Abstract
In an attempt to develop biodegradable, mechanically strong, biocompatible, and conductive nerve guidance conduits, pure magnesium (Mg) was used as the biodegradable substrate material to provide strength while the conductive polymer, poly(3,4-ethylenedioxythiophene) (PEDOT) was used as a conductive coating material to control Mg degradation and improve cytocompatibility of Mg substrates. This study explored a series of electrochemical deposition conditions to produce a uniform, consistent PEDOT coating on large three-dimensional Mg samples. A concentration of 1 M 3,4-ethylenedioxythiophene in ionic liquid was sufficient for coating Mg samples with a size of 5 × 5 × 0.25 mm. Both cyclic voltammetry (CV) and chronoamperometry coating methods produced adequate coverage and uniform PEDOT coating. Low-cost stainless steel and copper electrodes can be used to deposit PEDOT coatings as effectively as platinum and silver/silver chloride electrodes. Five cycles of CV with the potential ranging from −0.5 to 2.0 V for 200 s per cycle were used to produce consistent coatings for further evaluation. Scanning electron micrographs showed the micro-porous structure of PEDOT coatings. Energy dispersive X-ray spectroscopy showed the peaks of sulfur, carbon, and oxygen, indicating sufficient PEDOT coating. Adhesion strength of the coating was measured using the tape test following the ASTM-D 3359 standard. The adhesion strength of PEDOT coating was within the classifications of 3B to 4B. Tafel tests of the PEDOT coated Mg showed a corrosion current (ICORR) of 6.14 × 10−5 A as compared with ICORR of 9.08 × 10−4 A for non-coated Mg. The calculated corrosion rate for the PEDOT coated Mg was 2.64 mm/year, much slower than 38.98 mm/year for the non-coated Mg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Injury to the peripheral nervous system (PNS) is a significant cause of morbidity and disability today. Although direct suture repair may be effective for short (<5 mm) nerve gaps, Nerve Guidance Conduits (NGC) are necessary to mechanically support and direct axonal sprouting between the injured nerve stumps when nerve defects or gaps are larger [1]. The biomaterial used for NGC must be biocompatible, should provide a guidance cue that can enhance nerve regeneration, and must be biodegradable while maintaining a mechanically stable architecture during the regeneration process. Current NGCs that have been approved by the U.S. Food and Drug Administration (FDA) are made out of either natural polymers (e.g., Type I collagen, porcine small intestinal submucosa) or synthetic polymers (e.g. polyglycolic acid). Although these materials are biocompatible and biodegradable, there is still much room for improvement, such as greater mechanical and electrical properties and enhanced bioactive guidance cue.
Magnesium (Mg) and Mg alloys could potentially meet the requirements for NGCs and provide a promising alternative with improved mechanical and electrical properties as well as bioactivity. Mg inherently has much greater mechanical properties and electrical conductivity than polymers. Specifically, pure Mg has an elastic modulus of 40 GPa [2], a yield strength of 126 MPa [2], and an electrical conductivity of 22 × 106 S/m [3]. Furthermore, Mg ions, the degradation product of Mg in body fluids, have been clinically infused in the form of magnesium sulfate to serve as a neuro-protective agent [4–8]. Mg wires with a 99.8 % purity have been implanted into rats and rabbits, and the results showed that the nerves and muscle fibers in the close proximity to the Mg wires were preserved as the wires resorbed in 20 weeks [9]. Therefore, Mg is promising in providing bioactive cues to stimulate nerve regeneration. Although Mg is resorbable and its degradation products can be naturally metabolized, its rapid degradation (1.2 mg/cm2/day) in physiological environment [10] can cause an unfavorable increase in surrounding pH, which may induce adverse effects on surrounding cell functions. Although addition of other alloying elements can improve Mg degradation properties, their long-term toxicity effects are still questionable [11]. Alternatively, polymer coatings can simultaneously control Mg degradation and improve its biocompatibility [12]. Collectively, these findings make Mg a potential material for neural applications if its degradation can be properly controlled using polymer coating.
