
There may also be scope to develop therapeutic interventions for couples based on other aspects of disgust. Although, as we mentioned above, disgust sensitivity remains relatively stable across time, disgust propensity appears to be more malleable (Azlan et al., 2016). Indeed, disgust propensity shows evidence of habituation in a domain-specific manner via exposure to relevant disgust elicitors (Rozin 2008). It is possible that (for example) prior exposure to examples of disgust-eliciting stimuli ahead of treatment could lessen disgust propensity in partners, or at least inoculate them to the effect of upcoming elicitors. However, it must be remembered that in the present study partner’s disgust propensity played a less important role than their disgust sensitivity in patient’s anxiety and depression.
In more general terms, the present study’s focus on emotional factors in the genesis of anxiety and depression in people with cancer suggests that therapeutic approaches using “second wave” cognitive behavior therapy (CBT) based on challenging dysfunctional thoughts may be less appropriate in this group. Recently, Acceptance and Commitment Therapy (ACT) has been proposed as a useful approach for psychological distress in cancer patients (Angiola and Bowen 2013). Our findings here which stress the importance of emotional factors in psychological well-being in cancer patients adds further weight to this suggestion, given ACT’s focus on emotional acceptance. Early indications are that ACT is indeed more effective than CBT at lowering levels of depression and anxiety in people with breast cancer (Paez et al. 2007).
Limitations
The primary limitation in this study is the moderate sample size, which reflects the challenge of conducting a dyadic study involving people with cancer, with only around 10% of the organizations we approached being willing to share our advert with their members. This recruitment difficulty is the likely cause of an aspect of our participant sample that adds a challenge to how representative they were, namely nine of the couples (18%) in our study were same sex, a figure that is much higher than the proportion of same sex couples in any of the countries in which the recruiting organizations were based. In the UK for example, the most recent survey suggests that around 1% of couples are same sex (Office of National Statistics 2015). As a consequence, our sample may not be representative with respect to this dimension. In terms of the influence that this may have on relevant measures, as we mentioned above, self-disgust levels may be different in same sex and heterosexual couples given differences in attitudes towards these groups (Inbar et al., 2009) and the role of the attitudes of others in constructing self-disgust schema (Powell et al. 2015a).
A further limitation of the present research is that it relies entirely on self-report measures. However, self-report measures have been extensively used in research on disgust as they are inexpensive, easy to administer (in comparison to physiological and neurological measures), and are particularly useful in studies (such as this) that are concerned with the simultaneous assessment of multiple emotional states (Simpson et al. 2006).
Finally, this study was also limited by its cross-sectional design, although longitudinal studies are very difficult to conduct and interpret in people with cancer, who have a chronic progressive illness, the nature of which and the treatments associated with, change over time. Furthermore, we have found the attrition rate (particularly with negatively valenced studies like our own) to be high in this group.
References
Angiola, J. E., & Bowen, A. M. (2013). Quality of Life in advanced cancer: An Acceptance and Commitment Therapy view. The Counseling Psychologist, 41, 313–335. https://doi.org/10.1177/0011000012461955.
Angyal, A. (1941). Disgust and related aversions. Journal of Abnormal and Social Psychology, 36, 393–412. https://doi.org/10.1037/h0058254.
Azlan, H. A., Overton, P. G., Simpson, J., & Powell, P. A. (2016). Differential disgust responding in people with cancer and implications for psychological wellbeing. Psychology & Health, 32, 19–37. https://doi.org/10.1080/08870446.2016.1235165.
Baik, O. M., & Adams, K. B. (2011). Improving the well-being of couples facing cancer: A review of couples-based psychosocial interventions. Journal of Marital and Family Therapy, 37, 250–266. https://doi.org/10.1111/j.1752-0606.2010.00217.x.
Cisler, J. M., Olatunji, B. O., & Lohr, J. M. (2009). Disgust sensitivity and emotion regulation potentiate the effect of disgust propensity on spider fear, blood-injection-injury fear, and contamination fear. Journal of Behavior Therapy and Experimental Psychiatry, 40, 219–229. https://doi.org/10.1016/j.jbtep.2008.10.002.