The conductive polymer poly(3,4-ethylenedioxythiophene) (PEDOT) will be chosen as the coating material due to its superior electrical conductivity (from 2,000 to 13,200 S/m) and biocompatibility as compared with other polymers [13, 14]. PEDOT can serve as a viable alternative material to polypyrrole for long-term neural implants [15–17] since PEDOT contains sulfur and is electrochemically more stable than its counterpart—polypyrrole [15]. Electrochemical impedance spectroscopy and pulse testing demonstrated that charge injection capacities of PEDOT did not decrease after 10 million pulses, indicating good stability [18]. Moreover, PEDOT films showed low intrinsic cytotoxicity and did not provoke inflammatory responses upon implantation [15]. One study showed that PEDOT provided a significant increase in electroactive properties when coated on decellularized nerve scaffolds [19]. Another study showed that PEDOT coated agarose based hydrogel grafts enhanced nerve regeneration as compared with polydimethylsiloxane (PDMS) and plain agarose grafts [20]. Thus, the evidence suggested the suitability of PEDOT for neural applications. Clearly, since the properties of PEDOT and Mg complement each other, PEDOT coating on Mg will provide desirable integrated properties for neural applications. Specifically, it is hypothesized that PEDOT coatings on Mg will reduce the rapid degradation rate of Mg in body fluids and thus improve the overall biocompatibility for neural applications.
In this study, electrochemical deposition was used to polymerize EDOT (monomer form of PEDOT) onto Mg substrates. Although PEDOT coating has been successfully deposited on a 3.2 mm diameter Mg surface previously using an electrochemical method [12], the ability to reproduce the PEDOT coating consistently on much larger three-dimensional Mg samples with a lower cost is important for practical translation of these materials to neural applications. Therefore, the objective of this study was to investigate the effects of key parameters of electrochemical deposition (i.e., cyclic voltammetry vs chronoamperometry, pristine vs recycled EDOT in ionic liquid bath, the use of low-cost electrodes, and different post-coating treatment) on the formation of PEDOT coating on Mg, and evaluate the PEDOT coating properties and its effectiveness in controlling Mg degradation. Specifically, surface microstructure and composition of the PEDOT coating, adhesion strength of the coating, and the corrosion potential of PEDOT coated versus non-coated Mg were studied.
2 Materials and methods
2.1 Preparation of Mg substrate
As-rolled 250 μm thick Mg sheets of 99.9 % purity (Goodfellow USA) were cut into samples with a size of 5 × 10 mm, cleaned with ethanol (99.5 % purity, Sigma-Aldrich) by sonication at room temperature for 15 min, and air-dried at room temperature. As-rolled Mg sheets had an oxidized surface that was induced during processing. To determine whether the oxidized versus polished surface conditions of Mg would affect the electrochemical deposition and coating properties, some samples were polished using 600, 800, and 1,200 grit silicon carbide (SiC) paper (Ted Pella, Inc.) and cleaned with ethanol.
2.2 Electrochemical deposition of PEDOT on Mg substrate
PEDOT coating was electrochemically deposited onto the Mg samples with a dimension of 5 × 5 × 0.25 mm for further characterization and evaluation. A potentiostat (model 273A, EG&G Princeton Applied Research) operated by a PowerSuite software and an electrochemical cell were set up and connected for electrochemical deposition (Fig. 1). A 50 mL glass beaker with a stir bar was used for the electrochemical cell setup. The electrochemical cell included three electrodes and a bath of electrolyte. The electrolyte bath was prepared by mixing 3,4-ethylenedioxythiophene (EDOT, Sigma-Aldrich) and pristine 1-ethyl-3-methylimidazolium bis(trifluoro-methylsulfonyl)imide (ionic liquid or IL, electrochemical grade, >99.5 % purity, Covalent Associates, Inc.) to give a 1 M EDOT concentration. Potentiostat leads were connected to their respective electrodes, and the experimental parameters were set up for deposition using the PowerSuite software. When the voltage was applied to the working electrode (Mg sample), EDOT monomers in the electrolyte were oxidized and polymerized onto Mg. Since electron transfer was much faster than the diffusion of radical ions in solution, high local concentrations of ions near the working electrode allowed the polymerization to continue at the electrode surface. A stir bar was utilized to resuspend the PEDOT settled on the bottom of the electrochemical cell and enhance polymerization of EDOT onto Mg substrates. Half of the Mg sample (a 5 × 5 mm surface with a 0.25 mm thickness) was immersed in the EDOT/IL bath for coating.
2.3 Key parameters of electrochemical deposition
A variety of relevant deposition parameters were explored in this study, including the coating method (cyclic voltammetry vs chronoamperometry), the purity of bath solution (pristine vs recycled EDOT/IL bath), type of electrodes, process duration at particular voltages, concentrations of EDOT/IL, stir bar usage during deposition, and post-coating treatment.
2.3.1 Cyclic voltammetry and chronoamperometry
Both cyclic voltammetry (CV) and chronoamperometry methods were investigated for PEDOT coating. The CV method involved cycling between two different potentials for a number of times. One CV experiment involved cycling between −0.5 and 1.2 V, and another involved cycling between −0.5 and 2.0 V. Cycle duration varied between as short as 30 s per cycle to as high as 200 s per cycle. The scan rate was set as a constant of 100 mV/s. Chronoamperometry involved the application of a constant potential for the duration of the coating process. For the chronoamperometry experiments, the potential was tested at 1.0, 1.2 and 2.0 V, and the duration of the electrochemical deposition ranged between 1,000 and 2,000 s.
2.3.2 Pristine versus recycled EDOT/IL bath
The effects of pristine versus recycled EDOT/IL bath on the coatings were investigated in this study as an effort to reduce the deposition cost. Initially, a freshly prepared 1 M EDOT/IL electrolyte bath solution (called pristine bath) was used for deposition. After initial deposition, the same bath was recycled to coat other Mg substrates. As the EDOT polymerized onto the Mg substrates, the bath solution changed color from clear to light yellow, to opaque brown, and eventually to black. The color of the recycled baths turned darker with each reuse (Fig. 2). To compare the Pristine versus Recycled EDOT/IL Bath, Mg substrates were coated using CV ranging from −0.5 to +2.0 V for 5 cycles at 200 s per cycle with a constant scan rate of 100 mV/s.
2.3.3 Electrodes
Mg samples were utilized as the working electrodes. Counter electrodes for this experiment included platinum (Pt, CH Instruments) or stainless steel (SS, Miniscience). Reference electrodes included silver/silver chloride (Ag/AgCl, CH Instruments) or copper (Cu, Miniscience). The platinum electrode consisted of a wire that was coiled in a spring like shape to fit into the electrochemical cell. The silver/silver chloride electrode was enclosed in a glass sleeve with a permeable tip and was used directly as purchased. After establishing the coating parameters using the Pt and Ag/AgCl electrodes, the Cu and SS electrodes were investigated as alternative low-cost electrodes. A flat sheet of Cu was cut into a dimension of 10 × 8 × 0.1 mm to produce the Cu electrode. The SS electrode was prepared similarly, with a dimension of 14 × 5 × 0.2 mm. Both electrodes were ultrasonically washed in ethanol for 15 min. To compare the Pt and Ag/AgCl electrodes with the Cu and SS electrodes, Mg substrates were coated using CV ranging from −0.5 to +2.0 V for 5 cycles at 200 s per cycle with a constant scan rate of 100 mV/s.
2.3.4 Post-coating treatment
Three different procedures for post-coating treatment were investigated: (1) the coated Mg samples were ultrasonically washed in ethanol for 5 min; (2) the samples were quickly rinsed with deionized (DI) water (Millipore) followed by ethanol; and (3) the samples were not rinsed at all. Afterwards, all the PEDOT coated Mg samples were dried in a vacuum oven at room temperature for 24 h.