Crowther, K. C. (2010). Secondary stress and coping experiences of partners of persons with oral cancer: A descriptive study 2010 (Doctor of Philosophy’s thesis, The University of Texas Medical Branch, Texas, United States). https://utmb-ir.tdl.org/utmb-ir/bitstream/handle/2152.3/123/Crocker_Crowther_Final_6_18_10.pdf?sequence=1&isAllowed=http://yorkspace.library.yorku.ca/xmlui/handle/10315/28261.
Curtis, V., Aunger, R., & Rabie, T. (2004). Evidence that disgust evolved to protect from risk of disease. Proceedings of the Royal Society B: Biological Sciences, 271(Suppl 4), S131–S133. https://doi.org/10.1098/rsbl.2003.0144.
Deacon, B., & Olatunji, B. O. (2007). Specificity of disgust sensitivity in the prediction of behavioral avoidance in contamination fear. Behaviour Research and Therapy, 45, 2110–2120. https://doi.org/10.1016/j.brat.2007.03.008.
de Jong, P. J., & Borg, C. (2015). Self-directed disgust: Reciprocal relationships with sex and sexual dysfunction. In P. A. Powell, P. G. Overton & J. Simpson (Eds.), The revolting self: Perspectives on the psychological and clinical implications of self-directed disgust (pp. 89–112). London: Karnac Books.
Fessler, D. M. T., Eng, S. J., & Navarrete, C. D. (2005). Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evolution and Human Behavior, 26, 344–351. https://doi.org/10.1016/j.evolhumbehav.2004.12.001.
Fox, J. R. (2009). A qualitative exploration of the perception of emotions in anorexia nervosa: A basic emotion and developmental perspective. Clinical Psychology & Psychotherapy, 16, 276–302. https://doi.org/10.1002/cpp.631.
Goetz, A. R., Cougle, J. R., & Lee, H.-J. (2013). Revisiting the factor structure of the 12-item Disgust Propensity and Sensitivity Scale - Revised: Evidence for a third component. Personality and Individual Differences, 55, 579–584. https://doi.org/10.1016/j.paid.2013.04.029.
Grayer, J. (2016). Emotionally focused therapy for couples: a safe haven from which to explore sex during and after cancer. Sexual and Relationship Therapy, 31, 488–492. https://doi.org/10.1080/14681994.2015.1126668.
Green, S. B. (1991). How many subjects does it take to do a regression analysis? Multivariate Behavioral Research, 26, 499–510. https://doi.org/10.1207/s15327906mbr2603_7.
Hawkins, Y., Ussher, J., Gilbert, E., Perz, J., Sandoval, M., & Sundquist, K. (2009). Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nursing, 32, 271–280. https://doi.org/10.1097/NCC.0b013e31819b5a93.
Hayes, A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. https://doi.org/10.1097/NCC.0b013e31819b5a93.
Hodges, L. J., Humphris, G. M., & Macfarlane, G. (2005). A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Social Science and Medicine, 60, 1–12. https://doi.org/10.1016/j.socscimed.2004.04.018.
Inbar, Y., Pizarro, D. A., Knobe, J., & Bloom, P. (2009). Disgust sensitivity predicts intuitive disapproval of gays. Emotion, 9, 435–439. https://doi.org/10.1037/a0015960.
Kouznetsova, D., Stevenson, R. J., Oaten, M. J., & Case, T. I. (2012). Disease-avoidant behaviour and its consequences. Psychology & Health, 27, 491–506. https://doi.org/10.1080/08870446.2011.603424.
Mallinckrodt, B., Abraham, W. T., Wei, M., & Russell, D. W. (2006). Advances in testing the statistical significance of mediation effects. Journal of Counselling Psychology, 53, 372–378. https://doi.org/10.1037/0022-0167.53.3.372.
Moncrieff-Boyd, J., Byrne, S., & Nunn, K. (2014). Disgust and anorexia nervosa: Confusion between self and non-self. Advances in Eating Disorders, 2, 4–18. https://doi.org/10.1080/21662630.2013.820376.
Moretz, M. W., McKay, D., Bjorklund, F., de Jong, P. J., Olatunji, B. O., Moretz, M. W., … Schienle, A. (2009). Confirming the three-factor structure of the Disgust Scale-Revised in eight countries. Journal of Cross-Cultural Psychology, 40, 234–255.