2.4 Characterization of surface microstructure and composition of PEDOT coating
After post-coating treatment, the surface morphology of PEDOT coated Mg samples was characterized using a field-emission scanning electron microscope (SEM, Philips XL-30) at a 15 kV accelerating voltage. Energy dispersive X-ray spectroscopy (EDS) analysis was performed on the respective SEM image to determine the surface composition and elemental distribution.
2.5 Adhesion strength of PEDOT coating: ASTM D3359 tape test
The adhesion strength of PEDOT coating on the Mg samples was measured by the tape test following ASTM D3359 standards. Briefly, the PEDOT coated surface was first marked with 5 × 5 mm crosscut grids using a cutter. The 3 M No. 3710 tape was attached and pressed onto the etched surface. The tape was then peeled off at a 180° angle. The remaining PEDOT coating on Mg was characterized for adhesion strength according to the ASTM classification system that ranges from 0B to 5B. A strong adhesion between the coating and substrate is classified as 5B (no coating detaches from the substrate). A weak adhesion strength is classified as 0B (65 % or more of the coating detaches).
2.6 Corrosion potential of PEDOT coated Mg versus non-coated Mg
The PEDOT coating was deposited using the CV method for 5 cycles with the potential ranging from −0.5 to +2.0 V for 200 s. The scan rate was set as a constant of 100 mV/s. The corrosion rate of the PEDOT coated Mg versus non-coated Mg was measured according to the Tafel test. The test procedure was carried out in a very similar manner as a CV experiment with the potential ranging from −2.0 to 0.5 V for 30 s with a constant scan rate of 100 mV/s. All the samples were tested in a simulated body fluid (SBF) with a pH of 7.4. Using the potential versus current (reported in a log scale) potentiodynamic polarization curves, the corrosion current was extrapolated and plugged into the following equation to calculate the corrosion rate:
where CR = corrosion rate, ICORR = corrosion current, K = constant for converting units, EW = equivalent weight of Mg, ρ = density of corroding species (Mg), and A = area of substrate submerged in SBF.
3 Results
3.1 Morphology and composition of PEDOT coating
Scanning electron micrographs and EDS analyses showed that PEDOT coated Mg had different surface topography and composition as compared with non-coated, polished Mg (Fig. 3). The PEDOT coating was prepared by CV method with the potential ranging from −0.5 to +2.0 V for five cycles, 200 s per cycle. The PEDOT coating appeared to have a micro-porous morphology. The EDS spectrum of non-coated Mg showed a small amount of oxygen (<5 wt%) even after polishing since Mg was prone to oxidation. The EDS analysis of PEDOT coated Mg showed the presence of sulfur (S), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and a trace amount of Mg (<1 at.%).
3.2 The effects of coating methods on surface microstructure and composition
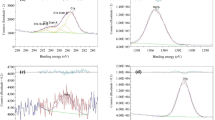
The PEDOT coating was deposited using both CV and chronoamperometry. During CV deposition, as the number of cycles or coating time increased, the coating thickness increased. Five cycles of CV with the potential ranging from −0.5 to +2.0 V yielded a uniform coating. Figure 4a shows the SEM image of the PEDOT coated Mg using the CV method for 5 cycles in a pristine EDOT/IL bath. Figure 4c shows the EDS analysis of Fig. 4a, indicating sufficient coating by the peaks of S, C, and O.
a SEM image of PEDOT coated Mg using CV method for 5 cycles (the potential ranging from −0.5 to +2.0 V, 200 s per cycle) in the pristine EDOT/IL bath. b SEM image of PEDOT coated Mg using chronoamperometry method at 1.2 V for 2,000 s in the pristine EDOT/IL bath. c EDS analysis conducted on image (a). d EDS analysis conducted on image (b). Scale bars = 200 μm
During chronoamperometric deposition, the greater the coating duration time, the thicker the coating. Higher voltages led to the appearance of coatings at a faster rate in the electrochemical cell. At voltages below 1.2 V, PEDOT coating did not form during the entire cycle of 2,000 s. At voltages of 2 V or greater, the coatings formed within the first few hundred seconds of the cycle. Figure 4b shows the SEM image of the PEDOT coated Mg using the chronoamperometry method at 1.2 V for 2,000 s in a pristine EDOT/IL bath. Figure 4d shows the EDS analysis of Fig. 4b, indicating the presence of PEDOT coating by the peaks of S, C, and O.
3.3 Comparison of PEDOT coatings produced in pristine versus recycled EDOT/IL bath
The PEDOT coatings were uniform with the use of a pristine EDOT/IL bath. A 1 M concentration of EDOT/IL was sufficient for forming the PEDOT coating on the Mg samples with a size of 5 × 5 × 0.25 mm. The pristine bath was clear and transparent before electrochemical deposition. When the recycled baths were used, the coatings were still able to form; however, the larger particles of previously polymerized EDOT were deposited onto the Mg substrates, leading to a non-uniform surface morphology. Figure 5 shows SEM images of PEDOT coated Mg samples using the CV method with the potential ranging from −0.5 to 2 V for 200 s per cycle for 5 cycles. In Fig. 5a, c), the PEDOT coating was deposited in a pristine bath of EDOT/IL and the coating morphology appeared uniform with well-defined particulate features. In contrast, the PEDOT coating that was deposited in a recycled bath of EDOT/IL showed non-uniform morphology, as shown in Fig. 5b, d). When comparing the EDS results in Fig. 5e, f), the elemental compositions of both PEDOT coatings were similar regardless of the type of bath used for deposition. That is, the presence of S, C, O, and a trace amount of Mg indicated the presence of PEDOT coating on Mg samples. Even though the PEDOT coating could be deposited on Mg samples in both the pristine and recycled bath, the SEM images showed significant differences in coating morphology and uniformity.
The PEDOT was deposited by the CV method with the potential ranging from −0.5 to 2 V for 200 s per cycle for 5 cycles in either pristine or recycled electrolyte bath. a, c SEM images of PEDOT coated Mg produced in a pristine bath at a magnification of a 80× and c 1,000×. b,d SEM images of PEDOT coated Mg produced in the recycled bath at a magnification of b 80× and d 1,000×. Scale bars = 500 μm for images (a, b). Scale bars = 50 μm for images (c, d). e EDS analysis conducted on image (a). f EDS analysis conducted on image (b). The microstructures of the PEDOT coating produced in the pristine bath appeared more uniform than that produced in the recycled bath. The EDS results showed that the elemental compositions of PEDOT coatings produced in both conditions were similar, and they both had high sulfur and low Mg content, indicating the presence of PEDOT coating on Mg samples
3.4 The effects of different electrodes on the PEDOT coating
To explore the potential of using low-cost electrodes for PEDOT deposition, Cu and SS electrodes were investigated as alternative reference and counter electrodes, respectively. Figure 6a shows PEDOT coated Mg using Cu and SS electrodes in a pristine EDOT/IL bath. EDS analysis (Fig. 6b) indicated the presence of PEDOT coating on the Mg samples, and no Cu ions were detected. The PEDOT coating produced using Cu and SS electrodes showed similar surface morphology and elemental composition as the coating previously produced using platinum and Ag/AgCl electrodes.
a SEM image of the PEDOT coating on Mg samples when the coating was electrochemically deposited using copper (Cu) and stainless steel (SS) electrodes. The PEDOT coating was deposited using CV method with the potential ranging from −0.5 to 2 V for 200 s per cycle for 5 cycles. b EDS analysis was conducted on image (a)
3.5 Other parameters affecting PEDOT deposition
The use of a polished Mg surface versus as-rolled oxidized surface as substrates for electrochemical deposition seemed to have no effect on the coating morphologies and compositions based on SEM and EDS analyses. Ultrasonic wash in ethanol immediately after electrodeposition led to removal of the coating. Before setting the samples to dry, a quick rinse with DI water followed by ethanol was necessary to remove excess IL. Once the samples were vacuum dried for at least 24 h, they were ready for further characterization and evaluation, such as SEM imaging, EDS analyses, adhesion strength testing, and corrosion testing.