Navon, L., & Morag, A. (2003). Advanced prostate cancer patients’ relationships with their spouses following hormonal therapy. European Journal of Oncology Nursing, 7, 73–80. https://doi.org/10.1016/S1462-3889(03)00022-X.
Neziroglu, F., Hickey, M., & McKay, D. (2010). Psychophysiological and self-report components of disgust in body dysmorphic disorder: The effects of repeated exposure. International Journal of Cognitive Therapy, 3, 40–51. https://doi.org/10.1521/ijct.2010.3.1.40.
Office of National Statistics (2015). Families and households 2014. Downloaded 20 March 2017 from https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/families/bulletins/familiesandhouseholds/2015-01-28.
Olatunji, B. O., Cisler, J. M., Deacon, B. J., Connolly, K., & Lohr, J. M. (2007). The disgust propensity and Sensitivity Scale-Revised: Psychometric properties and specificity in relation to anxiety disorder symptoms. Journal of Anxiety Disorders, 21, 918–930. https://doi.org/10.1016/j.janxdis.2006.12.005.
Olatunji, B. O., Lohr, J. M., Smits, J. A., Sawchuk, C. N., & Patten, K. (2009). Evaluative conditioning of fear and disgust in blood-injection-injury phobia: Specificity and impact of individual differences in disgust sensitivity. Journal of Anxiety Disorders, 23, 153–159. https://doi.org/10.1016/j.janxdis.2008.06.002.
Overton, P. G., Markland, F. E., Taggart, H. S., Bagshaw, G. L., & Simpson, J. (2008). Self-disgust mediates the relationship between dysfunctional cognitions and depressive symptomatology. Emotion, 8, 379–385. https://doi.org/10.1037/1528-3542.8.3.379.
Paez, M., Luciano, M. C., & Gutierrez, O. (2007). Psychological treatment for breast cancer: Comparison between acceptance based and cognitive control strategies. Psicooncologia, 4, 75–95.
Park, J. H., Schaller, M., & Crandall, C. S. (2007). Pathogen-avoidance mechanisms and the stigmatization of obese people. Evolution and Human Behavior, 28, 410–414. https://doi.org/10.1016/j.evolhumbehav.2007.05.008.
Persson, E., Severinsson, E., & Hellström, A. L. (2004). Spouses’ perceptions of and reactions to living with a partner who has undergone surgery for rectal cancer resulting in a stoma. Cancer Nursing, 27, 85–90. https://doi.org/10.1097/00002820200401000-00011.
Powell, P. A., Azlan, H. A., Simpson, J., & Overton, P. G. (2016). The effect of disgust-related side-effects on symptoms of depression and anxiety in people treated for cancer: A moderated mediation model. Journal of Behavioral Medicine, 39, 560–573. https://doi.org/10.1007/s10865-016-9731-0.
Powell, P. A., Overton, P. G., & Simpson, J. (2014). The revolting self: An interpretative phenomenological analysis of the experience of self-disgust in females with depressive symptoms. Journal of Clinical Psychology, 70, 562–578. https://doi.org/10.1002/jclp.22049.
Powell, P. A., Simpson, J., & Overton, P. G. (2015a). An introduction to the revolting self: Self-disgust as an emotion schema. In P. A. Powell, P. G. Overton & J. Simpson (Eds.), The revolting self (pp. 1–24). London: Karnac Books.
Powell, P. A., Simpson, J., & Overton, P. G. (2015b). Self-affirming trait kindness regulates disgust toward one’s physical appearance. Body Image, 12, 98–107. https://doi.org/10.1016/j.bodyim.2014.10.006.
Prokop, P., & Fančovičová, J. (2013). Self-protection versus disease avoidance. The perceived physical condition is associated with fear of predators in humans. Journal of Individual Differences, 34, 15–23. https://doi.org/10.1027/1614-0001/a000092.
Reynolds, L. M., Bissett, I. P., Porter, D., & Consedine, N. S. (2016). The “ick” factor matters: Disgust prospectively predicts avoidance in chemotherapy patients. Annals of Behavioral Medicine, 50, 935–945. https://doi.org/10.1007/s12160-016-9820-x.