3.6 Adhesion strength of PEDOT coating on Mg
The tape test can determine how well a coating adheres to the substrate of interest. The tape test results showed that the coating adhesion strength was within the classifications of 3B to 4B as only small flakes of the PEDOT coating were detached along the intersections of cuts (less than 15 % of the lattice area). Figure 7a shows the surface of PEDOT coated Mg sample before making a 5 × 5 mm crosscut grid. Figure 8b shows the grids created by a cutter according to ASTM D3359 standard. Figure 8c shows the sample after the tape removal, which was used to determine the coating adhesion strength as between 3B and 4B. Figure 8d shows the PEDOT remnants on the tape itself.
Adhesion strength of PEDOT coated Mg was evaluated by tape test following ASTM D3359 standard. a PEDOT coating deposited on Mg substrate using the CV method with the potential ranging from −0.5 to 2 V for 200 s per cycle for 5 cycles. b Grids of 5 × 5 mm crosscut were created on PEDOT coating using a cutter. c Grid appearance after tape removal. d PEDOT remnants on the tape. The adhesion strength of PEDOT coating on Mg was classified as between 3B and 4B
Potentiodynamic polarization curves of PEDOT coated Mg and non-coated Mg in simulated body fluid (SBF) at pH 7.4. The coating was deposited using the CV method with the potential ranging from −0.5 to +2.0 V for 5 cycles. Scanning parameters of the Tafel test ranged from −2.0 to +0.5 V at a scanning rate of 100 mV/s. After extrapolation, the corrosion current (ICORR) for PEDOT coated Mg was 6.14 × 10−5 amps, yielding a corrosion rate of 2.64 mm/year; ICORR for non-coated Mg was 9.08 × 10−4 amps, yielding a corrosion rate of 38.98 mm/year
3.7 Improved corrosion potential
Figure 8 shows the potentiodynamic polarization curves of PEDOT coated Mg and non-coated Mg in SBF. After extrapolation, the corrosion current (ICORR) for PEDOT coated Mg was 6.14 × 10−5 amps, yielding a corrosion rate of 2.64 mm/year. ICORR for non-coated Mg was 9.08 × 10−4 amps, yielding a corrosion rate of 38.98 mm/year. The PEDOT coating did decrease the corrosion rate of Mg according to the Tafel test.
4 Discussion
4.1 Morphology and composition of PEDOT coating
Electrochemical deposition parameters affected the microstructure of the PEDOT coatings on Mg. When coated in a pristine bath, SEM images of the PEDOT coatings showed a uniform micro-porous structure, which matched with the results observed in previous studies [12, 21, 22]. The microstructure of PEDOT determines its conductivity [23]. The conductivity decreases with loosely packed polymers that have high inter-chain distances [24]. The electrochemical deposition method allows for precise control of polymer growth on the working electrode. The EDS spectra of the PEDOT coated Mg showed high S, C, and O peaks and low Mg peaks, which indicated PEDOT coating on the substrate since PEDOT consists of S, C, and O. The F peaks indicated that the anion F− in the ionic liquid was transferred onto Mg surface during the electrochemical deposition [25].