Rodin, G., Walsh, A., Zimmermann, C., Gagliese, L., Jones, J., Shepherd, F. A., … Mikulincer, M. (2007). The contribution of attachment security and social support to depressive symptoms in patients with metastatic cancer. Psycho-Oncology, 16, 1080–1091. https://doi.org/10.1002/pon.1186.
Rohrmann, S., Hopp, H., & Quirin, M. (2008). Gender differences in psychophysiological responses to disgust. Journal of Psychophysiology, 22, 65–75. https://doi.org/10.1027/0269-8803.22.2.65.
Rozin, P. (2008). Hedonic “adaptation”: Specific habituation to disgust/death elicitors as a result of dissecting a cadaver. Judgment and Decision Making Journal, 3, 191–194.
Rozin, P., Haidt, J., & McCauley, C. R. (2008). Disgust. In M. Lewis, J. M. Haviland-Jones & L. F. Barrett (Eds.), Handbook of emotions (3rd edn., pp. 757–776). New York: Guilford Press.
Schäfer, A., Leutgeb, V., Reishofer, G., Ebner, F., & Schienle, A. (2009). Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neuroscience Letters, 465, 262–266. https://doi.org/10.1016/j.neulet.2009.09.030.
Schaller, M., & Park, J. H. (2011). The behavioral immune system (and why it matters). Current Directions in Psychological Science, 20, 99–103. https://doi.org/10.1177/0963721411402596.
Simpson, J., Carter, S., Anthony, S. H., & Overton, P. G. (2006). Is disgust a homogeneous emotion? Motivation and Emotion, 30, 31–41. https://doi.org/10.1007/s11031-006-9005-1.
Smith, A. B., Selby, P. J., Velikova, G., Stark, D., Wright, E. P., Gould, A., & Cull, A. (2002). Factor analysis of the Hospital Anxiety and Depression Scale from a large cancer. Psychology Psychotherapy, 75, 165–176. https://doi.org/10.1348/147608302169625.
van Overveld, M., de Jong, P. J., Peters, M. L., Cavanagh, K., & Davey, G. C. L. (2006). Disgust propensity and disgust sensitivity: Separate constructs that are differentially related to specific fears. Personality and Individual Differences, 41, 1241–1252. https://doi.org/10.1016/j.paid.2006.04.021.
Viar-Paxton, M. A., & Olatunji, B. O. (2012). Context effects on habituation to disgust-relevant stimuli. Behavior Modification, 36, 705–722. https://doi.org/10.1177/0145445512446189.
Wimberly, S. R., Carver, C. S., Laurenceau, J. P., Harris, S. D., & Antoni, M. H. (2005). Perceived partner reactions to diagnosis and treatment of breast cancer: Impact on psychosocial and psychosexual adjustment. Journal of Consulting and Clinical Psychology, 73, 300–311. https://doi.org/10.1037/0022-006X.73.2.300.
Wortman, C. B., & Dunkel-Schetter, C. (1979). Interpersonal relationships and cancer: A theoretical analysis. Journal of Social Issues, 35, 120–155. https://doi.org/10.1111/j.1540-4560.1979.tb00792.x.
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. https://doi.org/10.1002/pon.1660.
Acknowledgements
This research was supported by a postgraduate studentship grant to Haffiezhah Azlan from MARA Education Sponsorship Division, Malay, for Indigenous People’s Trust Council (MARA), MARA Head Office 21, Jalan Raja Laut 50609 Kuala Lumpur: 330408224812.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Haffiezhah A. Azlan, Paul G. Overton, Jane Simpson, and Philip A. Powell declare that they have no conflict of interest.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation, Institutional and/or National, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
All individuals included in the study provided informed consent.
Rights and permissions
About this article
Cite this article
Azlan, H.A., Overton, P.G., Simpson, J. et al. Effect of Partners’ Disgust Responses on Psychological Wellbeing in Cancer Patients. J Clin Psychol Med Settings 24, 355–364 (2017). https://doi.org/10.1007/s10880-017-9521-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10880-017-9521-z