4.2 Adhesion strength of PEDOT coating
The ASTM standard tape test is crucial for determining the PEDOT coating reliability for medical implant or device applications, particularly if it is under friction or wear [26]. The PEDOT coatings on Mg had acceptable adhesion strength on the Mg substrates for biomedical applications. High adhesion strengths have also been observed with PEDOT-PSS nano-composite films coated on titanium substrates [27]. Surface preparation has been shown to affect PEDOT adhesion strength on platinum, gold, indium tin oxide, and silver substrates [28]. The superior adhesion strength of coating was reported when the substrates were ultrasonically washed in acetone before deposition [28].
4.3 Corrosion potential of PEDOT coated Mg versus non-coated Mg
The corrosion potential of PEDOT coated Mg increased by about 250 mV. This yielded a slower corrosion rate for the coated Mg. Similar results were reported in literature; that is, the corrosion potential of PEDOT coated Mg increased by about 120 mV and the corrosion current decreased by about 50 % [12]. These findings indicated that PEDOT coatings indeed improved the corrosion resistance of Mg.
4.4 PEDOT coated Mg for potential neural applications
This study confirmed that the PEDOT coating could decrease Mg degradation in SBF and it had acceptable adhesion strength for medical applications. Combining the results of this study with the previous in vitro and in vivo biological studies [4–8], PEDOT coated Mg could potentially integrate the benefits of PEDOT and Mg for neural applications. To further improve biological connection with neurons, co-deposition of the peptide DCDPGYIGSR onto the surface may be needed to enhance neuron binding abilities of the PEDOT on the biodegradable Mg alloys. It has been reported that rat glial cells preferentially attached and grew on electrode sites containing the peptide after 24 h of in vitro culture [15]. PEDOT coated Mg should be further studied in cell culture systems and in animal models to evaluate its interactions with nerve cells and its capacity in stimulating nerve regeneration.
5 Conclusion
The PEDOT coating was electrochemically deposited on large Mg substrates in a 1 M EDOT/IL electrolyte solution successfully using either CV or chronoamperometry method. With longer coating time or more CV cycles, more EDOT can be polymerized onto the Mg substrate, resulting in a thicker coating. As the EDOT concentration increases in the pristine bath, the electrochemical deposition process can be used to coat larger Mg substrates for future cell studies. Alternative electrodes such as Cu and SS can be as effective as the more costly Pt and Ag/AgCl electrodes for this coating process. The electrochemically deposited PEDOT coating on Mg showed micro-porous structure and contained the elements of S, C, and O. The corrosion rate of the PEDOT coated Mg, calculated from Tafel tests, was 2.64 mm/year, much slower than 38.98 mm/year for the non-coated Mg. The adhesion strength of PEDOT coating was within the classifications of 3B to 4B. Future studies are needed to determine the in vitro degradation rate of PEDOT coated Mg implants and how relevant cells respond to the PEDOT coated Mg in vitro and in vivo.
References
Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–72.
Johnson I, Perchy D, Liu H. In vitro evaluation of the surface effects on magnesium-yttrium alloy degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2011;. doi:10.1002/jbm.a.33290.
Kainer KU, ed. Magnesium: proceedings of the 7th international conference on magnesium alloys and their applications; 2007. p 1126.
Muir KW. New experimental and clinical data on the efficacy of pharmacological magnesium infusions in cerebral infarcts. Magnes Res. 1998;11(1):43–56.
Clarkson AN. Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci. 2007;80(13):1157–75.
Gupta VK. Intravenous magnesium for neuroprotection in acute stroke: clinical hope versus basic neuropharmacology. Stroke. 2004;35(12):2758.
Saver JL, Kidwell C, Eckstein M, Starkman S, Investigators F-MPT. Prehospital neuroprotective therapy for acute stroke—Results of the field administration of stroke therapy-magnesium (FAST-MAG) pilot trial. Stroke. 2004;35(5):E106–8.
Campbell K, Meloni BP, Zhu HD, Knuckey NW. Magnesium treatment and spontaneous mild hypothermia after transient focal cerebral ischemia in the rat. Brain Res Bull. 2008;77(5):320–2.
Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–92.
Liu H. The effects of surface and biomolecules on magnesium degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2011;99(2):249–60.
Guan RG, Johnson I, Cui T, Zhao T, Zhao ZY, Li X, Liu H. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J Biomed Mater Res A. 2012;100(4):999–1015.
Luo XL, Cui XYT. Electrochemical deposition of conducting polymer coatings on magnesium surfaces in ionic liquid. Acta Biomater. 2011;7(1):441–6.
Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim DH, Martin DC. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28(8):1539–52.
Luo SC, Ali EM, Tansil NC, Yu HH, Gao S, Kantchev EAB, Ying JY. Poly(3,4-ethylenedioxythiophene) (PEDOT) nanobiointerfaces: thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir. 2008;24(15):8071–7.
Cui XY, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens Actuators B Chem. 2003;89(1–2):92–102.
Yang JY, Kim DH, Hendricks JL, Leach M, Northey R, Martin DC. Ordered surfactant-templated poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomater. 2005;1(1):125–36.
Bobacka J, Lewenstam A, Ivaska A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J Electroanal Chem. 2000;489(1–2):17–27.
Boretius T, Schuettler M, Stieglitz T. On the stability of poly-ethylenedioxythiopene as coating material for active neural implants. Artif Organs. 2011;35(3):245–8.
Urbanchek MG, Shim BS, Baghmanli Z, Wei B, Schroeder K, Langhals NB, Miriani RM, Egeland BM, Kipke, Martin DC, et al. Conduction properties of decellularized nerve biomaterials. IFMBE Proc. 2010;32:430–3.
Melanie Urbanchek MRA, Engeland B, Miriani RM, Schroeder K, Daneshvar E, Ewig K, Kuzon WM, Kipke DR, Cederna PS. Nerve Regeneration through PEDOT, an electrically conducting polymer nerve graft. Plast Surg; 2009. p. 124 (4S): 67.
Yang JY, Martin DC. Microporous conducting polymers on neural microelectrode arrays II Physical characterization. Sens Actuators A Phys. 2004;113(2):204–11.
Zhong YH, Bellamkonda RV. Biomaterials for the central nervous system. J R Soc Interface. 2008;5(26):957–75.
Tamburri E, Sarti S, Orlanducci S, Terranova ML, Rossi M. Study of PEDOT conductive polymer films by admittance measurements. Mater Chem Phys. 2011;125(3):397–404.
Chandrasekhar P. Conducting polymers fundamentals and applications. New York: Springer; 1999.
Macfarlane DR, Forsyth M, Howlett PC, Pringle JM, Sun J, Annat G, Neil W, Izgorodina EI. Ionic liquids in electrochemical devices and processes: managing interfacial electrochemistry. Acc Chem Res. 2007;40(11):1165–73.
Peter J, Blau KGB. Development and use of ASTM standards for wear testing. Wear. 1999;225–229(Part 2):1159–70.
Muto MIT, Kobayashi K, Miyasaka T. Conductive polymer-based mesoscopic counterelectrodes for plastic dye-sensitized solar cells. Chem Lett. 2007;36(6):804–5.
Kozak A. Effect of deposition characteristics on electrochemically prepared PEDOT films. NNIN REU Research Accomplishments 2010:20–21.
Acknowledgments
This material is based upon study supported by the National Science Foundation under Grant 1125801. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors would also like to thank the University of California for financial support. We would like to thank the Central Facility for Advanced Microscopy and Microanalysis (CFAMM) at the University of California, Riverside, thank Drs. X. Luo, X.T. Cui, and V. Vullev for their advice on electrochemistry, and thank Mitch Boretz for proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meriam A. Sebaa and Shan Dhillon contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sebaa, M.A., Dhillon, S. & Liu, H. Electrochemical deposition and evaluation of electrically conductive polymer coating on biodegradable magnesium implants for neural applications. J Mater Sci: Mater Med 24, 307–316 (2013). https://doi.org/10.1007/s10856-012-4796-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4796-y